Abstract
Arsenite is an important cancer chemotherapeutic. The liver is a major target tissue of arsenic toxicity and hepatotoxicity may limit its chemotherapeutic efficacy. O2-vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (V-PYRRO/NO) is a liver-selective nitric oxide (NO)-producing prodrug metabolized by hepatic P450 enzymes to release NO locally. V-PYRRO/NO protects against various organic or inorganic hepatotoxicants but any role in arsenic hepatotoxicity is undefined. Thus, we studied the effects of V-PYRRO/NO (0-1000 μM) pretreatment on inorganic arsenic-induced toxicity in cultured rat liver (TRL 1215) cells. These cells metabolized the prodrug to release NO, producing extracellular nitrite levels to 41.7-fold above control levels (7.50 ± 0.38 μM) after 24 h V-PYRRO/NO (1000 μM) exposure. The effect of pretreatment with V-PYRRO/NO (24 h) on the cytolethality of arsenic (as NaAsO2) exposure (24 h) was assessed. Arsenic was markedly less toxic in V-PYRRO/NO pretreated cells (LC50 = 30.3 μM) compared to control (LC50 = 20.1 μM) and the increases in LC50 showed a direct relationship to the level of NO produced (measured as nitrite). Consistent with the cytolethality data, V-PYRRO/NO pretreatment markedly reduced arsenic-induced apoptosis as assessed by DNA fragmentation. Activation of the c-Jun N-terminal kinase (JNK) pathway can be critical to apoptosis and pretreatment with V-PYRRO/NO suppressed arsenic-induced JNK activation. V-PYRRO/NO pretreatment modestly increased metallothionein (MT), a metal-binding protein, but greatly enhanced arsenic induction of MT. Thus, V-PYRRO/NO pretreatment directly mitigates arsenic toxicity in cultured liver cells, reducing cytolethality, apoptosis and related JNK pathway activation, apparently through generation of NO. The role of NO in reducing the hepatotoxicity of arsenical chemotherapeutics in vivo deserves additional study.
Keywords: V-PYRRO/NO, arsenic, apoptotic resistance, in vitro, liver cells
1. Introduction
Arsenic has been linked to various acute and chronic toxic effects in the liver [1]. Recently, arsenicals have shown great promise in the chemotherapy of various types of human cancers, and can be curative of acute promyelocytic leukemia even in patients with multiple relapses [2,3]. Although very effective clinically, concerns have been raised about the potential for fatal hepatotoxicity in patients undergoing arsenical chemotherapy for cancer [4]. The individual susceptibility to arsenical hepatotoxicity varies widely in humans, possibly due to differences in arsenic methylation [5]. Thus, an agent or agents that would effectively limit arsenical hepatotoxicity may be a valuable adjunct to arsenical chemotherapy.
Nitric oxide (NO) is a ubiquitous biomolecule with a wide variety of critical functions [6]. The cellular effects of NO have been studied by various means, such as through treatment with various NO-donating compounds [7,8]. O2-vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (V-PYRRO/NO) is a stable diazeniumdiolate, which can circulate freely in the body until it is metabolized to release NO by cytochrome P450 in the liver, providing a significant level of local hepatic NO [9]. V-PYRRO/NO mitigates the hepatotoxicity of various compounds in vivo and in vitro [10-12]. For instance, V-PYRRO/NO can protect against LPS-induced liver injury in mice, an effect that is linked to suppression of apoptosis and NF-κB expression [11]. V-PYRRO/NO also reduces TNF-α-induced liver injury associated with reduced hepatocellular apoptosis [9]. V-PYRRO/NO pretreatment can similarly reduce both the hepatotoxic effects of acetaminophen and cadmium in mice [12,13]. Furthermore, V-PYRRO/NO reduces cadmium toxicity and apoptosis directly on the cellular level in liver cells [14], indicating that it's protective effects are not just based in organ-level alterations in vivo toxicokinetics, potentially due to enhanced vascular capacity from NO-induced vasodilation.
Metallothionein (MT) is a thiol-rich, low-molecular weight, metal-binding protein that can be induced by metals [15]. Arsenic has been shown to induce MT expression both in vitro and in vivo [16-18]. Furthermore, Jiang et al. reported that arsenic and its methylated metabolites interacted with MT in a stoichiometric fashion [16]. Recent evidence indicates that human populations poorly expressing MT may be more sensitive to chronic arsenic intoxication [19]. Thus, the existing data indicate MT may be able reduce arsenic toxicity in many instances. Furthermore, NO treatment can also increase MT levels, at least indirectly [20].
Thus, the purpose of this study is to define the potential protective effects of excess NO, using the liver-specific NO prodrug, V-PYRRO/NO, on arsenic-induced toxicity and apoptosis at the cellular level in the liver cell line TRL 1215.
2. Materials and methods
2.1. Chemicals
Sodium arsenite was purchased from Sigma Chemical Company (St. Louis, MO). V-PYRRO/NO was synthesized as described previously [9]. The structure of V-PYRRO/NO and the mode of NO release have been reported previously [7,9]. SAPK/JNK assay kits were purchased from New England Biolabs, Inc. (Beverly, MA).
2.2. Cell culture
The TRL 1215 liver cell line was originally derived from the liver of 10-day old rats and cells were cultured in William's E medium containing 10% fetal bovine serum. These cells metabolically resemble hepatocytes and are diploid. They are normally nontumorigenic.
2.3. Metabolic integrity assay
The Promega Cell Titer 96 Non-Radioactive Cell Proliferation Assay kit was used to determine acute cytotoxicity of arsenic in cells as defined by metabolic integrity. This assay measures the amount of formazan produced by metabolic conversion of Owen's reagent [(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt, MTS] by dehydrogenase enzymes found in the mitochondria of metabolically active cells. The quantity of formazan product, as measured by absorbance at 490 nm, is directly proportional to the number of living cells. A minimum of 4 replicates of 10,000 cells per well were plated in 96-well plates and allowed to adhere to the plate for 24 h, at which time the media were removed and replaced with fresh media with or without V-PYRRO/NO (500 or 1000 μM). These levels of V-PYRRO/NO were selected because preliminary study showed them to be non-toxic while prior work indicated they were very effective in inducing NO production in hepatocytes [9]. At the end of this period arsenic was added in fresh media. Cells were then incubated for an additional 24 h and cell viability was determined. The LC50 values were determined from analysis of the linear portion of four separately derived metabolic integrity curves.
The levels of V-PYRRO/NO used in these studies (500 or 1000 μM) produced levels of NO (as measured by extracellular nitrite-see below) that are approximately 2∼ to 8∼ fold higher than the normal plasma levels of NO observed in control human subjects [21].
2.4. Nitrite measurement
A Griess reagent-based system (Promega, Madison, WI) was used to determine nitrite concentration in cell culture medium as an indication of NO generated from V-PYRRO/NO. A minimum of 4 replicates of 10,000 cells per well were plated in 96-well plates and allowed to adhere to the plate for 24 h. Cells were pretreated with V-PYRRO/NO for 24 h. Extracellular media were collected and nitrite was measured.
2.5. Quantification of apoptotic cell death
DNA fragmentation, as an indication of apoptotic cell death, was assessed by determination of cytoplasmic histone-DNA fragments using the Cell Death Detection ELISA kit (Roche, Indianapolis, IN). In all cases, cells were seeded in 96-well plates at 10,000 cells per well in 200 μl medium and treatments were initiated 24 h after plating. To examine the effects of V-PYRRO/NO on arsenite-induced apoptosis, cells were pretreated with V-PYRRO/NO for 24 h and then incubated with arsenic for an additional 24 h. Apoptosis was evaluated in both floating and adherent cells.
2.6. JNK activity assay
The enzymatic activity of JNKs was determined by using the JNK assay kits from New England Biolabs. Cells were pretreated with 500 or 1000 μM V-PYRRO/NO for 24 h, followed by arsenic (300 μM) treatment for 30 minutes. Cell lysates containing equal amount of total protein (250 μg, 250 μl) were incubated overnight at 4°C with same amount of N-terminal c-Jun (1-89) fusion protein (2 μg, 20 μl) for assay of JNK kinase activity. This N-terminal c-Jun (1-89) fusion protein bound to glutathione sepharose beads allows selective “pull down” of SAPK/JNK from cell lysates. c-Jun (1-89) fusion contains a high affinity binding site for SAPK/JNK, just N-terminal to the two phosphorylation sites, Ser63 and Ser73. After selectively pulling down SAPK using the c-Jun fusion protein beads, the beads were washed to remove nonspecifically bound proteins. The kinase reaction was performed by adding 100 μM of ATP to the suspension. Phosphorylation of c-Jun was measured by western blot analysis with a phospho-specific c-Jun antibody that specifically detects Ser63-phosphorylated c-Jun, a site important for c-Jun-dependent transcriptional activity [22]. Since this assay measures the end-product of an enzymatic reaction, activity can be compared across groups based on the use of equal amounts of total cellular protein.
2.7. Metallothionein (MT) quantitation
Cellular MT concentrations were measured by the Cd-hemoglobin radioassay method [23]. Values were adjusted to cell number and are expressed as nanograms of MT per 106 cells.
2.8. Statistical analysis
Data are expressed as mean ± SEM. Student's t-test or ANOVA with subsequent Dunnett's test were used as appropriate. Linear (Pearson) correlations were used to determine statistical significance of correlations between V-PYRRO/NO concentrations or NO production and LC50 for arsenic. Values are derived from 3 or more replications. Differences were considered significant at a level of p < 0.05.
3. Results
3.1. V-PYRRO/NO-induced tolerance to arsenite
TRL 1215 rat liver cells were pretreated with V-PYRRO/NO then incubated with various levels of arsenic for 24 h and cytolethality was assessed. V-PYRRO/NO pretreatment significantly reduced arsenic-induced cytolethality (Table 1). The LC50 for arsenic in V-PYRRO/NO pretreated cells was ∼50% higher than that in control cells. The V-PYRRO/NO pretreatment levels alone had no cytotoxic effects.
Table 1.
V-PYRRO/NO-induced tolerance to arsenic-induced cytotoxicity
| Pretreatment | Arsenic LC50 (μM) |
|---|---|
| None (control) | 20.1 ± 1.9 |
| V-PYRRO/NO | 30.3 ± 2.9* |
TRL 1215 cells were pretreated with V-PYRRO/NO (1000 μM) for 24 h or left untreated. Cells were then incubated with various levels of arsenic for an additional 24 h and cytotoxicity was measured by the MTS assay. Lethal concentration 50% (LC50) values were derived from analysis of 4 separate cytotoxicity curves. Results are presented as the mean ± SEM, n = 4.
The asterisk indicates a significant difference from control (p ≤ 0.05).
3.2. Nitrite formation after V-PYRRO/NO exposure
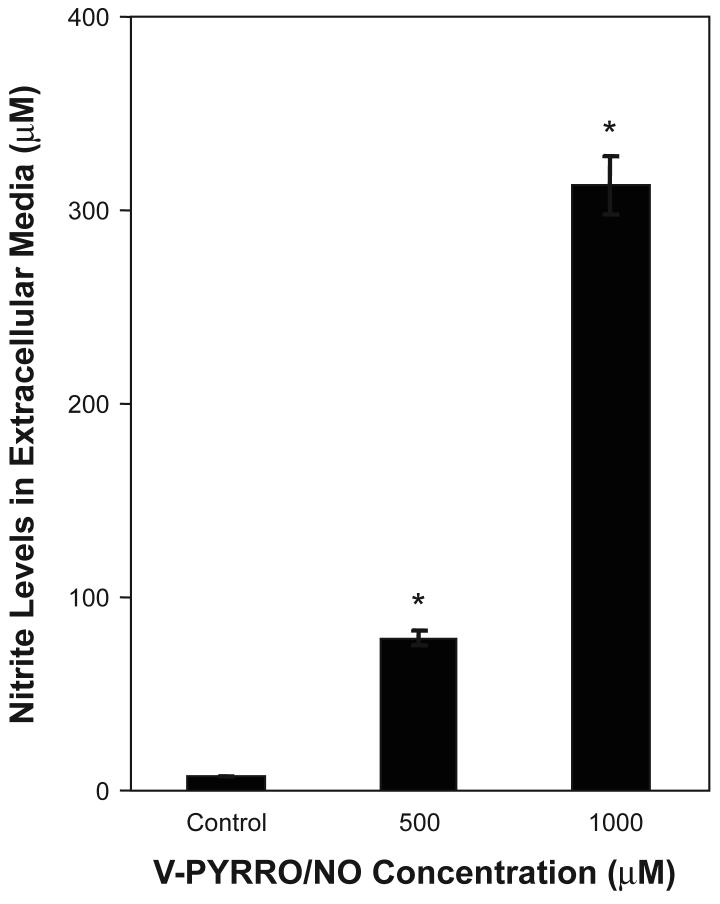
To confirm that V-PYRRO/NO was acted upon by liver cells to release NO, cells were exposed to V-PYRRO/NO and nitrite was measured by Griess assay in extracellular fluid as an indirect measurement of NO production. These liver-derived cells clearly acted upon the prodrug to release NO, producing nitrite (Fig. 1). Addition of V-PYRRO/NO to medium in the absence of cells did not generate nitrite (data not shown).
Figure 1. Nitrite formation after V-PYRRO/NO exposure in TRL 1215 cells.
Cells were exposed to V-PYRRO/NO for 24 h. Extracellular media were then collected and nitrite was measured by Griess assay as an indirect measurement of NO production. Results are presented as the mean ± SEM (n = 3). An asterisk (*) indicates a significant difference from control.
3.3. Correlation between V-PYRRO/NO, NO production and LC50 for arsenic
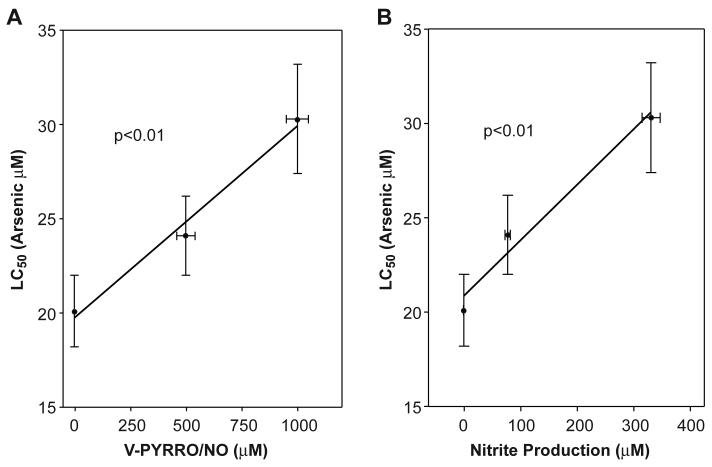
In cells pretreated with V-PYRRO/NO, analysis revealed a highly significant (p < 0.01) correlation between increased LC50 for arsenic and the pretreatment level of V-PYRRO/NO (Fig. 2A). The relationship between reduced arsenic cytolethality and NO production after V-PYRRO/NO treatment was also assessed by measurement of nitrite products. The results showed that increasing NO production also was highly correlated with increase LC50 for arsenic (Fig. 2B). These strong correlations suggest that, when exposed to V-PYRRO/NO, these liver cells produce NO, which then protects against arsenic-induced toxicity.
Figure 2. Correlation between V-PYRRO/NO, NO production and LC50 for arsenic.
Cells were first treated with various levels of V-PYRRO/NO for 24 h followed by arsenic treatment for 24 h. Cytotoxicity was measured as metabolic integrity by the MTS assay and LC50 values were determined. Nitrite produced after V-PYRRO/NO treatment as an indication of NO production was measured by the Griess reagent. Results are the mean ± SEM of four separate determinations. Arsenic LC50 values were significantly (p < 0.01) correlated with V-PYRRO/NO concentration (A) or NO production (B).
3.4. Effect of V-PYRRO/NO on arsenic-induced apoptosis and JNK1/2 kinase activation
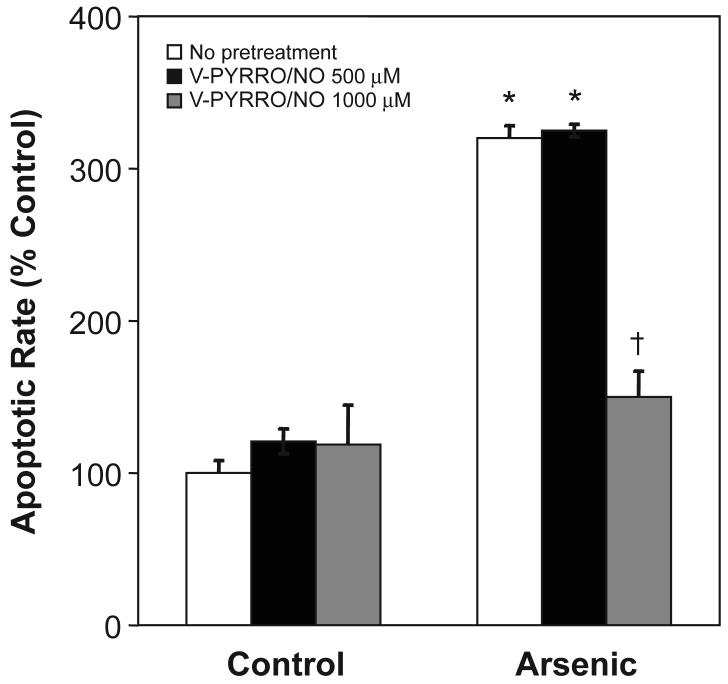
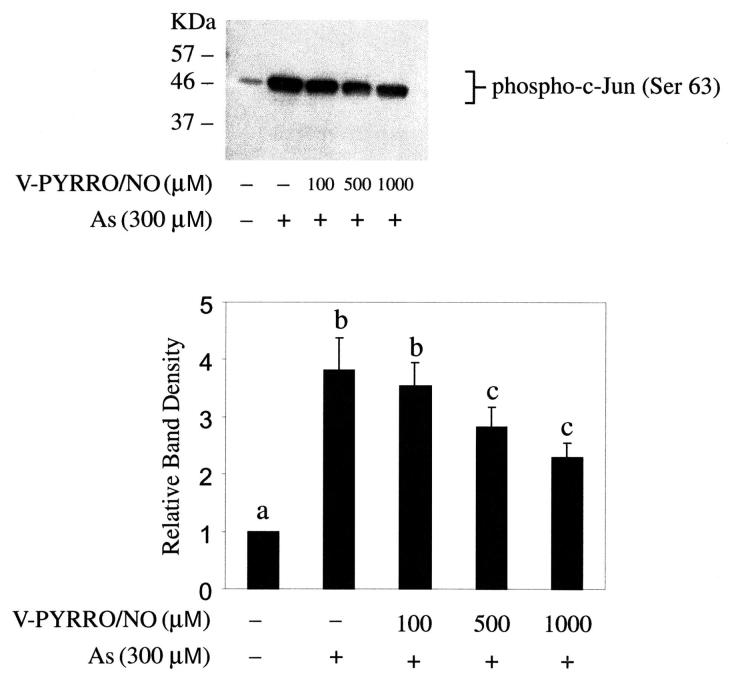
Cells were pretreated with V-PYRRO/NO then exposed to arsenic and apoptotic cell death was measured (Fig. 3). V-PYRRO/NO (1000 μM) completely blocked arsenic-induced apoptosis. V-PYRRO/NO itself did not induce apoptosis at the concentrations employed. V-PYRRO/NO pretreatment also mitigated arsenic-induced activation of JNK1/2 (Fig. 4), which is likely a key event important in reducing arsenic-induced apoptotic cell death. V-PYRRO/NO treatment alone had no effect on activation of JNK1/2 (data not shown).
Figure 3. Effect of V-PYRRO/NO pretreatment on arsenic-induced apoptosis.
Cells were pretreated with V-PYRRO/NO for 24 h then incubated with arsenic (15 μM) for an additional 24 h and the apoptotic rate of cells was measured by ELISA. Data are expressed as a percent of control (no V-PYRRO/NO or arsenic). Results are presented as the mean ± SEM (n = 4). *, significantly different (p < 0.05) from control cells treated with the same level of V-PYRRO/NO. †, significantly different (p < 0.05) from the arsenite-alone control (no V-PYRRO/NO).
Figure 4. Effect of V-PYRRO/NO on activation of c-Jun N-terminal kinase (JNK1/2) by arsenic.
Cells were pretreated with V-PYRRO/NO for 24 h, followed by arsenic treatment (300 μM, 30 minutes; top). Cell extracts were incubated overnight with c-Jun fusion protein. Phosphorylation of c-Jun at Ser 63 was measured by Western blot using Phospho-c-Jun (Ser 63) antibody. Blots represent a typical result of three independent experiments. Protein immunoblots were analyzed by scanning densitometry. Results are presented as the mean ± SEM (n = 3). Differing superscripts (a, b, c) indicate a significant (p < 0.05) difference between respective groups.
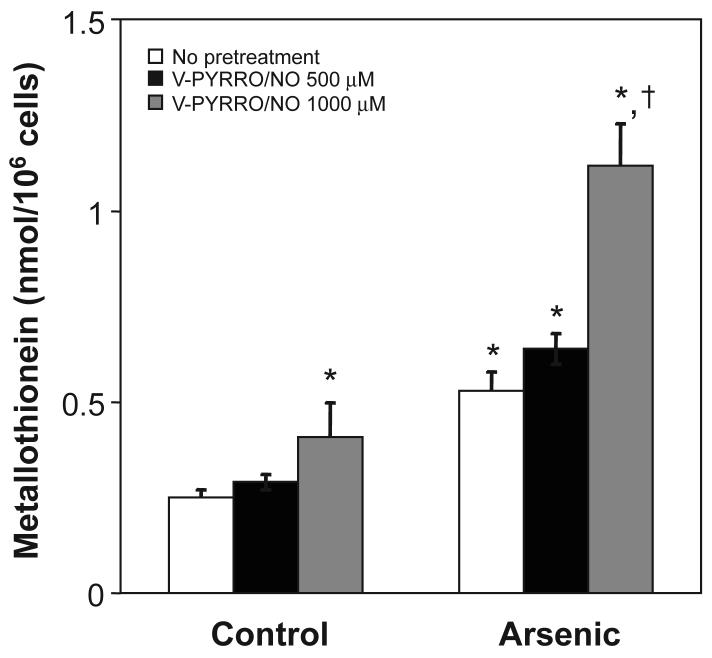
3.5. Effect of V-PYRRO/NO on MT
V-PYRRO/NO pretreatment by itself caused a modest increase in MT as did arsenic alone (Fig. 5). Pretreatment with V-PYRRO/NO significantly increased MT levels after subsequent arsenic exposure.
Figure 5. Effect of V-PYRRO/NO pretreatment on cellular MT levels.
Cells were pretreated with V-PYRRO/NO, followed by arsenic treatment (15 μM, 24 h). MT levels were measured by the cadmium-hemoglobin assay. Results are presented as the mean ± SEM (n = 3). *, significantly different (p < 0.05) from untreated (no V-PYRRO/NO and no arsenic) control. †, significantly different (p < 0.05) from the arsenic-alone control (no V-PYRRO/NO).
4. Discussion
Based on the biological importance of NO, several NO donor prodrugs have been designed that allow its controlled release [7]. V-PYRRO/NO, a NO-releasing prodrug, is thought to deliver NO to the liver in a selective fashion via hepatic metabolism and in vitro cultured hepatocytes clearly metabolize V-PYRRO/NO to produce NO [9]. V-PYRRO/NO improves porcine liver hemodynamics after ischemia reperfusion [8], likely due to effects on the hepatic vasculature. In mice, V-PYRRO/NO blocks cadmium-induced hepatic congestion [13], a lesion likely vascular in origin, but V-PYRRO/NO also protects against cadmium cytolethality and apoptosis at the cellular level in liver cells [14]. Thus, the protection of the liver from hepatotoxins by V-PYRRO/NO [10] may include a vascular component as well as direct actions within hepatocytes. The liver is a key target tissue of arsenic toxicity and this may limit its chemotherapeutic efficacy [4]. The present study investigated the effects of V-PYRRO/NO treatment on arsenic-induced toxicity in liver cells in vitro and showed that the NO-releasing prodrug had a number of mitigating effects directly within these cells. This included reduction of apoptotic cell death as well as molecular events induced by arsenic leading to apoptosis. VPYRRO/NO-induced tolerance to arsenic was clearly related to NO release, likely by the metabolic action of the liver cells on the NO prodrug. Thus, it appears that a significant part of the protection from arsenic toxicity afforded by NO may occur directly within liver cells. Further work will assess the ability of V-PYRRO/NO to reduce arsenic toxicity in vivo.
MT plays an important role in the detoxification of various inorganics, probably through sequestrational binding, and arsenic binds readily to thiols within MT [24]. The binding stoichiometry indicates that each MT molecule binds with up to six inorganic arsenic molecules [16]. MT plays a protective role in chronic inorganic arsenic exposure, as in MT–I/II double knock-out (MT-null) mice where are hypersensitive to chronic arsenic hepatotoxicity [25]. Arsenic can induce MT in various cell lines [1,17,18]. The present study showed that pretreatment with V-PYRRO/NO greatly enhanced arsenic-induced MT levels directly in liver cells, which appears an important factor in NO-induced arsenic tolerance. Thus, the protective effect of V-PYRRO/NO might in part be a result of increased MT levels facilitating sequestration of arsenic.
MAPKs comprise a family of serine/threonine phosphorylating proteins that mediate a variety of signal transduction pathways [22,26]. Of the major MAPKs, the c-Jun NH2-terminal kinases (JNKs) result in stress responses, growth arrest, and/or apoptosis [22]. Arsenic can induce JNKs activity in various cells [22,26] and activation of JNK by arsenic contributes to apoptosis [27,28]. Interestingly, arsenic-transformed cells become highly resistant to arsenic-induced apoptosis from the perturbation of JNK1/2 activity [22]. Thus, the JNK pathway appears to play an important role in arsenic-induced apoptosis. In this regard, NO can function as an intracellular signaling regulator [29] and, in particular, can impact JNK signaling [30-33]. NO is a thiol-reactive molecule and JNK has a cysteine residue that is sensitive to thiol-modifying agents and endogenously produced NO negatively regulates the JNK pathway by means of a thiol-redox mechanism [29]. The present results indicate that V-PYRRO/NO generates NO within liver cells, which in turn likely suppresses arsenic-induced JNK activity. Thus, the release of NO from VPYRRO/NO appears to be related to a reduction in arsenic-induced apoptosis potentially via perturbed signaling events.
In summary, the present work shows that V-PYRRO/NO, a liver-selective NO-producing prodrug, protects against arsenic-induced cytotoxicity and apoptosis at the cellular level in cultured liver cells. This protection is apparently through generation of NO and the concurrent blockade of arsenic-activation of the apoptosis-related JNK pathway. V-PYRRO/NO facilitated arsenic induction of MT, which may be important in NO-induced arsenic tolerance. The highly effective use of inorganic arsenic against certain leukemias is well established [2,3,5] and it's utility against solid tumors is under study. A limiting side effect, a least in some subpopulations, could be hepatotoxicity [4,5]. Thus, a liver specific prodrug that reduces inorganic arsenic hepatotoxicity could have significant utility as an adjuvant in arsenical chemotherapy.
Acknowledgments
The authors thank Drs. Erik Tokar and Chikara Kojima for critical review of this manuscript. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by NCI contract NO1-C012400 with SAIC Frederick, Inc.
Abbreviations
- ERKs
extracellular signal-regulated kinases
- cGMP
cyclic guanosine 3',5'-monophosphate
- LC50
Lethal Concentration 50%
- JNK
c-Jun N-terminal kinase
- MAPKs
Mitogen-activated protein kinases
- MT
Metallothionein
- NO
Nitric oxide
- TRL 1215
rat liver epithelial cell line
- V-PYRRO/NO
O2-vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flora SJ, Tripathi N. Hepatic and renal metallothionein induction following single oral administration of gallium arsenide in rats. Biochem. Mol. Biol. Int. 1998;45:1121–1127. doi: 10.1080/15216549800203342. [DOI] [PubMed] [Google Scholar]
- 2.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PMLRaα/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 3.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL). II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 4.Douer D, Tallman MS. Arsenic trioxide: new clinical experience with an old medication in hematologic malignancies. J. Clin Oncol. 2005;23:2396–2410. doi: 10.1200/JCO.2005.10.217. [DOI] [PubMed] [Google Scholar]
- 5.Mathews V, Desire S, George B, Lakshmi KM, Rao GJ, Viswabandya A, Bajel A, Srivastava VM, Srivastava A, Chandy M. Hepatotoxicity profile of single agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia, its impact on clinical outcome and the effect of genetic polymorphisms on the incidence of hepatotoxicity. Leukemia. 2006;20:881–883. doi: 10.1038/sj.leu.2404165. M. [DOI] [PubMed] [Google Scholar]
- 6.Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L185–L192. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- 7.Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu. Rev. Pharmacol. Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 8.Ricciardi R, Foley DP, Quarfordt SH, Saavedra JE, Keefer LK, Wheeler SM, Donohue SE, Callery MP, Meyers WC. V-PYRRO/NO: an hepato-selective nitric oxide donor improves porcine liver hemodynamics and function after ischemia reperfusion. Transplantation. 2001;71:193–198. doi: 10.1097/00007890-200101270-00004. [DOI] [PubMed] [Google Scholar]
- 9.Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-alpha-induced apoptosis and toxicity in the liver. J. Med. Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Waalkes MP. Nitric oxide and chemically induced hepatotoxicity: beneficial effects of the liver-selective nitric oxide donor, V-PYRRO/NO. Toxicology. 2005;208:289–297. doi: 10.1016/j.tox.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Saavedra JE, Lu T, Song JG, Clark J, Waalkes MP, Keefer LK. O(2)-vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate protection against D-galactosamine/endotoxin-induced hepatotoxicity in mice: genomic analysis using microarrays. J. Pharmacol. Exp. Ther. 2002;300:18–25. doi: 10.1124/jpet.300.1.18. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Li C, Waalkes MP, Clark J, Myers P, Saavedra JE, Keefer LK. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced hepatotoxicity in mice. Hepatology. 2003;37:324–333. doi: 10.1053/jhep.2003.50063. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Qu W, Saavedra JE, Waalkes MP. The nitric oxide donor, O2-vinyl 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (V-PYRRO/NO), protects against cadmium-induced hepatotocity in mice. J. Pharmacol. Exp. Ther. 2004;310:18–24. doi: 10.1124/jpet.103.065003. [DOI] [PubMed] [Google Scholar]
- 14.Qu W, Liu J, Fuquay R, Shimoda R, Sakurai T, Saavedra JE, Keefer LK, Waalkes MP. The nitric oxide prodrug, V-PYRRO/NO, protects against cadmium toxicity and apoptosis at the cellular level. Nitric Oxide. 2005;12:114–120. doi: 10.1016/j.niox.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- 16.Jiang G, Gong Z, Li XF, Cullen WR, Le XC. Interaction of trivalent arsenicals with metallothionein. Chem. Res. Toxicol. 2003;16:873–880. doi: 10.1021/tx034053g. [DOI] [PubMed] [Google Scholar]
- 17.Kreppel H, Bauman JW, Liu J, McKim JM, Jr, Klaassen CD. Induction of metallothionein by arsenicals in mice. Fundam. Appl. Toxicol. 1993;20:184–189. doi: 10.1006/faat.1993.1025. [DOI] [PubMed] [Google Scholar]
- 18.Falnoga I, Stibilj E, Tusek-Znidaric M, Slejkovec Z, Mazej D, Jacimovic R, Scancar J. Effect of arsenic trioxide on metallothionein and its conversion to different arsenic metabolites in hen liver. Biol. Trace. Elem. Res. 2001;78:241–254. doi: 10.1385/bter:78:1-3:241. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Cheng ML, Yang Q, Shan KR, Shen J, Zhou Y, Zhang X, Dill AL, Waalkes MP. Blood metallothionein transcript as a biomarker for metal sensitivity: Low blood metallothionein transcripts in arsenicosis patients from Guizhou, China, Environ. Health Perspect. 2007 doi: 10.1289/ehp.10035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katakai K, Liu J, Nakajima K, Keefer LK, Waalkes MP. Nitric oxide induces metallothionein (MT) gene expression apparently by displacing zinc bound to MT. Toxicol. Lett. 2001;119:103–108. doi: 10.1016/s0378-4274(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 21.Lluch P, Torondel B, Medina P, Segarra G, Del Olmo JA, Serra MA, Rodrigo JM. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J. Hepatol. 2004;41:55–59. doi: 10.1016/j.jhep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Qu W, Bortner CD, Sakurai T, Hobson MJ, Waalkes MP. Acquisition of apoptotic resistance in arsenic-induced malignant transformation: role of the JNK signal transduction pathway. Carcinogenesis. 2002;23:151–159. doi: 10.1093/carcin/23.1.151. [DOI] [PubMed] [Google Scholar]
- 23.Eaton DL, Toal BF. Evaluation of the Cd/hemoglobin affinity assay for the rapid determination of metallothionein in biological tissues. Toxicol. Appl. Pharmacol. 2001;66:134–142. doi: 10.1016/0041-008x(82)90068-0. [DOI] [PubMed] [Google Scholar]
- 24.Ngu TT, Stillman MJ. Arsenic binding to human metallothionein. J. Am. Chem. Soc. 2006;128:12473–12483. doi: 10.1021/ja062914c. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol. Sci. 2000;55:460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 26.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 27.Samet JM, Graves LM, Quay J, Dailey LA, Devlin RB, Ghio AJ, Wu W, Bromberg PA, Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Ma WY, Li J, Dong Z. Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res. 1999;59:3053–3058. [PubMed] [Google Scholar]
- 29.Park HS, Huh SH, Kim MS, Lee SH, Choi EJ. Nitric oxide negatively regulates c-Jun N-terminal kinase/stress-activated protein kinase by means of S-nitrosylation. Proc. Natl. Acad. Sci. USA. 2000;97:14382–14387. doi: 10.1073/pnas.97.26.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of cJun NH2-terminal kinases. J. Biol. Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Yu X, Brecher P. Nitric oxide and N-acetylcysteine inhibit the activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J. Biol. Chem. 1998;273:33027–33034. doi: 10.1074/jbc.273.49.33027. [DOI] [PubMed] [Google Scholar]
- 32.Lander HM, Jacovina AT, Davis RJ, Tauras JM. Differential activation of mitogen-activated protein kinases by nitric oxide-related species. J. Biol. Chem. 1996;271:19705–19709. doi: 10.1074/jbc.271.33.19705. [DOI] [PubMed] [Google Scholar]
- 33.Jun CD, Oh CD, Kwak HJ, Pae HO, Yoo JC, Choi BM, Chun JS, Park RK, Chung HT. Overexpression of protein kinase C isoforms protects RAW 264.7 macrophages from nitric oxide-induced apoptosis: involvement of c-Jun N-terminal kinase/stress-activated protein kinase, p38 kinase, and CPP-32 protease pathways. J. Immunol. 1999;162:3395–3401. [PubMed] [Google Scholar]