Summary
We examined planning and execution of precision grasp in eight right-handed patients with a right pure motor or sensorimotor lacunar syndrome after a subcortical stroke, and eight age-matched controls, as they grasped and lifted an instrumented object whose weight could be varied without altering its visual appearance. Grip (normal) and load (tangential) forces at the fingertip-object interface were measured and the grip force rate (GFR) and load force rate (LFR) were derived. Planning of precision grasp was assessed by measurement of anticipatory scaling of peak GFR and peak LFR to object weight. Execution of precision grasp was assessed by measurement of the timing and efficiency of grip-load force coordination with the preload phase duration (PLD) and load phase duration (LPD), and the grip force at load force onset (GFO) and at lift-off (GFL), respectively. Subjects lifted a light and heavy object five times first with the RIGHT hand, then with the LEFT hand, and then once more with the RIGHT AFTER LEFT hand. Patients with stroke did not scale the peak LFR or peak GFR to object weight with the RIGHT hand even with repeated attempts; however, they scaled the peak LFR to object weight on the first lift with the RIGHT AFTER LEFT hand (p = 0.01). Patients also prolonged the PLD and LPD and produced excessive GFO and GFL for RIGHT hand lifts, but decreased the GFL for the heavy object (p = 0.016) with the RIGHT AFTER LEFT hand. Correlation of precision grasp variables from lifts with the RIGHT hand with clinical measures showed that anticipatory scaling of peak LFR and peak GFR did not correlate with clinical measures of hand function, whereas the PLD did (r = 0.88, p = 0.004). The results suggest that patients with right hemiparesis from a subcortical lesion of the corticospinal tract have a higher-order motor planning deficit. This planning deficit is dissociable from deficits in motor execution, is not captured by routine clinical assessment, and is correctable by transfer of information from the unaffected hemisphere. A rehabilitation strategy that involves practice with the left hand prior to practice with the right hand may improve planning of grasping behavior in patients with right hemiparesis.
Keywords: hand, motor planning, internal model, grasp, interlimb transfer
Introduction
Hemiparesis is the most common impairment after stroke and typically affects the upper more than the lower extremity. Studies indicate that upper-extremity weakness, spasticity, and abnormal motor synergies are insufficient to explain the impairment in reaching movements after stroke (Roby-Brami et al., 1997; Twitchell, 1959; Wing et al., 1990), and suggest that additional higher-order control deficits may be present (Beer et al., 1999). Impairments in execution of precision grasp after damage to the primary motor cortex or corticospinal tract have been extensively described in non-human and human primates (Aruin, 2005; Denny-Brown, 1966; Duque et al., 2003; Golge et al., 2004; Grichting et al., 2000; Hepp-Reymond, 1988; Hepp-Reymond and Wiesendanger, 1972; Hermsdörfer et al., 2003; Hermsdörfer and Mai, 1996; Lawrence and Kuypers, 1968; Muir and Lemon, 1983; Nowak et al., 2003; Porter and Lemon, 1993; Quaney et al., 2005; Wenzelburger et al., 2005). However, it has not been established whether higher-order abnormalities in precision grasp are present after stroke, comparable to those found in reaching (Beer et al., 1999; Takahashi and Reinkensmeyer, 2003).
A well-characterized paradigm for the study of higher-order sensorimotor integration in hand motor control is to measure subjects’ ability to anticipate the fingertip forces required to grasp and lift objects (Johansson, 1996). Anticipatory (feed-forward) fingertip force control ensures the generation of appropriate grip and load forces so as to avoid crushing delicate objects or dropping heavy ones, and is thought to be based on the formation, in the central nervous system, of internal models of object properties (Davidson and Wolpert, 2004; Flanagan, 1999; Gordon et al., 1993; Johansson and Westling, 1988). Anticipatory control of grasp is reflected in the ability to scale peak grip force rates (GFR) and peak load force rates (LFR) to the texture and weight of objects before confirmatory feedback becomes available (Flanagan et al., 2001; Johansson and Westling, 1988). Healthy subjects are able to appropriately scale peak force rates to object properties after just one or two lifts, and accurately recall them 24 hours later (Flanagan et al., 2001; Gordon et al., 1993).
Anticipatory scaling of peak GFR and peak LFR for novel objects is impaired in children with hemiplegic cerebral palsy (CP) (Eliasson et al., 1992; Gordon et al., 1999; Gordon and Duff, 1999a; Gordon and Duff, 1999b). The impairment is thought to result from an inability to form or access internal models of object properties due to either disrupted sensory feedback from the affected hand (Gordon and Duff, 1999a; Gordon and Duff, 1999b), or a higher-order deficit in sensorimotor integration (Eliasson et al., 1992). However, anticipatory scaling of the peak force rates in these children is improved in the affected hand when preceded by lifts with the unaffected hand (Gordon et al., 1999). Such improvement would not be expected if the impairment in anticipatory scaling were solely due to an execution deficit. Here we ask if anticipatory scaling of peak GFR and peak LFR is impaired in patients with adult onset stroke, and if it is separate from deficits in motor execution.
Both impaired anticipatory control and abnormal timing of grip-load force coordination during grasping may contribute to poor manual dexterity in children with CP (Duque et al., 2003; Forssberg et al., 1999; Gordon and Duff, 1999b). A recent study in patients with stroke (Wenzelburger et al., 2005) examined the relationship between precision grasp and clinical measures, and found a strong correlation between timing of grip-load force coordination and dexterity. However, to the best of our knowledge, a comparative analysis of the relationship of precision grasp planning and execution variables to clinical measures has not been done in adult patients with hemiparesis.
In the present study, we examined planning and execution of precision grasp in patients with a right pure motor or sensorimotor lacunar syndrome after a subcortical stroke. Planning of precision grasp was assessed by measurement of anticipatory scaling of peak LFR and peak GFR to object weight, as the peak amplitude of these variables is scaled to the expected weight of the object before sensory feedback signaling the object’s weight is available at lift-off; scaling of peak force rate ensures that the time to produce lifting forces does not increase linearly with object weight (Flanagan et al., 2001; Gordon et al., 1993; Johansson and Westling, 1988). Precision grasp execution was assessed by measurement of the timing and efficiency of grip-load force coordination, as these variables indicate the degree of fine motor control necessary for precision grasp (Forssberg et al., 1999). We hypothesized that both anticipatory scaling and grip-load force coordination would be impaired in the involved right hand of patients. In order to confirm that deficits in anticipatory scaling are separate from those associated with grip-load force coordination, we examined whether anticipatory scaling could transfer to the involved right hand after prior lifts with the left hand. We hypothesized that anticipatory control would transfer across hands, whereas grip-load force coordination would not. Finally, we examined the relationship of anticipatory scaling and grip-load force coordination variables with conventional clinical measures of impairment (tactile sensation, spasticity, and grip strength) and tests of hand function.
Methods
Subjects
Eight adult patients with right hemiparesis (six women and two men, 27–88 yrs, mean = 65.4) and an equal number of age-matched (+/− 2 yrs) control subjects (29–90 yrs, mean = 67.2), without evidence of neurological deficit or orthopedic abnormality participated in the study. All subjects were right-handed as confirmed by a laterality quotient of > +80 on the 10 point Edinburgh Inventory (Oldfield, 1971). All patients had sustained a single subcortical stroke at least three months previously, and met the following inclusion criteria: (1) presentation with either a pure motor or a sensorimotor lacunar syndrome; (2) score of ≤ 25/33 on the wrist and hand subcomponents of the Fugl-Meyer Scale (Fugl-Meyer et al., 1975) suggesting at least 25% motor impairment; (3) score of > 24 on the Folstein’s Mini Mental Status Examination (Cockrell and Folstein, 1988); (4) absence of aphasia that would interfere with testing; (5) ability to bisect a straight line within 5% of the midpoint (Schenkenberg et al., 1980); (6) negative screening for ideomotor apraxia by accurate demonstration of the use of scissors (O’Hare et al., 1999); (7) clinically intact joint proprioception suggested by the ability to perceive the direction of passive displacements of the metacarpophalangeal joints of all five digits with eyes closed; (8) subcortical location of stroke verified by brain magnetic resonance imaging, FLAIR sequence, by JWK (patients 3, 4, and 8), from official radiology reports (patients 2, 5 and 6), and from the patient’s medical record (patients 1 and 7); and (9) ability to complete the experimental protocol with the involved hand. Patients were excluded if their history suggested (1) coexistent neurological problems such as Parkinson’s disease; (2) arthritis, surgery or other significant injury to the upper extremities; (3) botulinum toxin injections in the upper extremity musculature in the last three months; or (4) treatment with intrathecal baclofen. Patient characteristics are shown in Table 1.
Table 1.
Clinical characteristics of patients with stroke
| Pt | Age (yrs) | Lesion location1 | TSS2 (mos) | FMS3 | Tactile sensation4 | MAS5 | Grip stgth6 (kg/cm3) | PPT7 | WMFT8 (s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | L BG & IC | 36 | 24/48 | cnd | 1,1+,1 | 14 | 2.4 | 10.3/6.2 |
| 2 | 34 | L putaminal hemorrhage | 109 | 20/56 | 5.7 | 0,1+,0 | 42.7 | 5.2 | 10.0/5.8 |
| 3 | 54 | L PLIC | 3 | 16/44 | 4.7 | 0,0,1 | 7.3 | 4.4 | 6.5/4.0 |
| 4 | 75 | L PLIC & thalamus | 69 | 18/46 | cnd | 0,0,1 | 14 | 7.2 | 4.8/3.7 |
| 5 | 79 | L IC | 37 | 20/40 | 7.8 | 0,1+,1 | 2.3 | 1.6 | 12.5/7.2 |
| 6 | 82 | L BG & PLIC | 5 | 25/55 | 4.0 | 0,0,0 | 30.7 | 9.0 | 4.0/2.7 |
| 7 | 88 | L IC | 18 | 25/52 | 4.6 | 1,0,0 | 12.3 | 8.0 | 3.8/2.7 |
| 8 | 84 | L BG & IC | 14 | 13/32 | 3.3 | 2,1+,1 | 6.7 | 4.8 | 11/7.5 |
All lesions refer to infarcts except in patient no. 2, L = Left, BG = basal ganglia, IC = internal capsule, PLIC = Posterior limb of the internal capsule;
TSS = Time Since Stroke, in months;
FMS = Fugl-Meyer Scale, scores of the wrist and hand out of a maximum of 33 over those of the total upper extremity out of a maximum of 66;
tactile sensation was measured by the mean Two-point discrimination scores of the thumb and index fingers over three trials, cnd = could not detect;
MAS = Modified Ashworth Scale, scores across the involved shoulder, elbow, wrist joints;
grip strength;
PPT = Purdue pegboard test, scores represent the average number of pegs inserted in 30 seconds over five trials;
WMFT = Wolf Motor Function Test, scores represent the average time taken to complete tasks 8–13 involving fine motor skills over the average for all 15 tasks.
Physicians and therapists specializing in the treatment of stroke in the New York metropolitan area referred the patients. Control subjects were recruited by public advertisement. Subjects were reimbursed for their travel expenses. Experiments were conducted in the Hand Motor Control Laboratory at Teachers College, Columbia University, and the Teachers College institutional review board approved the study protocol. All subjects provided informed consent in accordance with the declaration of Helsinki.
Clinical Measures
Standard neurological tests of impairment measured: (1) tactile sensation over the grasping surfaces of the thumb and index finger of each hand with the Two-Point Discrimination Test (Mackinnon and Dellon, 1985) – this test correlated best with anticipatory control in children with CP (Gordon and Duff, 1999b); (2) grip and pinch strength with standard JAMAR dynamometers (Pro Med Products, Atlanta, GA); and (3) spasticity in the affected shoulder, elbow and wrist joints with the Modified Ashworth Scale (MAS) (Bohannon and Smith, 1987). Hand function was assessed with tasks 8 to 13 of the Wolf Motor Function Test (WMFT) (Wolf et al., 2001), and the Purdue pegboard test (PPT) (Desrosiers et al., 1995; Hurvitz et al., 2003).
Procedure
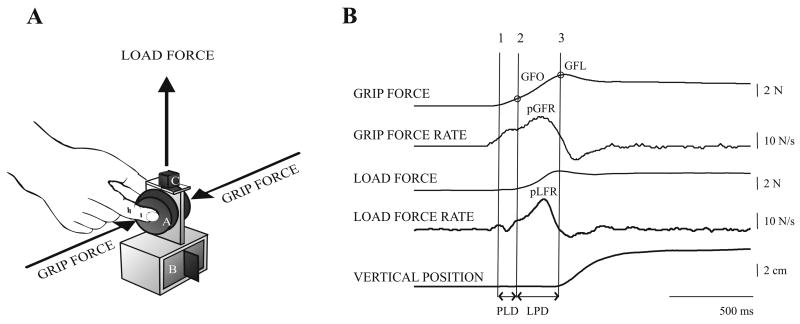
Fig. 1A shows the grasp instrument used in the experiment (for a detailed description of the apparatus see Muratori et al., 2003). Subjects first washed their hands with soap and water and dried them thoroughly. At trial onset they sat with their elbow flexed to 90 degrees, aligned with the shoulder, and hand resting on a height-adjustable table with the forearm parallel to the floor. Following an auditory cue, subjects reached forward at their preferred speed to a distance of 75% of their arm’s length, grasped the object with the thumb and index finger over the force sensors so that the load was perpendicular to the long axis of the fingers (Fig. 1A), lifted it to a height of 5 cm corresponding to a vertical marker by flexing the shoulder, and held it briefly (~ 3 seconds) before lowering it to the table. The task was first demonstrated to subjects and then they practiced lifting the object without a weight in its core. For the experimental trials we changed the weight of the object behind a screen, while all other object features such as shape, size, and frictional property at the grip surface (200-grit sandpaper) remained unchanged. Subjects kept their eyes open throughout the protocol. The experiment took place over a single session and subjects were given rest breaks to prevent fatigue. The experiment consisted of two series of lifts to test the following:
Figure 1.
1A: Schematic diagram of the grip instrument with exchangeable grip surfaces (A) covering strain-gauge force transducers for measuring grip and load force, exchangeable mass (B) in base and electromagnetic position sensor (C).1B: Precision grasp variables related to planning and execution in a healthy subject. Anticipatory scaling (planning) was measured by the peak grip force rate (pGFR) and peak load force rate (pLFR). Timing and efficiency of grip and load force coordination (execution) was measured at specific lift events: (1) point at which object contact is made and the grip force begins to increase (>0.1N); (2) point at which load force becomes positive (>0.1N); and (3) point at object lift-off (vertical position > 0.1 cm and load force = weight of object). Temporal coordination of grip and load forces was measured by the interval between (1) and (2), the preload phase duration (PLD), and that between (2) and (3), the load phase duration (LPD); and efficiency of grip-load force coordination was measured by the grip force at (2) and at (3) – the grip force at onset of positive load force (GFO), and grip force at lift-off (GFL), respectively.
I: Anticipatory control
This series of lifts examined if subjects showed anticipatory control to object weight. Subjects first lifted either a light (300g) or a heavy (500g) object five times with their right hand. The weight in the core of the object was then changed behind a screen, and subjects lifted the object with the second weight five times with the same hand. The order of presentation of the light and heavy object was counterbalanced across subjects in each group to prevent an order effect. Variables related to precision grasp planning and execution (defined below) from the fifth lift with the light and heavy object were used for subsequent analyses.
II: Transfer of anticipatory control
This series of lifts examined if subjects could transfer anticipatory control to the right hand after lifts with the left hand. To avoid a practice confound from lifting the same weights, we used a different set of light and heavy weights from that used in condition I. However, to facilitate comparison, the light and heavy object differed by 200g in both experimental conditions. Subjects first lifted either a light (400g) or heavy (600g) object five times with their left hand. Then, the experimenter slid the object across the table, aligned it with the right hand, and the subject lifted the same weight once more. The weight in the core of the object was then changed behind a screen, and the above protocol was repeated for the second weight. The order of presentation of the light and heavy object was counterbalanced across subjects in each group. Variables related to precision grasp planning and execution from the fifth lift with the left hand and the first lift with the right hand, with the light and heavy object, were used for subsequent analyses.
Data Analysis
Force and position data were sampled with 12-bit resolution at 400 Hz and 120 Hz respectively, using a flexible data acquisition/analysis system (SC/ZOOM, Umeå University, Sweden), and were filtered with a second-order Butterworth filter with zero phase lag using a cutoff frequency of 8 Hz. Load forces at the thumb and index finger sensors were summed, and grip forces at these sensors were averaged. Load force rates (LFR) and grip force rates (GFR) were derived from the summed load and average grip forces using a ± five-point numerical differentiation (i.e., calculated with a ±12.5-ms window). Anticipatory scaling (planning) of fingertip forces was measured by the peak LFR and GFR (Fig.1B, pLFR and pGFR) (Gordon et al., 1993; Johansson and Westling, 1988). The peak force rate was defined as the highest point in the force rate profile that was followed by a subsequent drop of at least 50%; this definition controlled for false peaks due to hand tremors. Execution of grasp was assessed by the timing and efficiency of grip-load force coordination at specific lift events (Duque et al., 2003; Forssberg et al., 1999). Temporal coordination was measured by the preload phase duration (PLD), which is the interval between (1) onset of grip force and (2) onset of positive load force, both defined as the point at which the force exceeded 0.1N (Fig. 1B, PLD) and indicates the time taken for grasp stabilization; and the load phase duration (LPD) which is the interval between (2) and object lift-off (3) – which occurred when the object’s vertical position exceeded 0.1cm and the load force exceeded the force corresponding to the object’s weight (Fig. 1B, LPD), and indicates the time needed to develop appropriate lifting forces. The grip force at load force onset (GFO), and the grip force at lift-off (GFL) measured the efficiency of grip-load force coordination (Fig. 1B, GFO and GFL).
Statistics
Hand condition (RIGHT hand, LEFT hand, and RIGHT AFTER LEFT hand) × weight (light and heavy) repeated measures analysis of variance (ANOVA) was performed on each dependent variable for the stroke and control groups separately. To test our hypotheses, we performed a priori pairwise comparisons of the difference scores of light and heavy object lifts between the RIGHT hand and RIGHT AFTER LEFT hand (R-R) using an orthogonal contrast for the hand condition × weight interaction – these results are reported as ‘R-R contrast’.
The relationship between precision grasp variables (obtained with the RIGHT hand) and clinical measures in patients with stroke were analyzed by Pearson’s correlation tests. The precision grasp variables used were: the difference scores of lifts with the light and heavy object for the peak LFR, peak GFR, LPD and GFL; and the average scores of lifts with the light and heavy object for the PLD and GFO (since these two variables are not influenced by object weight, see results).
Given the number of multiple comparisons with several dependent variables, we adjusted the p-value to reduce the likelihood of type I statistical errors. However, since many of our variables are correlated (e.g., GFR and LFR), traditional Bonferroni correction of p-values is overly conservative (O’Brien and Shampo, 1988) and may result in type II errors. Thus, we used Keppel’s Modified Bonferroni correction (Keppel, 1991), which takes into account that the measures may not be independent of each other, and reduces the likelihood of type II errors while still controlling for type I errors. Accordingly, the significance threshold of the p-value for the ANOVA [α = df (0.05)/c, where α is adjusted p value, df is degrees of freedom, and c is number of comparisons] and the correlation analyses [α = (number of variables * 0.05)/c] was set to p < 0.017.
Results
Planning of precision grasp: anticipatory scaling of peak load and grip force rates
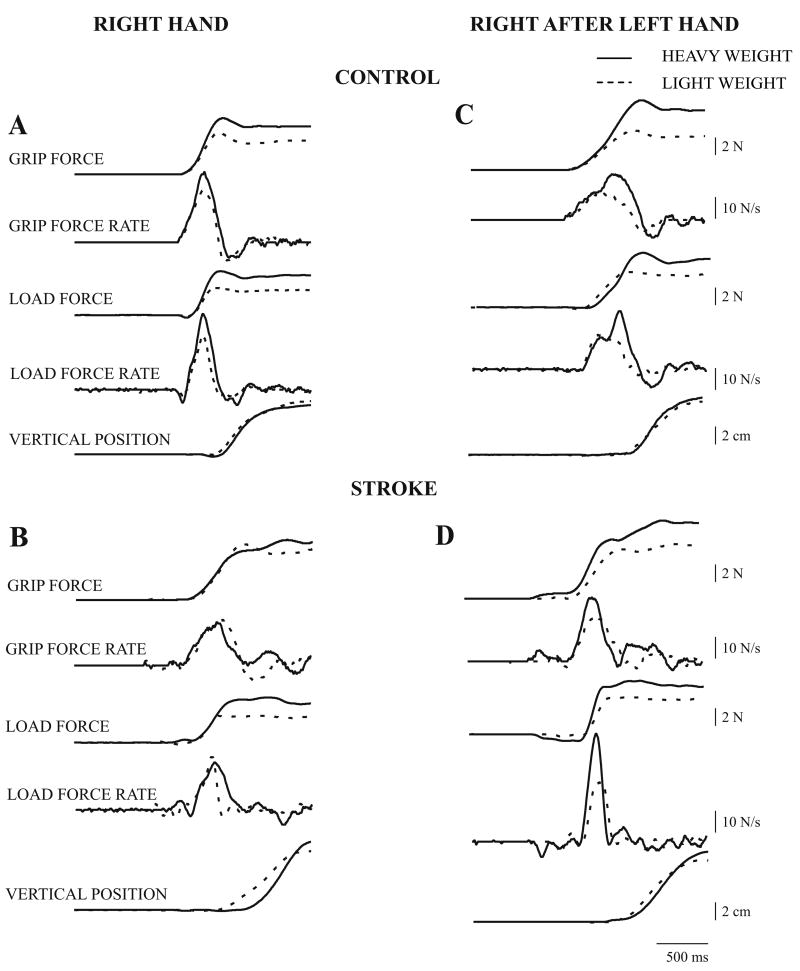
Planning of precision grasp was first investigated by examining anticipatory scaling of peak LFR and peak GFR to object weight with the right hand. In order to separate anticipatory scaling from execution-related measures we then examined transfer of anticipatory scaling from the left to the right hand (described below). Fig. 2 shows force, force rate, and position trajectories for lifting the light and heavy object on the fifth lift with the RIGHT hand, and the first lift with the RIGHT AFTER LEFT hand, in a control subject and a patient with stroke. On lifting with the RIGHT hand, the control subject (Fig. 2A) produced higher peak LFR and peak GFR for the heavy object (solid lines); however, the patient with stroke (Fig. 2B) produced similar peak LFR and peak GFR for both the light and heavy object, even after four prior lifts. Thus, although the force-rate profiles are single peaked, anticipatory scaling to object weight is not seen. On lifting with the RIGHT AFTER LEFT hand, the control subject (Fig. 2C) produced higher peak LFR and peak GFR for the heavy object, implying transfer of anticipatory scaling. The patient with stroke (Fig. 2D) also produced higher peak LFR and peak GFR for the heavy object, in contrast to that produced with the RIGHT hand. However, unlike scaling of peak LFR, scaling of peak GFR did not transfer consistently across all subjects in both the control and stroke groups as detailed below.
Figure 2.
Fingertip force, force rate and object position trajectories from a control subject and a patient with stroke (no. 3) are shown for lifting the light (dashed traces) and heavy (solid traces) object, on the fifth lift with the right hand (2A and C), and on the first lift with the right hand after five lifts with the left hand (2B and D).
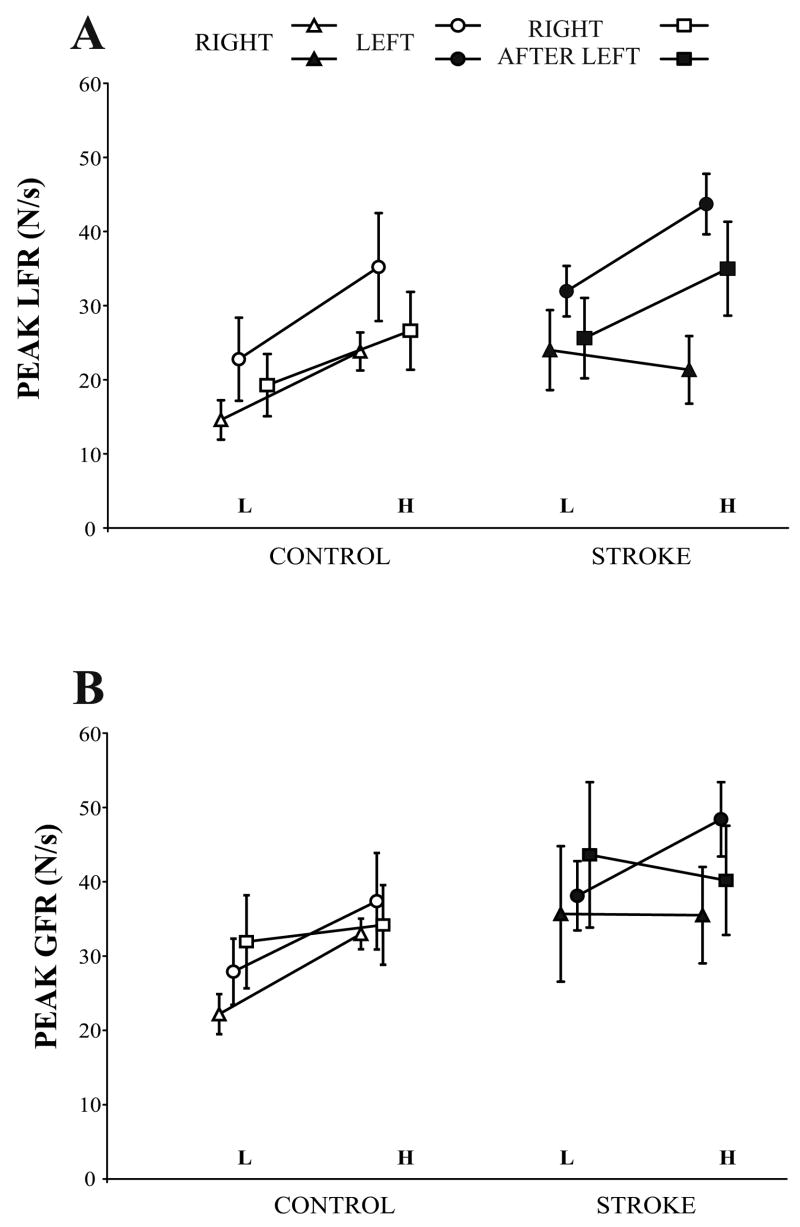
Scaling of load force rate
The peak LFR for light and heavy object lifts is shown in Fig. 3A, and the slopes represent the mean difference scores for the two weights. Controls scaled the peak LFR to object weight for the three conditions: they showed higher peak LFR for the heavy object with the RIGHT, LEFT, and RIGHT AFTER LEFT hand conditions (main effect for weight, F (1, 7) = 57.463, p = 0.001); scaling was similar with the RIGHT AFTER LEFT and RIGHT hand conditions (R-R contrast, F (1, 7) = 0.511, p = 0.498). In contrast, patients were unable to scale with their RIGHT hand: the slope was, if anything, negative with a similar peak LFR for light and heavy object lifts. However, the patients were able to scale with their LEFT hand and their RIGHT AFTER LEFT hand (main effect for weight, F (1, 7) = 27.385, p = 0.001). The R-R contrast confirmed that scaling was significantly improved with the RIGHT AFTER LEFT hand compared with the RIGHT hand (F (1, 7) = 12.204, p = 0.01), which indicates that patients transferred scaling of peak LFR to the right hand after lifts with their unaffected left hand.
Figure 3.
Peak load force rate (mean ± SEM) (3A), and peak grip force rate (3B) for lifting the light (L) and heavy (H) object on the fifth lift with the RIGHT and LEFT hand, and the first lift with the RIGHT AFTER LEFT hand in control subjects and patients with stroke are shown. The slopes represent the difference in peak force rates for the light and heavy weights.
Scaling of grip force rate
The peak GFR for light and heavy object lifts is shown in Fig. 3B, and the slopes represent the mean difference scores for the two weights. Controls showed scaling of peak GFR with both the RIGHT and LEFT hand conditions (main effect for weight, F (1, 7) = 32.210, p = 0.001). However, the slope for RIGHT AFTER LEFT hand is essentially flat, suggesting lack of transfer of peak GFR scaling. However, a comparison of the slopes between RIGHT and RIGHT AFTER LEFT hand conditions did not quite reach significance (R-R contrast, F (1, 7) = 7.224, p = 0.031). Patients were unable to scale peak GFR with their RIGHT hand, similar to their failure to scale peak LFR. As in controls, the patients were able to scale peak GFR with their LEFT hand but not with their RIGHT AFTER LEFT hand; this failure to scale to object weight in two out of the three conditions was reflected in a non-significant main effect of weight (F (1, 7) = 0.559, p = 0.479). Scaling was not significantly improved with the RIGHT AFTER LEFT hand compared with the RIGHT hand (R-R contrast, F (1, 7) = 0.122, p = 0.738). Thus, patients were unable to scale GFR with their affected right hand and neither patients nor controls were able to transfer scaling from the left to the right hand.
Execution of precision grasp: timing and efficiency of grip-load force coordination
Temporal coordination of grip and load forces
In controls, the PLD (data not shown) was similar for both light and heavy object lifts (mean across the two weights = 110–130 ms) with no significant main effect of weight across the three conditions (F (1, 7) = 1.339, p = 0.285). Patients also showed a similar PLD for light and heavy object lifts (main effect for weight, F (1, 7) = 0.469, p = 0.516), but it was prolonged compared to controls for the RIGHT hand (mean = 300 ms) and RIGHT AFTER LEFT hand (mean = 230 ms). However, the normal PLD for the LEFT hand (mean = 100 ms) did not transfer to the RIGHT AFTER LEFT hand (R-R contrast, F (1, 7) = 5.489, p = 0.052).
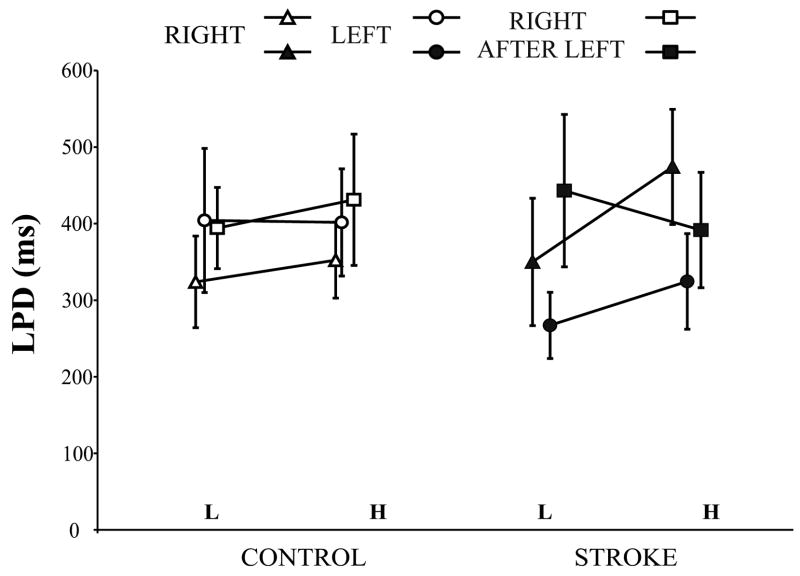
The LPD for light and heavy object lifts is shown in Fig. 4, and the slopes represent the mean difference scores for the two weights. Although the figure shows that controls increased the LPD slightly for the heavy object, there was no significant main effect of weight (F (1, 7) = 1.136, p = 0.322). In patients, the LPD was increased considerably (by 120 ms) for the heavy object with the RIGHT hand, but not with the LEFT or RIGHT AFTER LEFT hand conditions. Overall however, there was no main effect for weight (F (1, 7) = 1.944, p = 0.206). The slope of the LPD for the RIGHT AFTER LEFT hand was almost flat compared with that for the RIGHT hand and the R-R contrast trended towards significance (F (1, 7) = 6.442, p = 0.039).
Figure 4.
Load phase duration (mean ± SEM) for lifting the light (L) and heavy (H) object on the fifth lift with the RIGHT and LEFT hands, and the first lift with the RIGHT AFTER LEFT hand in control subjects and patients with stroke is shown. The slopes represent the difference in load phase duration for the light and heavy weights.
Efficiency of grip-load force coordination
There was no difference in GFO (data not shown) between light and heavy object lifts in both controls (main effect for weight, F (1, 7) = 0.305, p = 0.589) and patients (main effect for weight, F (1, 7) = 0.036, p = 0.855). However, the mean GFO (across the two weights) was elevated in patients for all three hand conditions (mean = 2–3 N) compared to controls (mean = 1.2 N).
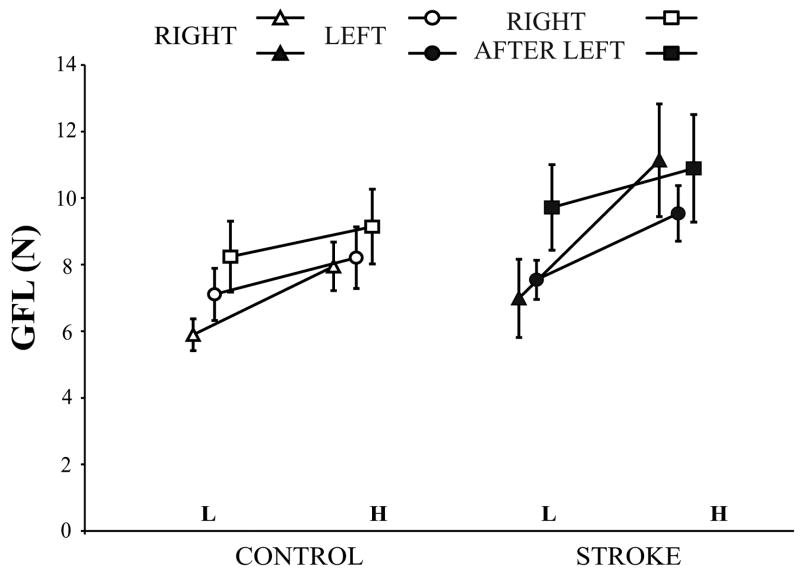
The GFL for light and heavy object lifts is shown in Fig. 5, and the slopes represent the mean difference scores of the two weights. The GFL was higher for heavy object lifts across the three conditions for both controls (main effect for weight, F (1, 7) = 13.996, p = 0.007) and patients (main effect for weight, F (1, 7) = 13.280, p = 0.008). In patients, the GFL increase with the heavy object was more marked (~ 4 N) for lifts with the RIGHT hand compared to lifts with the LEFT and RIGHT AFTER LEFT hand conditions. The slope of the GFL for the RIGHT AFTER LEFT hand was less steep compared with that for the RIGHT hand (R-R contrast, F (1, 7) = 9.898, p = 0.016).
Figure 5.
Grip force at lift-off (mean ± SEM) for lifting the light (L) and heavy (H) object on the fifth lift with the RIGHT and LEFT hands, and the first lift with the RIGHT AFTER LEFT hand in control subjects and patients with stroke is shown. The slopes represent the difference in grip force at lift-off for the light and heavy weights.
Correlations between precision grasp planning and execution variables with clinical measures
Correlations of precision grasp variables (from the RIGHT hand) with clinical measures related to impairment (tactile sensation, elbow spasticity, grip strength) and hand function (WMFT and PPT) in patients with stroke are shown in Table 2. Measures of precision grasp planning – peak LFR and GFR – did not correlate with any of the clinical measures. However, measures of precision grasp execution correlated with measures of both impairment and hand function. Specifically, the GFO and GFL correlated with tactile sensation (r = 0.800 with GFO, and r = 0.906 with GFL), and the PLD correlated strongly with elbow spasticity (r = 0.950) and hand function assessed with the WMFT (r = 0.880).
Table 2.
Correlation of precision grasp variables with clinical measures in patients
| Variable1 | Tactile sensation2 | Elbow spasticity3 | Grip strength (kg/cm3) | PPT4 | WMFT5 |
|---|---|---|---|---|---|
| Peak LFR (N/s) | 0.062 (0.885) | 0.422 (0.298) | 0.141 (0.739) | −0.379 (0.355) | 0.405 (0.320) |
| Peak GFR (N/s) | 0.551 (0.158) | 0.464 (0.247) | 0.137 (0.746) | −0.473 (0.237) | 0.463 (0.248) |
| PLD (ms) | 0.141 (0.740) | 0.950 (0.001) | 0.124 (0.770) | −0.628 (0.096) | 0.880 (0.004) |
| LPD (ms) | −0.537 (0.171) | −0.389 (0.341) | −0.220 (0.601) | 0.342 (0.408) | −0.231 (0.583) |
| GFO (N) | 0.800 (0.017) | 0.237 (0.572) | −0.153 (0.718) | −0.216 (0.608) | 0.110 (0.795) |
| GFL (N) | 0.906 (0.002) | −0.026 (0.952) | −0.080 (0.850) | −0.021 (0.962) | −0.078 (0.855) |
LFR = load force rate; GFR = grip force rate; PLD = preload phase duration; LPD = load phase duration; GFO = grip force at load force onset; GFL = grip force at lift-off. Values represent Pearson’s correlation coefficients (r) with the corresponding significance (p-values) in parenthesis; p values ≤ 0.017 are in boldface.
The difference scores of the peak LFR, peak GFR, LPD, and GFL for light and heavy object lifts, and the mean scores of the PLD and GFO for light and heavy object lifts with the RIGHT hand were used for the correlation analyses;
Tactile sensation was measured with the Two-point discrimination test;
Elbow spasticity was measured by the Modified Ashworth Scale;
PPT = Purdue pegboard test; and
WMFT = Wolf Motor Function Test.
Discussion
Impaired anticipatory scaling
Healthy subjects, including those in our study, appropriately scale fingertip force rates within one to three lifts (Johansson, 1996), through presumed formation of internal models of object weight, center of mass, and surface friction (Gordon et al., 1993; Johansson and Westling, 1987; Johansson and Westling, 1988; Salimi et al., 2003; Salimi et al., 2000). Patients with stroke, however, did not scale the peak LFR or peak GFR with their involved right hand despite repeated lifts. Our findings are similar to those described previously in children with CP. Corticospinal tract damage has been shown to lead to a slowed rate of motor neuron recruitment and thus a slowed rate of force development (Chae et al., 2002a; Chae et al., 2002b; Hepp-Reymond and Wiesendanger, 1972). Thus, the inability to attain peak LFR and peak GFR required for anticipatory scaling in these patients could result from a deficit in motor execution and give the false impression of impaired anticipatory control. However, as will be discussed below, transfer of anticipatory scaling to the right hand after lifts with the non-involved left hand suggests that a motor execution deficit is not sufficient to explain the impairment in anticipatory scaling.
Transfer of anticipatory scaling
Age-matched controls in our study, and healthy subjects studied previously (Gordon et al., 1994), transfer anticipatory scaling of peak LFR across hands. Patients with stroke also scaled the peak LFR to object weight on a single lift with the affected right hand after similar lifts with the unaffected left hand, as observed in children with hemiplegic CP (Gordon et al., 1999). However, scaling of peak GFR did not transfer consistently in the control subjects or the patients. This is consistent with previous studies in healthy subjects that have shown that scaling of peak LFR is more specific than scaling of peak GFR for anticipatory control of object weight (Flanagan et al., 2001; Gordon et al., 1994) and that scaling of peak GFR to object weight transfers poorly from the left to the right hand in healthy subjects (Gordon et al., 1994). Thus our main finding was that patients could not initially scale peak LFR to object weight with their affected right hand, even after repeated attempts, but were able to do so immediately after lifts with their unaffected left hand. This result is not consistent with an execution deficit but suggests instead that patients with right hemiparesis also have a higher-order deficit in anticipatory control.
Why is anticipatory control impaired in the affected hand of patients with right hemiparesis? In patients with CP (Eliasson et al., 1992; Gordon and Duff, 1999a) the deficit is thought to be related to impaired tactile sensation (Gordon and Duff, 1999b), or a higher-order deficit in sensorimotor integration (Eliasson et al., 1992). However, tactile sensation did not correlate with anticipatory scaling of peak LFR or peak GFR in our study. Although it is possible that the impairment in anticipatory scaling was due to clinically undetected proprioceptive deficits, we hypothesize an alternative mechanism: the subcortical lesion interrupts output from primary motor cortex (M1) and thereby prevents short-latency integration of the motor output with sensory input from the primary sensory cortex (S1). This M1-S1 sensorimotor integration hypothesis is supported by a recent study that showed disruption of scaling of fingertip force rates with the right hand by repetitive transcranial magnetic stimulation over left M1 in healthy subjects (Chouinard et al., 2005).
How can prior lifts with the left hand restore anticipatory scaling of peak LFR in the affected right hand? Damage to the corticospinal tract is associated with increased contribution from ipsilesional premotor areas (Fries et al., 1993; Johansen-Berg et al., 2002; Seitz et al., 1998; Weiller et al., 1992), which unlike M1, do not have direct access to ipsilateral primary sensory input (Asanuma and Arissian, 1984) needed to form sensorimotor associations. However, sensorimotor associations formed in right M1 as a result of lifting objects with the left (non-involved) hand can feed the right premotor regions and then the left premotor cortex via callosal connections, which have been shown to be necessary for transfer (Gordon et al., 1994). Thus, access to updated internal representations of object weight formed in the contralesional (undamaged) cortex may facilitate anticipatory scaling of peak LFR during subsequent lifts with the involved right hand.
Execution of grasp
Measures of temporal coordination – the PLD and LPD – were prolonged for lifts with the affected hand in patients with stroke, and the PLD remained prolonged even after prior lifts with the left hand. The PLD was also increased in children with hemiplegic CP (Duque et al., 2003; Forssberg et al., 1999), and in adult patients with chronic pure motor hemiparesis (Wenzelburger et al., 2005). In addition, it correlated highly with both damage to the posterior limb of the internal capsule, thought to carry corticospinal projections related to upper extremity movement (Morecraft et al., 2002), and upper extremity dysfunction in patients with small capsular infarcts (Wenzelburger et al., 2005). The similarity of our results to these studies supports our contention that our patients sustained damage primarily to the corticospinal tract.
Grip-load force coordination, measured by the GFO and GFL, was inefficient for lifts with the involved right hand in patients with stroke. Deficits in tactile sensation could account for these high grip forces (Nowak and Hermsdorfer, 2003; Nowak et al., 2001); indeed, tactile sensation correlated significantly with the GFO and GFL in our study. However, the GFO in patients was also increased for lifts with the non-involved left hand, which had intact sensation. Abnormalities in grip force control have been observed in the ipsilesional hand of patients with stroke in the absence of sensory deficits (Quaney et al., 2005), suggesting that grip force execution, but not planning, may be impaired in the unaffected hand after stroke. The GFL, which was markedly increased for heavy object lifts with the affected right hand, became more similar for the two weights with the same hand after lifts with the unaffected left hand; this suggests that the initial increase in GFL was due to online compensation for inadequate anticipation of object weight (Johansson and Westling, 1988). Thus, both impaired tactile sensation and inadequate anticipatory scaling may lead to excessive grip forces, although improved anticipatory control can reduce the grip force applied at lift-off despite concurrent tactile deficits.
Relationship of clinical measures to planning and execution of precision grasp
Measures of precision grasp planning (anticipatory scaling of peak LFR and peak GFR) did not correlate with any of the clinical measures; however, measures of precision grasp execution correlated with clinical measures of both impairment and hand dysfunction – the GFO and GFL correlated with tactile sensation, and the PLD correlated strongly with elbow spasticity and hand function tested with the WMFT. This suggests that conventional clinical measures used in the assessment of patients with stroke are primarily measures of execution; they are unable to detect, or are unrelated to higher-order planning deficits that could influence grasping behavior underlying everyday manual activities.
Some caution is required in interpreting correlation analyses when the number of subjects is small with respect to the number of comparisons. However, the main danger is in type I errors, and we only found a few significant correlations, which are consistent with previous findings in the literature. Abnormal grip force control has repeatedly correlated with impaired tactile sensation in previous studies (Nowak and Hermsdorfer, 2003; Nowak et al., 2001). The PLD has also been shown to be correlated with elbow spasticity in children with CP (Gordon and Duff, 1999b). Interestingly, the PLD correlated with performance on the WMFT, but not with performance on the PPT. Both the precision grasp task and hand tasks of the WMFT involved a single grasp and hold movement, whereas the PPT involved repetitive and alternating grasp and release movements – this difference could account for the dissociation, and suggests that task specificity may be critical to understanding relationships between motor impairment and functional motor behavior.
How relevant is anticipatory control to functional motor behavior?
Simple reaching movements in everyday tasks require prediction of inter-joint forces to preserve smooth movement paths and end-point accuracy (Pigeon et al., 2003; Sainburg et al., 1999). Such prediction or anticipatory control, which requires internal models of limb dynamics (Conditt et al., 1997), is impaired in patients with hemiparesis (Beer et al., 1999). Planning of grasp in everyday tasks also requires the acquisition of internal models of limb-object interactions (Haaland et al., 1999; Jeannerod et al., 1995; Wolpert et al., 2001). Limb apraxia, which has traditionally been understood and tested in terms of a semantic understanding of a motor act (Geschwind and Damasio, 1985), has more recently been characterized as a manifestation of a more general impairment in sensorimotor integration (Leiguarda and Marsden, 2000). Thus, our findings of impaired anticipatory control of precision grasp, independent of an execution deficit, parallel findings for reaching movements in patients with stroke and also suggest that higher-order motor planning deficits may be present even when patients do not show clinical evidence of apraxia. However, since motor planning deficits occur predominantly in individuals with left-hemisphere damage (Haaland et al., 2000), these deficits may not be seen in individuals with right-brain damage. Future studies should contrast planning and execution of precision grasp in patients with left and right hemiparesis.
Conclusions
Our findings have a number of important implications for patients with hemiparesis and their subsequent rehabilitation. First, patients with a right pure motor or sensorimotor lacunar syndrome after subcortical stroke demonstrate deficits in motor planning that are separate from deficits in motor execution. Second, these motor-planning deficits may not be fully apparent unless psychophysical tests of sensorimotor integration are performed in addition to conventional clinical measures. Finally, transfer paradigms are likely to give us a better understanding of how information is exchanged between the two hemispheres and may have important implications for the development of rehabilitation strategies that incorporate practice with the non-involved hand prior to practice with the involved hand to improve grasping behavior after stroke. Future investigations of the deficits in planning and execution of precision grasp should be conducted in larger groups of patients with left and right hemiparesis, controlled for lesion location, to shed further light on the relationship between quantitative behavioral measures, clinical tests of hand function, and cerebral localization.
Acknowledgments
This work was supported by National Institutes of Health grant 2K12 HD0197-7 through the Rehabilitation Medicine Scientist Training Program (RMSTP). The work is also supported, in part, by National Institutes of Health grant NS02138 (JWK).
Abbreviations
- ANOVA
analysis of variance
- CP
cerebral palsy
- GFL
grip force at lift-off
- GFO
grip force at onset of positive load force
- GFR
grip force rate
- LFR
load force rate
- LPD
load phase duration
- M1
primary motor cortex
- MAS
Modified Ashworth Scale
- PLD
prelaod phase duration
- PPT
Purdue pegboard test
- R-R contrast
RIGHT - RIGHT AFTER LEFT hand comparison
- S1
primary sensory cortex
- WMFT
Wolf Motor Function Test
Footnotes
Parts of this work were presented at the annual conference of the Society of Neuroscience in New Orleans, 2003.
References
- Aruin AS. Support-specific modulation of grip force in individuals with hemiparesis. Arch Phys Med Rehabil. 2005;86:768–75. doi: 10.1016/j.apmr.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Arissian K. Experiments on functional role of peripheral input to motor cortex during voluntary movements in the monkey. J Neurophysiol. 1984;52:212–27. doi: 10.1152/jn.1984.52.2.212. [DOI] [PubMed] [Google Scholar]
- Beer R, Dewald J, Rymer Z. Disturbances of voluntary movement coordination in stroke: problems of planning or execution? Prog Brain Res. 1999;123:455–60. doi: 10.1016/s0079-6123(08)62881-2. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Chae J, Yang G, Park BK, Labatia I. Delay in initiation and termination of muscle contraction, motor impairment, and physical disability in upper limb hemiparesis. Muscle Nerve. 2002a;25:568–75. doi: 10.1002/mus.10061. [DOI] [PubMed] [Google Scholar]
- Chae J, Yang G, Park BK, Labatia I. Muscle weakness and cocontraction in upper limb hemiparesis: relationship to motor impairment and physical disability. Neurorehabil Neural Repair. 2002b;16:241–8. doi: 10.1177/154596830201600303. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T. Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci. 2005;25:2277–84. doi: 10.1523/JNEUROSCI.4649-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacol Bull. 1988;24:689–92. [PubMed] [Google Scholar]
- Conditt MA, Gandolfo F, Mussa-Ivaldi FA. The motor system does not learn the dynamics of the arm by rote memorization of past experience. J Neurophysiol. 1997;78:554–60. doi: 10.1152/jn.1997.78.1.554. [DOI] [PubMed] [Google Scholar]
- Davidson PR, Wolpert DM. Internal models underlying grasp can be additively combined. Exp Brain Res. 2004;155:334–40. doi: 10.1007/s00221-003-1730-z. [DOI] [PubMed] [Google Scholar]
- Denny-Brown . The Cerebral Control of Movement. Liverpool: Liverpool University Press; 1966. [Google Scholar]
- Desrosiers J, Hebert R, Bravo G, Dutil E. The Purdue Pegboard Test: normative data for people aged 60 and over. Disabil Rehabil. 1995;17:217–24. doi: 10.3109/09638289509166638. [DOI] [PubMed] [Google Scholar]
- Duque J, Thonnard JL, Vandermeeren Y, Sebire G, Cosnard G, Olivier E. Correlation between impaired dexterity and corticospinal tract dysgenesis in congenital hemiplegia. Brain. 2003;126:732–47. doi: 10.1093/brain/awg069. [DOI] [PubMed] [Google Scholar]
- Eliasson AC, Gordon AM, Forssberg H. Impaired anticipatory control of isometric forces during grasping by children with cerebral palsy. Dev Med Child Neurol. 1992;34:216–25. doi: 10.1111/j.1469-8749.1992.tb14994.x. [DOI] [PubMed] [Google Scholar]
- Flanagan J. The notion of internal models in sensorimotor control - an overview. Acta Physiol Scand. 1999;167:A9–A10. doi: 10.1046/j.1365-201x.1999.0600j.x. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, King S, Wolpert DM, Johansson RS. Sensorimotor prediction and memory in object manipulation. Can J Exp Psychol. 2001;55:87–95. doi: 10.1037/h0087355. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Eliasson AC, Redon-Zouitenn C, Mercuri E, Dubowitz L. Impaired grip-lift synergy in children with unilateral brain lesions. Brain. 1999;122(Pt 6):1157–68. doi: 10.1093/brain/122.6.1157. [DOI] [PubMed] [Google Scholar]
- Fries W, Danek A, Scheidtmann K, Hamburger C. Motor recovery following capsular stroke. Role of descending pathways from multiple motor areas. Brain. 1993;116(Pt 2):369–82. doi: 10.1093/brain/116.2.369. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- Geschwind N, Damasio AR. Apraxia. In: Vinken PJ, Bruyn PW, Klawans HL, editors. Handbook of clinical neurology. Vol. 45. Amsterdam: Elsevier; 1985. pp. 423–32. [Google Scholar]
- Golge M, Muller M, Dreesmann M, Hoppe B, Wenzelburger R, Kuhtz-Buschbeck JP. Recovery of the precision grip in children after traumatic brain injury. Arch Phys Med Rehabil. 2004;85:1435–44. doi: 10.1016/j.apmr.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Charles J, Duff SV. Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. II: bilateral coordination. Dev Med Child Neurol. 1999;41:176–85. doi: 10.1017/s0012162299000365. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Duff SV. Fingertip forces during object manipulation in children with hemiplegic cerebral palsy. I: anticipatory scaling. Dev Med Child Neurol. 1999a;41:166–75. doi: 10.1017/s0012162299000353. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Duff SV. Relation between clinical measures and fine manipulative control in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 1999b;41:586–91. doi: 10.1017/s0012162299001231. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Forssberg H, Iwasaki N. Formation and lateralization of internal representations underlying motor commands during precision grip. Neuropsychologia. 1994;32:555–68. doi: 10.1016/0028-3932(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Westling G, Cole KJ, Johansson RS. Memory representations underlying motor commands used during manipulation of common and novel objects. J Neurophysiol. 1993;69:1789–96. doi: 10.1152/jn.1993.69.6.1789. [DOI] [PubMed] [Google Scholar]
- Grichting B, Hediger V, Kaluzny P, Wiesendanger M. Impaired proactive and reactive grip force control in chronic hemiparetic patients. Clin Neurophysiol. 2000;111:1661–71. doi: 10.1016/s1388-2457(00)00355-2. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Spatial deficits in ideomotor limb apraxia. A kinematic analysis of aiming movements. Brain. 1999;122(Pt 6):1169–82. doi: 10.1093/brain/122.6.1169. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123(Pt 11):2306–13. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond MC. Functional organization of motor cortex and its participation in voluntary movements. In: Seklis HD, Erwin J, editors. Comparative Primate Biology. Vol. 4. New York: Liss; 1988. pp. 501–624. [Google Scholar]
- Hepp-Reymond MC, Wiesendanger M. Unilateral pyramidotomy in monkeys: effect on force and speed of a conditioned precision grip. Brain Res. 1972;36:117–31. doi: 10.1016/0006-8993(72)90770-6. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Hagl E, Nowak DA, Marquardt C. Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol. 2003;114:915–29. doi: 10.1016/s1388-2457(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Mai N. Disturbed grip-force control following cerebral lesions. J Hand Ther. 1996;9:33–40. doi: 10.1016/s0894-1130(96)80009-3. [DOI] [PubMed] [Google Scholar]
- Hurvitz EA, Conti GE, Brown SH. Changes in movement characteristics of the spastic upper extremity after botulinum toxin injection. Arch Phys Med Rehabil. 2003;84:444–54. doi: 10.1053/apmr.2003.50001. [DOI] [PubMed] [Google Scholar]
- Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–20. [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–23. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS. Sensory control of dextrous manipulation in humans. In: Wing AM, Haggard P, Flanagan JR, editors. Hand and Brain. San Diego: Academic Press; 1996. pp. 381–414. [Google Scholar]
- Johansson RS, Westling G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp Brain Res. 1987;66:141–54. doi: 10.1007/BF00236210. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res. 1988;71:59–71. doi: 10.1007/BF00247522. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A researchers handbook. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey.I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–13. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Leiguarda RC, Marsden CD. Limb apraxias: higher-order disorders of sensorimotor integration. Brain. 2000;123(Pt 5):860–79. doi: 10.1093/brain/123.5.860. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, Dellon AL. Two-point discrimination tester. J Hand Surg [Am] 1985;10:906–7. doi: 10.1016/s0363-5023(85)80173-8. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, et al. Localization of arm representation in the corona radiata and internal capsule in the non-human primate. Brain. 2002;125:176–98. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- Muir RB, Lemon RN. Corticospinal neurons with a special role in precision grip. Brain Res. 1983;261:312–6. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- Muratori LM, Reilmann R, Gordon AM. Coordination of fingertip forces during precision grasping in multiple system atrophy. Neuropsychologia. 2003;41:1498–508. doi: 10.1016/s0028-3932(03)00092-7. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdorfer J. Selective deficits of grip force control during object manipulation in patients with reduced sensibility of the grasping digits. Neurosci Res. 2003;47:65–72. doi: 10.1016/s0168-0102(03)00182-2. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdörfer J, Glasauer S, Philipp J, Meyer L, Mai N. The effects of digital anaesthesia on predictive grip force adjustments during vertical movements of a grasped object. Eur J Neurosci. 2001;14:756–62. doi: 10.1046/j.0953-816x.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Hermsdörfer J, Topka H. Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol. 2003;250:850–60. doi: 10.1007/s00415-003-1095-z. [DOI] [PubMed] [Google Scholar]
- O’Brien PC, Shampo MA. Statistical considerations for performing multiple tests in a single experiment. 4. Performing multiple statistical tests on the same data. Mayo Clin Proc. 1988;63:1043–5. doi: 10.1016/s0025-6196(12)64922-2. [DOI] [PubMed] [Google Scholar]
- O’Hare A, Gorzkowska J, Elton R. Development of an instrument to measure manual praxis. Dev Med Child Neurol. 1999;41:597–607. doi: 10.1017/s0012162299001255. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pigeon P, Bortolami SB, DiZio P, Lackner JR. Coordinated turn-and-reach movements. I. Anticipatory compensation for self-generated coriolis and interaction torques. J Neurophysiol. 2003;89:276–89. doi: 10.1152/jn.00159.2001. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal function and voluntary movement. Oxford: Oxford University Press; 1993. [Google Scholar]
- Quaney BM, Perera S, Maletsky R, Luchies CW, Nudo RJ. Impaired grip force modulation in the ipsilesional hand after unilateral middle cerebral artery stroke. Neurorehabil Neural Repair. 2005;19:338–49. doi: 10.1177/1545968305282269. [DOI] [PubMed] [Google Scholar]
- Roby-Brami A, Fuchs S, Mokhtari M, Bussel B. Reaching and grasping strategies in hemiparetic patients. Motor Control. 1997;1:72–91. [Google Scholar]
- Sainburg RL, Ghez C, Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol. 1999;81:1045–56. doi: 10.1152/jn.1999.81.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi I, Frazier W, Reilmann R, Gordon AM. Selective use of visual information signaling objects’ center of mass for anticipatory control of manipulative fingertip forces. Exp Brain Res. 2003;150:9–18. doi: 10.1007/s00221-003-1394-8. [DOI] [PubMed] [Google Scholar]
- Salimi I, Hollender I, Frazier W, Gordon AM. Specificity of internal representations underlying grasping. J Neurophysiol. 2000;84:2390–7. doi: 10.1152/jn.2000.84.5.2390. [DOI] [PubMed] [Google Scholar]
- Schenkenberg T, Bradford DC, Ajax ET. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology. 1980;30:509–17. doi: 10.1212/wnl.30.5.509. [DOI] [PubMed] [Google Scholar]
- Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081–8. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- Takahashi CD, Reinkensmeyer DJ. Hemiparetic stroke impairs anticipatory control of arm movement. Exp Brain Res. 2003;149:131–40. doi: 10.1007/s00221-002-1340-1. [DOI] [PubMed] [Google Scholar]
- Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1959:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–72. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Wenzelburger R, Kopper F, Frenzel A, Stolze H, Klebe S, Brossmann A, et al. Hand coordination following capsular stroke. Brain. 2005;128:64–74. doi: 10.1093/brain/awh317. [DOI] [PubMed] [Google Scholar]
- Wing AM, Lough S, Turton A, Fraser C, Jenner JR. Recovery of elbow function in voluntary positioning of the hand following hemiplegia due to stroke. J Neurol Neurosurg Psychiatry. 1990;53:126–34. doi: 10.1136/jnnp.53.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–9. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends Cogn Sci. 2001;5:487–494. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]