Abstract
Several indole ethyl isothiocyanate (IEITC) analogs were designed, synthesized and screened to evaluate their cytotoxicity against neuroblastoma (NB) cells in-vitro. In NB, predominantly a tumor of early childhood, survival remains low despite aggressive treatments. Therefore, novel treatment strategies are greatly needed. The objective of the present study was to study the therapeutic potential of IEITC by analyzing the cytotoxic, anti-proliferative and apoptotic effects on NB cell lines. 7-methyl-indole-3-ethyl isothiocyanate (7Me-IEITC) proved to be cytotoxic to various NB cell lines (SMS-KCNR, SK-N-SH, SH-SY5Y, IMR-32) with an IC50 at 2.5-5.0 μM, while primary control cells (lung fibroblasts) were not affected. 7Me-IEITC led to the activation of apoptotic markers caspase-3, - 8 and -9, caused activation of pro-apoptotic p38 MAPK and SAP/JNK, and down-regulated pro-survival factor AKT in SMS-KCNR cells. Moreover, 7Me-IEITC displayed anti-proliferative effects (IC50 at 600 nM) and caused an arrest in cell cycle progression. This wide effect of 7Me-IEITC on NB cell signaling and survival suggests that it could be developed as a therapeutic agent against neuroblastoma.
Neuroblastoma (NB), predominantly a tumor of early childhood is the most common extracranial solid tumor. Two-thirds of the cases occur in children younger than 5 years of age. NB account for 7–10% of all childhood cancers; in the majority of patients older than 1 year of age the disease is fatal.1 There are approximately 500–1000 new cases of NB in the U.S. each year.2 Treatment methods currently available include surgery, radiation therapy, chemotherapy, and autologous stem-cell transplantation.3–5 However, despite intensive multimodality treatment, more than 50% of children with high-risk disease relapse, due to drug-resistant residual disease.6–8 Eradication of refractory microscopic disease remains one of the most significant challenges in the treatment of the high-risk NB and innovative treatments for children with neuroblastoma need to be developed.
Isothiocyanates (ITC) are currently being investigated as anti-tumor agents and in animal models ITC have been shown to inhibit chemically induced tumor genesis in the lung, stomach, colon, liver, esophagus, bladder, and mammary glands.9 Natural ITCs exist as glucosinolates in plants and their release is catalyzed by myrosinase enzymes. Several mechanism for the activities of ITC in cancer treatment have been proposed, such as (i) induction of apoptosis and G2/M cell cycle block10, (ii) inhibition of phase-I and –II carcinogen-activating enzyme9 (iii) reduction of NF-kB binding to DNA11, (iv) inhibition of histone deacetylase12 and (vi) up-regulation of thioredoxin reductase-1.13 Various other effects such as disruption of microtubulin polymerization14 and disruption of the mitochondrial membrane potential have been reported.15 Interestingly, various ITC such as naphthyl ITC (NITC), phenethyl ITC (PEITC) and benzyl ITC (BITC) (Figure 1a) inhibit activation and/or function of factors implicated in emergence of multi-drug resistance.16
Figure 1. Naturally occurring ITC; design and structure of novel Indole ethyl ITC (IEITC).
(a) Various naturally occurring ITC: (i): BITC; (ii): PEITC; (iii) Sulforaphane
(b) Design of novel IEITC including 7Me-IEITC (2g)
(c) Synthesis of IEITC: Tryptamines, Thiophosgene (1.1 eq), 20% NaHCO3, EtOAC+H20, 1 hr; yield: 70–75% after purification through preparative thin layer chromatography.
Naturally occurring non-ITC indole derivatives exhibit potent anti-proliferative activity, induce apoptosis and cause cell cycle arrest in many human solid and non-solid tumors.17 The objective of the present study was to identify an isothiocyanate class (based on an indole scaffold) with improved anti-cancer activity. We observed that indole ethyl isothiocyanates (IEITC) are structurally very close to benzyl isothiocyanate (BITC) and phenyl ethyl isothiocyanate (PEITC) that display anti-cancer activity.16,18,19 The present report describes the syntheses and cytotoxic activities of seven IEITC analogs in SK-N-SH NB cell lines to determine if IEITCs are potential anti-NB drugs. We analyzed the effect of the highly cytotoxic compound 7Me-IEITC on the viability of four NB cell lines. In addition, we investigated the therapeutic potential of 7Me-IEITC by analyzing its effects on caspase activation, activation of pro-apoptotic markers (JNK, p38), suppression of pro-survival marker Akt and on cell proliferation and cell cycle progression in SMS-KCNR NB cells.
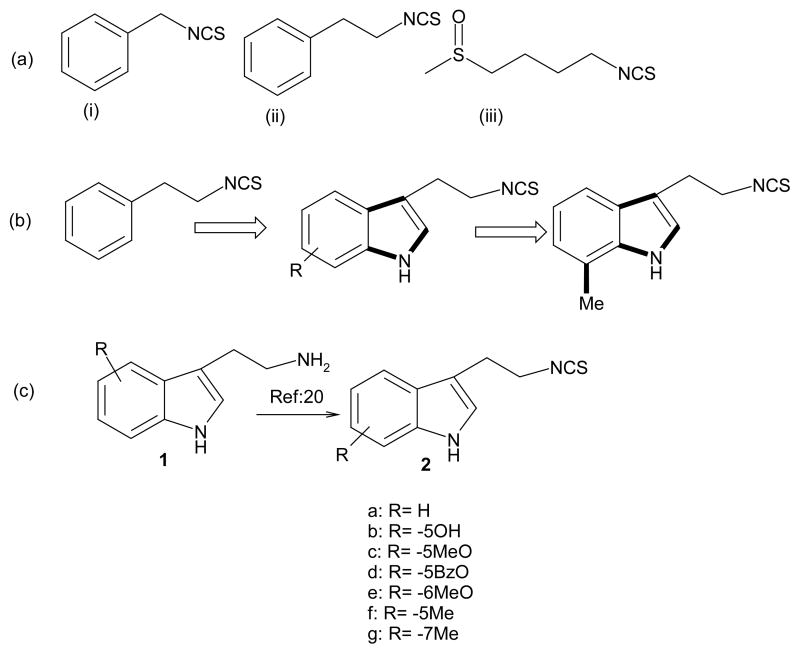
Various commercially available tryptamine derivatives were converted to corresponding isothiocyanates in one step (70–75% yield after purification) by following a recent protocol.20 The derivatives (Figure 1c) were characterized by IR, NMR and mass spectrometry. As an initial approach to evaluation of the cytotoxicity of indole ethyl ITC analogs, all derivatives were screened against the SK-N-SH NB cell line21 in a cell viability assay (CellTiter 96® AQueous Cell Assay; Promega Corp, Madison, WI).22 An interesting IEITC structure-activity relationship emerged. Substituting a phenyl ring (PEITC; Figure 1a) for an indole ring (compound 2a) leads to a significant increase in cytotoxicity (Figure 2). The nature and position of further substitutions on the indole moiety affected the cytotoxic activity significantly: we observed that polar groups such as -OH (2b) and -OMe (2c) did not significantly increase the cytotoxicity (2a), whereas Me- (2e, 2g) and Benzyloxy (BzO) (2d) substituents dramatically increased the cytotoxic activity against NB cells (IC50 below 2.5 μM; Figure 2). We hypothesize that non-polar group substitutions at the 5- or 7-positions are essential for the biological activity of this family of compounds.
Figure 2. Comparative analysis of the cytotoxic effect of various IEITC analogs in a human NB cell line (SK-N-SH).
SK-N-SH NB cells were treated with various concentrations (2.5 μM to 40μM) of IEITCs 2a–2g for 48hrs. A viability assay (Promega Corp, Madison, WI) was carried out.21 Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X±SD) of a representative experiment in % cell viability of untreated cells [100%].
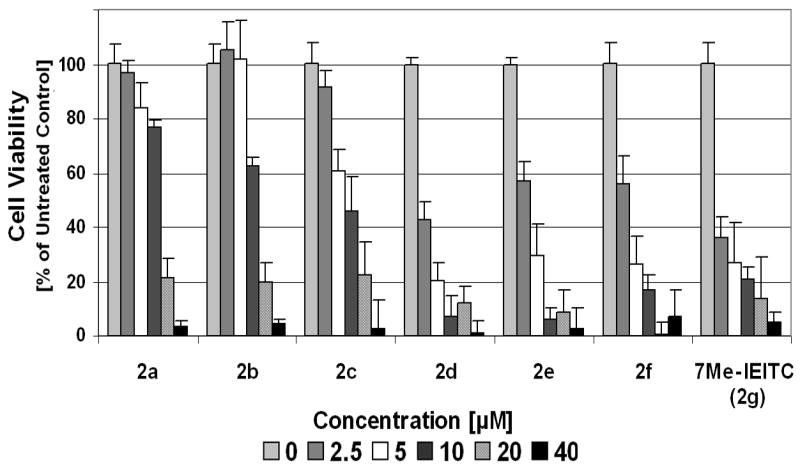
Compounds 7Me-IEITC (2g) and 5BzO-IEITC (2d) were subsequently screened against three additional neuroblastoma cell lines21 (SMS-KCNR, IMR-32, SH-SY5Y; Figure 3). Both compounds 2d and 7Me-IEITC (2g) dose-dependently reduced the viability of all NB cell lines. 7Me-IEITC (2g) was selectively cytotoxic for NB cells. The viability of primary lung fibroblasts (LF, passage 10), which like NB cell lines possess a high metabolism and growth rate and, thus, were used as controls, were not affected by 7Me-IEITC treatment (Figure 3). Even though 7Me-IEITC (2g) displayed IC50 cytotoxicity values (50% death as compared to the untreated control) between 2.5 and 5.0 μM in various NB cell lines, fibroblasts were not significantly affected even at drug concentrations as high as 20 μM (Figure 3).
Figure 3. Comparative analysis of the cytotoxic effect of 5BzO-IEITC and 7Me-IEITC in various NB cell lines.
NB cells (SMS-KCNR, SH-SY5Y, IMR-32) and primary fibroblasts (LF, control cells) were treated with various concentrations (2.5 μM to 40 μM) of 5BzO-IEITC (2d) or 7MeIEITC (2g) for 48 hrs and the MTS viability assay was carried out. Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X±SD) of a representative experiment in % cell viability of untreated cells [100%].
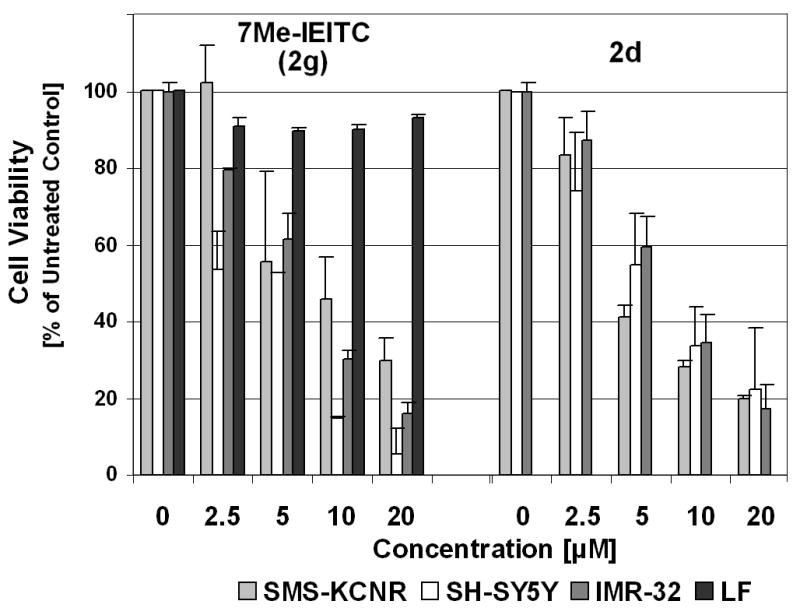
To define the cellular response of NB cells to treatment with 7Me-IEITC (2g) we next analyzed the expression and/or activation of cellular markers that are hallmarks of pro-survival (Akt), pro-apoptotic signaling (JNK, p38 MAPK) or directly indicate apoptotic responses (such as caspase-3, -8, -9, and PARP-1).
The effect of 7Me-IEITC (2g) on the activity of JNK, p38 MAPK and Akt was studied by immunblotting of PAGE separated lysates of treated cells using antibodies23 specifically recognizing the inactive, as well as the phosphorylated active form of these proteins. 7Me-IEITC (2g) caused a rapid (within 1hr), strong and sustained activation (peak at 18 hrs) of p38 and JNK, along with a delayed up-regulation of non-phosphorylated p38 and JNK (Figure 4A). This figure also shows that SMS-KCNR cells show a high basic level of activation (phosphorylation) of the pro-survival factor Akt in untreated cells, which within 36 hrs of 7Me-IEITC (2g) treatment was down-regulated.
Figure 4.
Panel A, expression of apoptotic and pro-survival markers in NB cells after 7Me-IEITC treatment: SMS-KCNR NB cells were treated with 3μM 7Me-IEITC (2g). Detection of proteins in the lysates of treated and untreated cells by PAGE and Western Blot analysis was carried out as described.23 Caspase activation: primary antibodies against pro- and activated Caspase-3, -8, -9, and inactivated/cleaved PARP-1. As an internal standard for equal loading, blots were probed with an anti-beta-Actin antibody. Kinase activation: primary antibodies against phosphorylated and inactive JNK, p38 and AKT.
Panel B, effect of various caspase inhibitors on the cytotoxicity of 7Me-IEITC in SMS-KCNR cells: SMS-KCNR NB cells were pre-incubated with 40 μM caspase inhibitors (Z-VAD-FM/pancaspase, Z-DEVD-FMK/caspase-3, Z-IETD-FMK/caspase-8 and Z-LEHD-FMK/caspase-9) for 2 hrs. 7Me-IEITC (5.0 μM) was added to the cells for 48 hrs and cell viability assessed. Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X±SD) of a representative experiment in % cell viability of untreated cells [100%].
Akt plays important role in cell survival and proliferation and has been strongly implicated in development of resistance against chemotherapy agents such as Paclitaxel, Cisplatin, Vincristine and Rapamycin in various human solid tumors.24 In contrast, JNK and p38 MAPK are involved in the apoptotic response to cytotoxic agents.25 Activation of p38 and JNK has been observed in human breast cancer cells treated with AplidinTM26 a depsi-peptide molecule undergoing Phase-II clinical trials. JNK mediates the apoptosis induced by DNA-damaging drugs such as Etoposide (VP-16) and Camptothecin in human myeloid leukemia cells27 and of Vinblastine in KB3 lung carcinoma cells.28 In MDA-MB-231 breast cancer cells, Taxol induced apoptosis via JNK, which caused inactivation of the anti-apoptotic Bcl-2 protein.29 Taxol has also been shown to increase p38 MAPK, ERK, and JNK activities in human breast cancer cells.30 Similarly, our present study suggests that 7Me-IEITC (2g) suppresses pro-survival signaling and induces pro-apoptotic signaling in SMS-KCNR NB cells (Figure 4A).
Apoptosis is executed by caspases: initiator caspases (such as caspase-2, -8, -9, and -10) function mainly as upstream apoptotic signals. Once activated, the initiator caspases cleave and activate downstream effector caspases (such as caspase-3, -6, and -7), which are responsible for the cleavage of many intracellular proteins, leading to the morphological and biochemical changes associated with apoptosis.31,32 Accordingly, immunblotting of lysates of SMS-KCNR NB cells confirmed that 7Me-IEITC (2g) treatment resulted in strong activation/cleavage of caspase-3, -8 and 9 (Figure 4A) and PARP-1, another hallmark of apoptosis. The direct consequence of the induction of apoptosis by 7Me-IEITC (2g) is the reduction of viability in NB cells as demonstrated in (Figure 4B). We employed various caspase inhibitors (Calbiochem, LaJolla, CA), which were added to NB cell cultures 2 hrs prior to treatment with 7Me-IEITC (2g) (at IC50 concentration of 5.0 μM). Cytotoxicity of the drug was reduced by ~2/3 using a specific pancaspase inhibitor (Z-VAD-FMK), by ~1/2 using a specific caspase-3 inhibitor (caspase-3), and by 1/3 with specific caspase-8 (Z-IETD-FMK) or -9 (Z-LEHD-FMK) inhibitors (Figure 4B).
The strong activation of Caspase-8 in SMS-KCNR cells by 7Me-IEITC (2g), evident in our western blotting and cytotoxicity/inhibitor studies, is of special significance. Caspase-8 regulates the survival and invasive capacity of neuroblastoma cells.33 Suppression of caspase-8 expression results in metastasis of neuroblastoma in vivo, and reconstitution of caspase-8 expression in deficient neuroblastoma cells suppressed their metastasis.33 Our experiments indicate that 7Me-IEITC (2g) exhibited the potential to activate caspase-8 and, thus, can potentially be considered to correct aberrations in caspase-8 expressions of aggressive neuroblastoma cells.
As described in the previous section 7Me-IEITC (2g) acts as a cytotoxic drug and leads to a protein expression profile characteristic for apoptotic events. To investigate if 7Me-IEITC (2g) affects the proliferation of NB cells, (particularly at the drug concentration of 3μM, when cell viability is only partially reduced), we performed BrdU incorporation assays and cell cycle analysis. The BrdU/Proliferation assay is a colorimetric assay34 and spectroscopic data directly correlate to the amount of BrdU incorporated into the DNA, which in turn represents proliferation. Figure 5A demonstrates that 7Me-IEITC (2g) dose-dependently reduced SMS-KCNR proliferation. Even at low drug concentrations (600 nM) BrdU incorporation was dramatically suppressed when compared to untreated cells (Figure 5A). Cell cycle analysis35 revealed that 7Me-IEITC (2g) treatment of NB cells at 3.0 μM lead to a high percentage of cells in the apoptotic sub-G0/G1 (Figure 5B) as early as 12 hrs following treatment. The apoptotic sub-G0/G1 population represents cells with significant DNA damage. This observation directly correlates with the reduction of SMS-KCNR viability by 7Me-IEITC (2g) in concentrations between 2.5 and 5.0 μM (Figure 3). With respect to the cycling cells, 7Me-IEITC (2g) increased the number of cells in G0/G1 and caused a prominent arrest in the G2/M phase of the cell cycle (Figure 5B) in this asynchronous NB cell population. Accordingly, the block of cell cycle progression resulted in a diminished cell count in the S-phase. Even though not the objective of the present report, further studies emphasizing cancer related cell cycle features36,37 could focus on the specific checkpoints in G0/G1 and G2/M phase affected by 7Me-IEITC (2g) treatment. This would require to study the expression profile of cell cycle regulators (cyclin-dependent kinases and cyclins)38,39 in synchronized NB cultures.
Figure 5. 7Me-IEITC effect on cell proliferation and cell cycle progression in NB cells.
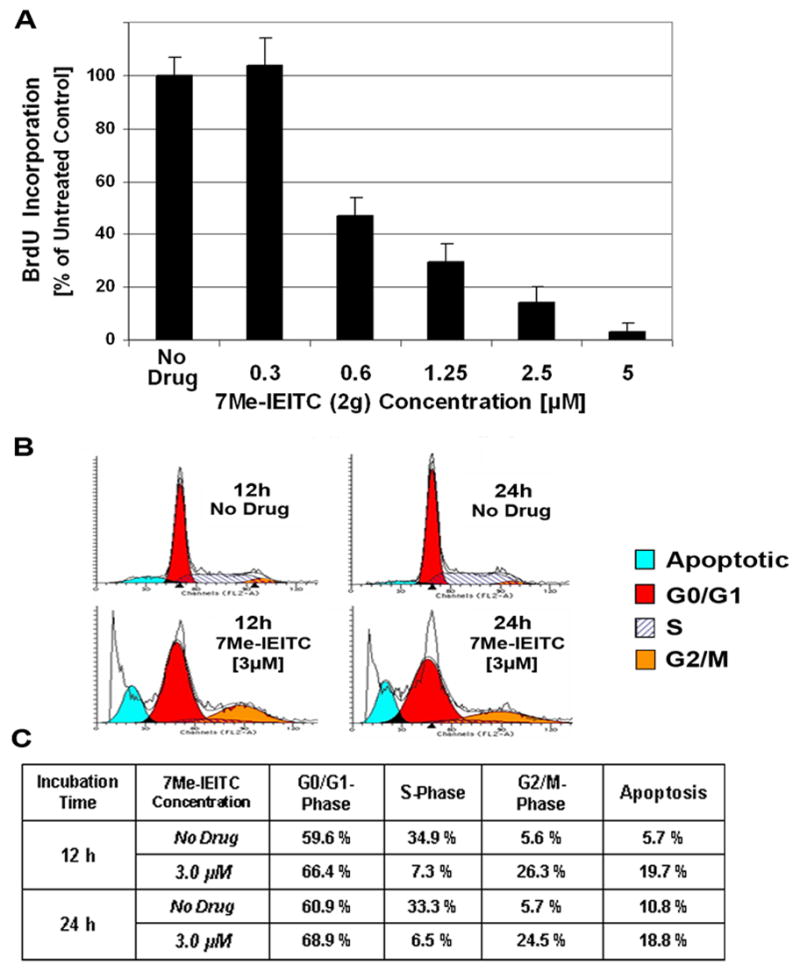
Panel A, BrdU Incorporation: NB cells (SMS-KCNR) were treated with various concentrations (300 nM to 5μM) of 7Me-IEITC (2g) for 48 hrs. The proliferation assay was carried out as described.34 Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X±SD) in % cell proliferation of untreated cells [100%].
Panel B & C, Cell Cycle Analysis by FACS: SMS-KCNR NB cells were treated with 3.0 μM 7Me-IEITC (2g) for 12 or 24 hrs. Cell cycle analysis of treated and untreated cells was carried out.35 Data are presented as the relative fluorescence intensity of cell sub-populations in the 2-dimensional FACS profile (panel B) or in % of cells in a given sub-population (panel C).
Conclusion
For the present report several indole ethyl isothiocyanate (IEITC) analogs were designed, synthesized and screened in viability assays against neuroblastoma (NB) cells in vitro. The nature and position of substitutions on the indole moiety significantly affected the cytotoxic activity. We observed that the cytotoxicity of IEITCs with non-polar groups such as -Me and -Benzyloxy (BzO) was significantly higher than of IEITCs with polar groups such as -OH and -OMe. Substitution at the 5- and 7-position (2d, 2g) resulted in an additional improvement of the cytotoxic activity against NB cells. The present report suggests that 7Me-IEITC is a potent and growth-suppressing agent in various NB cell lines (stromal S-type SMS-KCNR as well as neuronal N-type SH-SY5Y, SK-N-SH and IMR-32). 7Me-IEITC inhibited NB (SMS-KCNR) cell proliferation and cell viability along with caspase activation, inhibition of survival marker Akt and activation of pro-apoptotic p38/JNK MAPKs and could be developed as treatment for neuroblastoma.
Acknowledgments
This work was supported by funds from a Brown University Seed Grant and a NICHD, K12 HD043447 BIRCWH Scholar Grant to Dr. Brard. The authors thank Dr. Sunil K. Shaw for advice and technical guidance and NIH COBRE Grant 1-P20RR018728 for providing instrumentation support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Brodeur GM, Pizzo PA, Poplack DG. In Principles and Practice of Pediatric Oncology (Neuroblastoma) 4. Lippencott-Raven; Philadelphia: 2001. pp. 895–937. [Google Scholar]
- 2.Weinstein JL, Katzenstein HM, Cohn SL. Oncologist. 2003;8:278. doi: 10.1634/theoncologist.8-3-278. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Perez C, Seeger RC, Brodeur GM, Shimada H, Atkinson JB, Black CT, Gerbing R, Haase GM, Stram DO, Swift P, Lukens JN. J Clin Oncol. 1998;16:1256. doi: 10.1200/JCO.1998.16.4.1256. [DOI] [PubMed] [Google Scholar]
- 4.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. N Engl J Med. 1999;341:1165. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 5.Perez CA, Matthay KK, Atkinson JB, Seeger RC, Shimada H, Haase GM, Stram DO, Gerbing RB, Lukens JN. J Clin Oncol. 2000;18:18. doi: 10.1200/JCO.2000.18.1.18. [DOI] [PubMed] [Google Scholar]
- 6.Maris JM, Matthay KK. J Clin Oncol. 1999;17:2264. doi: 10.1200/JCO.1999.17.7.2264. [DOI] [PubMed] [Google Scholar]
- 7.Goldsby RE, Matthay KK. Paediatr Drugs. 2004;6:107. doi: 10.2165/00148581-200406020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Matthay KK, Atkinson JB, Stram DO, Selch M, Reynolds CP, Seeger RC. J Clin Oncol. 1993;11:2226. doi: 10.1200/JCO.1993.11.11.2226. [DOI] [PubMed] [Google Scholar]
- 9.Conaway CC, Yang-ming Y, Fung LC. Curr Drug Metabol. 2002;3:233. doi: 10.2174/1389200023337496. [DOI] [PubMed] [Google Scholar]
- 10.Jackson SJ, Singletary KW, Venema RC. Vascul Pharmacol. 2007;46:77. doi: 10.1016/j.vph.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Exp Biol Med. 2007;232:27. [PMC free article] [PubMed] [Google Scholar]
- 12.Jakubikova J, Bao Y, Sedlak J. Anticancer Res. 2005;25:3375. [PubMed] [Google Scholar]
- 13.Johnson CR, Chun J, Bittman R, Jarvis WD. J Pharmacol Exp Ther. 2004;309:452. doi: 10.1124/jpet.103.060665. [DOI] [PubMed] [Google Scholar]
- 14.Wu XJ, Hua X. Cancer Biol Ther. 2007 May 1;6:5. doi: 10.4161/cbt.6.5.4092. [DOI] [PubMed] [Google Scholar]
- 15.Yuesheng Z, Tang L, Gonzalez V. Mol Canc Therp. 2003:1045. [Google Scholar]
- 16.Heintz APM, Odicino F, Maisonneuve P, Beller U, Benedet JL, Creasman WT, Nga HYS, Pecorelli S. FIGO Annual Report. 1996–1998 [Google Scholar]
- 17.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. J Biol Chem. 2007 doi: 10.1074/jbc.M701978200. in press. [DOI] [PubMed] [Google Scholar]
- 18.Kalkunte S, Swamy N, Dizon DS, Granai CO, Brard L. J Exp Ther Oncol. 2006;5:287. [PubMed] [Google Scholar]
- 19.Satyan KS, Swamy N, Dizon DS, Singh R, Granai CO, Brard L. Gynecol Oncol. 2006;103:261. doi: 10.1016/j.ygyno.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Posner GH, Cho CG, Green JV, Zhang Y, Talalay P. J Med Chem. 1994;37:170. doi: 10.1021/jm00027a021. 2(a): IR (DCM): 3156, 2153, 2018, 1629, 1479, 1045 cm−1; 1 7.98 H NMR (CDCl3) δ (bs, 1H, NH), 7.313-7.297 (d, 2H, J=3.9 Hz, Ar), 7.131-7.129 (m, 1H, J=2.7 Hz, Ar), 6.96-6.95 (d, 1H, J=2.7 Hz, Ar), 6.79-6.74 (m, 2H, J=2.4 Hz, Ar), 3.719-3.694 (t, 2H, J=6.9Hz, CH2-Ar), 3.10-3.05 (t, 2H, J=6.3 Hz, CH2NCS); MS (ES): m/z 203 [M+H]+ [This compound has been previously reported; reference: Park S, Hayes BL, Marankan F, Mulhearn DC, Wanna L, Mesecar AD, Santarsiero BD, Johnson ME, Venton DL. J Med Chem. 2003;46:936. doi: 10.1021/jm020361k. 2(b): 1 7.978 (bs, 1H, NH), 7.29-7.27 (d, 1H, J=3.6 Hz, Ar), 7.10-H NMR (CDCl3) δ 7.09 (d, 1H, J= 2.4 Hz, Ar), 6.968-6.961 (d, 1H, J=2.1 Hz, Ar), 6.853-6.816 (dd, 1H, J=2.4 Hz, Ar), 3.737-3.692 (t, 2H, J=6.9Hz, CH2-Ar), 3.101-3.056 (t, 2H, J=6.3 Hz, CH2NCS); MS (ES): m/z 219 [M+H]+. 2(c): 1H NMR (CDCl3) δ 7.93 (bs, 1H, NH),7.22-7.215 (d, 1H, J=1.5 Hz, Ar), 7.049 (s, 1H, Ar), 6.939-6.932 (d, 1H, J=1.8 Hz, Ar), 6.862-6.825 (dd, 1H, J=2.4 Hz, Ar), 3.83 (s, 3H, OCH3), 3.746-3.701 (t, 2H, J=6.9Hz, CH2-Ar), 3.118-3.073 (t, 2H, J=6.6 Hz, CH2NCS); MS (FAB) m/z: 233 [M+H]+, 255 [M+Na]+. 2(d): 1H NMR (CDCl3) δ 8.01 (bs, 1H, NH), 7.43-7.40 (d, 2H, J=7.2 Hz, Ar), 7.351-7.187 (m, 4H, Ar), 7.024-7.0 (d, 1H, J=7.2 Hz, Ar), 6.91-6.88 (d, 1H, J=9 Hz, Ar), 5.059 (s, 2H, OCH2), 3.69-3.65 (t, 2H, J=6.6Hz, CH2-Ar), 3.076-3.031 (t, 2H, J=6.6 Hz, CH2NCS); MS ( FAB) m/z: 308 [M+H]+, 331 [M+Na]+. 2(e): 1H NMR (CDCl3) δ 8.09 (bs, 1H, NH), 7.29-7.265 (d, 1H, J=1.8 Hz, Ar), 7.049 (s, 1H, Ar), 6.94-6.93 (d, 1H, J=2.4 Hz, Ar), 6.857-6.819 (dd, 1H, J=2.1 Hz, Ar), 3.863 (s, 3H, OCH3), 3.79-3.701 (t, 2H, J=6.9Hz, CH2-Ar), 3.12-3.07 (t, 2H, J=7.2 Hz, CH2NCS); MS (FAB) m/z: 233 [M+H]+, 255 [M+Na]+. 2(f): 1H NMR (CDCl3) δ 8.11 (bs, 1H, NH), 7.93 (bs, 1H, NH), 7.41-7.44 (d, 1H, J=6.9 Hz, Ar), 7.217-7.21 (d, 1H, J=2.1 Hz, Ar), 7.033-7.131 (m, 2H, Ar), 3.733-3.752 (t, 2H, J=5.7Hz, CH2-Ar), 3.159-3.176 (t, 2H, J=5.1, CH2NCS), 2.65 (t, 3H, CH3); MS (FAB) m/z: 216 [M]+, 239 [M+Na]+. 2(g): IR (DCM): 3145, 2940, 2167, 2040, 1629, 1479, 1045 cm−1; 1H NMR (CDCl3) δ 8.08 (bs, 1H, NH), 7.39-7.41 (d, 1H, J=6.6 Hz, Ar), 7.25-7.26 (d, 1H, J=2.7 Hz, Ar), 7.041-7.13 (m, 2H, Ar), 3.76-3.77 (t, 2H, J=5.1Hz, CH2-Ar), 3.15-3.17 (t, 2H, J=5.1, CH2NCS), 2.52 (t, 3H, CH3); MS (ES) m/z: 219 [M+H]+.
- 21.Cell Culture: LF1 (primary human lung fibroblasts) and NB cell lines SK-N-SH, SH-SY5Y, IMR-32 were obtained from American Type Culture Collection (Manassas, VA). SMS-KCNR (NB) cells were provided by John Maris (CHOP, Philadelphia, PA). All cells were seeded at 5 × 105 cells/T75 cell culture flask (Corning, New York, NY) and cultured to ~ 80% confluency according to the suppliers recommendation.
- 22.Malich G, Markovic B, Winder C. Toxicology. 1997;124:179. doi: 10.1016/s0300-483x(97)00151-0. [DOI] [PubMed] [Google Scholar]
- 23.Western Blot Analysis: Cells were seeded into 100 mm2 tissue culture dishes (5 × 105 cells/dish), cultured to ~ 80% confluency, and treated as indicated, rinsed in PBS, pH 7.4, scraped off, spun down in a microcentrifuge (10,000g, 5min) and pellets resuspended in lysis buffer (1%NP-40, 20mM Tris pH 8.0, 137 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM activated sodium orthovanadate, 10μg/mL Aprotinin, 10μg/mL Leupeptin, Inhibitor Cocktail P-2714; Sigma-Aldrich, St. Louis, MO). Lysates were rocked at 4ºC for 5 min, sonicated (10 pulses 5 sec), centrifuged at 14,0000g for 10 min, and the protein concentration of the supernatant quantitated (BioRad protein estimation kit, Hercules, CA). The samples were boiled in the presence of 5X SDS-PAGE sample buffer and 50 μg total protein/lane were separated on 12% SDS-polyacrylamide gels and blotted onto PVDF membranes. The blots were blocked with 5% nonfat dry milk in PBST for 1hr at room temperature and incubated overnight at 4ºC with antibodies (purchased from Cell Signaling Technology, Beverly, MA) at a 1:1000 dilution in 5% BSA in PBST on a rotating platform. After washing in PBST the blots were incubated with secondary antibody (peroxidase-conjugated antibodies; Amersham-Pharmacia Biotech, Piscataway, NJ). The bands were visualized by enhanced chemiluminescence and autoradiography (F-Bx810 Film, Pheonix, Hayward, CA).
- 24.Kim D, Cheng GZ, Lindsley CW, Yang H, Cheng JQ. Curr Opin Investig Drugs. 2005;6:1250. [PubMed] [Google Scholar]
- 25.Mansouri A, Ridgway LD, Korapati AL, Zhang Q, Tian L, Wang Y, Siddik ZH, Mills GB, Claret FX. J Biol Chem. 2003;278:19245. doi: 10.1074/jbc.M208134200. [DOI] [PubMed] [Google Scholar]
- 26.Cuadrado A, Garcia-Fernandez LF, Gonzalez L, Suarez Y, Losada A, Alcaide V, Martinez T, Fernandez-Sousa JM, Sanchez-Puelles JM, Munoz A. J Biol Chem. 2003;278:241. doi: 10.1074/jbc.M201010200. [DOI] [PubMed] [Google Scholar]
- 27.Brozovic A, Osmak M. Cancer Lett. 2007;251:1. doi: 10.1016/j.canlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Brozovic A, Fritz G, Christmann M, Zisowsky J, Jaehde U, Osmak M, Kaina B. Int J Cancer. 2004;112:974. doi: 10.1002/ijc.20522. [DOI] [PubMed] [Google Scholar]
- 29.Zanke BW, Boudreau K, Rubie E, Winnett E, Tibbles LA, Zon L, Kyriakis J, Liu F-F, Woodgett JR. Curr Biol. 1996;6:606. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]
- 30.Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. J Biol Chem. 1996;271:31929. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 31.Salvesen GS, Abrams JM. Oncogene. 2004;23:2774. doi: 10.1038/sj.onc.1207522. [DOI] [PubMed] [Google Scholar]
- 32.Thornberry NA, Lazebnik Y. Science. 1998;281:1312. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 33.Stupack DG, Teitz T, Potter MD, Mikolon D, Houghton PJ, Vincent JK, Lahti JM, Cheresh DA. Nature. 2006;439:95. doi: 10.1038/nature04323. [DOI] [PubMed] [Google Scholar]
- 34.Cell proliferation was assessed by a BrdU assay, which measures incorporation of the pyrimidine analog, 5-bromo-2′-deoxyuridine (BrdU) during DNA synthesis (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s recommendations. Briefly, cells (5 × 103/well) were plated into 96 well flat bottom plates (Corning, Inc., Corning, NY) and allowed to attach overnight before treatment as indicated in fresh complete medium. BrdU (10 μM final concentration) was added and the cells grown for additional 6hrs. After various wash steps the cells were fixed and incubated for 2 hrs at 37ºC with an anti-BrdU antibody-peroxidase conjugate and immune complexes were detected by addition of a tetramethyl-benzidine substrate solution according to the manufacturer’s recommendation. The reaction was stopped by adding 50 μL of 1M sulfuric acid, and the absorbance was measured with an ELISA plate reader (Thermo Labsystems, Waltham, MA) at 450 nm. Blank wells were incubated with the anti-BrdU antibody and the background absorbance was subtracted from all other values. In this assay, the color intensity correlates directly to the amount of BrdU incorporated into the DNA, which in turn represents proliferation. Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X±SD) of a representative experiment in % of absorbance of untreated cells [100%]; Ref: Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science. 1982;218:474. doi: 10.1126/science.7123245.
- 35.Cell Cycle Analysis: Cell cycle analysis and quantification of apoptosis was carried out by flow cytometry. Cells were seeded into 100 mm2 tissue culture dishes (7.5 × 105 cells/dish), allowed to attach overnight and treated for 48hrs as indicated. At the end of the incubation period detached cells were collected in 15 mL polypropylene centrifuge tubes along with the medium; culture dishes were washed once with PBS, adherent cells scraped off and combined in the same tube. After centrifugation (250 g, 5 min) cells were fixed (ice-cold 70% ethanol for 30 min) followed by incubation with 50μg/mL of propidium iodide and 100μg/mL of RNase for 30min at 37ºC in the dark. Data was acquired on a BD FACSort flow cytometer using CellQuest software (BD Immunocytometry Systems, San Jose, CA) and analyzed using ModFit LT software (Verity Software House, Inc., Topsham, ME). Ten thousand events were analyzed for each sample. Appropriate gating was used to select the single cell population NB cells. The same gate was used on all samples, ensuring that the measurements were made on a standardized cell population.
- 36.Mazumder S, DuPree EL, Almasan A. A dual role of cyclin E in cell proliferation and apoptosis may provide a target for cancer therapy. Curr Cancer Drug Targets. 2004;4:65. doi: 10.2174/1568009043481669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gladden AB, Diehl JA. Cell cycle progression without cyclin E/CDK2: breaking down the walls of dogma. Cancer Cell. 2003;4:160. doi: 10.1016/s1535-6108(03)00217-4. [DOI] [PubMed] [Google Scholar]
- 38.Pines J. Four-dimensional control of the cell cycle. Nature Cell Biol. 1999;1:73. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- 39.Stillman B. Cell cycle control of DNA replication. Science. 1996;274:1659. doi: 10.1126/science.274.5293.1659. [DOI] [PubMed] [Google Scholar]