Abstract
Dysadherin is a cancer-associated cell membrane glycoprotein that promotes experimental cancer metastasis. Here we review recent work that has provided insights into possible mechanisms of action of this newly recognized player in the cancer progression process. Dysadherin modulates cell phenotype in a number of ways, including down-regulation of E-cadherin-mediated cell adhesion, and up-regulation of chemokine production. In this way, expression of dysadherin in a tumor can influence both the tumor cell itself and the stromal compartment, so as to create conditions that are more permissive for metastasic spread. Dysadherin expression is also an independent prognostic indicator of metastasis and survival for many different types of human cancer. Thus, dysadherin may represent a new molecular target for the visualization, prevention or treatment of advanced cancer.
Keywords: Dysadherin, E-cadherin, Chemokine (C-C motif) ligand 2, metastasis, breast cancer
1. Introduction
One major goal of cancer research is to identify molecules that are differentially expressed in tumors compared with normal tissues. If these molecules are causally involved in sustaining or promoting tumorigenesis, they represent potential molecular targets for the development of novel therapeutics. If they are merely associated with the tumorigenic process rather than driving it, differentially expressed molecules on the surface of the cancer cell can still be exploited for directing therapeutics or imaging agents specifically to the tumor cell. Dysadherin is a cell surface molecule that was recently identified as the target of a monoclonal antibody that was developed to selectively react with a wide variety of cancer cells, but relatively few normal cells [1]. In this original study, dysadherin was found to promote experimental metastasis, and dysadherin expression correlated with poor prognosis in a small cohort of breast cancer patients, suggesting a causal role for the newly-discovered molecule in tumor progression. The aim of the present review is to discuss recent evidence implicating dysadherin as a key player in cancer progression, to address likely mechanisms of action, and to discuss the clinical significance of dysadherin expression in different types of cancer.
2. Dysadherin structure, distribution and regulation.
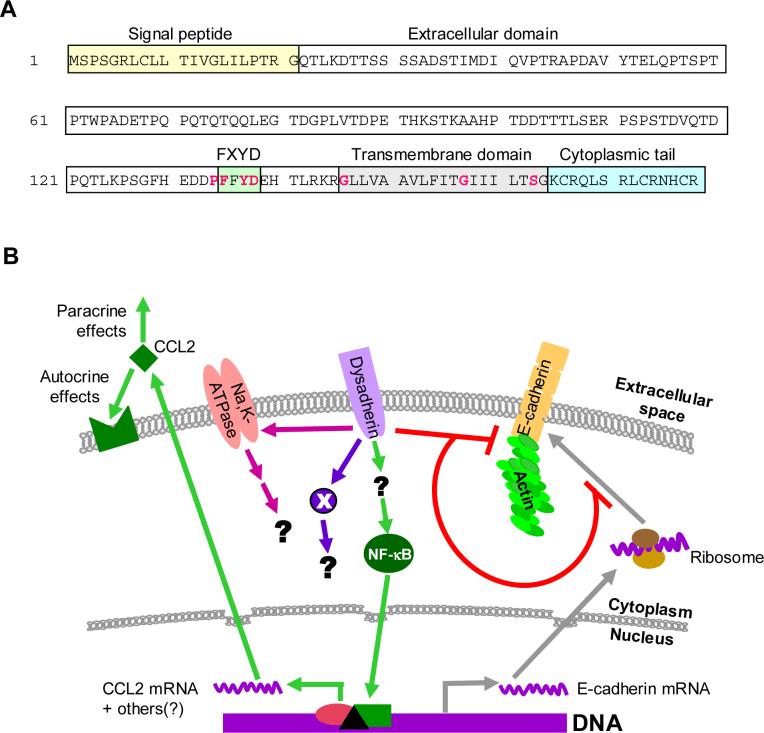
Dysadherin was originally identified by immunoscreening of a cDNA expression library to find the molecular target of a monoclonal antibody, NCG-3G10, that bound primarily to cancer and not to normal cell lines [1]. Sequence analysis showed that human dysadherin was closely related to the mouse gene RIC (related to ion channels). Dysadherin is now known to be identical to FXYD5 (Fxyd domain containing ion transport regulator 5), the human homolog of RIC. FXYD5 is a structurally divergent member of the FXYD family proteins, which are generally single-pass membrane proteins that interact with and modulate properties of the Na,K-ATPase [2]. The cDNA of dysadherin/FXYD5 encodes 178 amino acids, which include a putative signal sequence, an extracellular domain that is longer than that of the other FYXD members, a transmembrane domain, and a short cytoplasmic tail ([1,3], and see Figure 1A). The native form of the protein in tumor cells is heavily glycosylated on the extracellular domain [4].
Fig. 1. Dysadherin structure, and a model for the role of dysadherin in cancer progression.
A. Primary sequence and domain structure for human dysadherin. Dysadherin is a single-pass transmembrane protein of 178 amino acids, with a long extracellular domain, a transmembrane domain and a short cytoplasmic tail. The highest conservation between FXYD family members lies in the region of the FXYD motif and the transmembrane domain. Residues that are absolutely conserved between all family members are shown in red. The extracellular domain is heavily and heterogeneously glycosylated, carrying up to 30KDa of O-linked carbohydrate in some tumor cells. B. Model for dysadherin action. Dysadherin predominantly resides in the plasma membrane. There is convincing evidence that dysadherin can reduce cell-cell adhesion by blocking expression of E-cadherin at a post-transcriptional level, and/or by down-regulating the association of E-cadherin with the actin cytoskeleton. Dysadherin can also affect invasion and metastasis by E-cadherin-independent mechanisms. One such mechanism involves upregulating expression of the chemokine CCL2, which then exerts autocrine and paracrine tumor-promoting effects in the tumor bed. The detailed molecular mechanisms that couple dysadherin expression to down-stream events that promote tumor progression are currently not known. However, dysadherin expression is associated with enhanced activity of the NFκB pathway in a breast cancer model system. In non-transformed cells, dysadherin interacts with and modulates the activity of the Na+,K+-ATPase. It remains to be determined whether this interaction occurs in tumor cells or what the functional consequences may be. Finally, it is likely that dysadherin also regulates the activity of additional downstream effector molecules (indicated by X) that have yet to be identified.
In the original paper describing its discovery, dysadherin was shown to be expressed to various extents in many different types of tumors, such as stomach, colon, pancreatic, and breast tumors [1]. In contrast, only a limited number of normal cell types, including lymphocytes, endothelial cells, and basal cells of stratified squamous epithelium, showed dysadherin expression [1]. Subsequently however, dysadherin/FXYD5 expression was also detected by Western blotting in kidney, duodenum, spleen and lung, possibly reflecting a normal role in regulating ion transport [2]. Clues as to the basis for the overexpression in tumor tissue came from observations that expression of the mouse homolog of dysadherin, the RIC gene, was induced in NIH 3T3 fibroblasts transformed by a variety of oncogenes, including E2a-Pbx1, v-Ras, Neu and v-Src [5]. In silico data mining of published microarray studies using the Oncomine tool (www.oncomine.org) shows that dysadherin is also significantly upregulated in human primary mammary epithelial cells transfected with the activated H-ras, c-Src, c-Myc, E2F3 or activated β-catenin oncogenes ([6], and see Fig. 2A), and in human lung cancer cell lines that were p53 null compared with those that were p53 wildtype [7]. These data suggest that the upregulation of dysadherin following oncogene activation or loss of a tumor suppressor may be a general phenomenon that could underlie the preferentially high expression of dysadherin in tumor tissues.
Figure 2. Dysadherin expression in oncogene transformed breast epithelial cells and clinical breast cancer samples, from in silico datamining of published expression array data.
A. Dysadherin mRNA is upregulated in human breast epithelial cells transformed by several different oncogenes. Data were derived by datamining expression array results in [6]. β-Cat: activated β-catenin. * P<0.05 when compared with untransformed control cells. B. Dysadherin mRNA is upregulated in estrogen receptor (ER) negative compared with ER positive breast cancer samples (datamining from clinical microarray results in [30]). C. Dysadherin mRNA is upregulated in breast cancers with mutations in the BrCa1 gene when compared with sporadic breast cancers (datamining from clinical microarray results in [31]). N indicates the number of patients in each clinical subgroup.
3. The role of dysadherin in cancer progression: experimental evidence
Following the cloning of dysadherin, preliminary functional analysis showed that transfection of a liver cancer cell line with the cDNA of dysadherin resulted in reduced cell-cell adhesiveness in vitro and enhanced the formation of intrahepatic metastases following intrasplenic injection of the transfectants in vivo [1]. Similarly, overexpression of dysadherin in Capan-1 human pancreatic cells promoted metastasis [8], while knockdown of dysadherin in the MDA-MB-231 human breast cancer cell line suppressed metastatic potential ([9] and see Fig. 3). Dysadherin was highly expressed in a subset of cell lines (m2615 and m2691 cells) from human gastric scirrhous carcinoma that possessed the potential to metastasize spontaneously in nude mice [10], and in a xenograft model of breast cancer progression based on derivatives of the MCF10A cell line, dysadherin protein was only increased in the high grade metastatic cell line, and not in low-grade tumorigenic or premalignant lines [9]. These studies all suggested a role for dysadherin specifically in the metastatic process, and possible contributing mechanisms are discussed below.
Figure 3. Endogenous dysadherin is required for efficient metastasis of human breast cancer cells.

A. Schematic of experimental design. B. Functional knockdown of dysadherin in MDA-MB231 human breast cancer cells using siRNA results in decreased metastasis to the lungs following injection of genetically modified cells into the tail vein of nude mice (see [9]).
3.1. E-cadherin dependent mechanisms
The inactivation of the E-cadherin cell adhesion system, which can occur by various genetic and epigenetic mechanisms, is thought to be a necessary step for cancer invasion and metastasis [11]. Overexpression of dysadherin in a dysadherin-negative liver cancer cell line caused morphological changes suggestive of decreased cell-cell adhesiveness, and dysadherin was shown to cause a dose-dependent down-regulation of E-cadherin by a posttranscriptional mechanism [1]. Expression of α-catenin was also significantly reduced in this study. Thus it was plausibly suggested that dysadherin might promote metastasis by downregulating E-cadherin, thereby decreasing cell-cell adhesion and permitting expression of a motile phenotype. As discussed in a later section, expression of E-cadherin is inversely correlated with dysadherin in tumor specimens in a number of clinical studies, confirming the likely importance of this mechanism in at least some tumor types. Modulation of dysadherin expression in pancreatic cancer cell lines also affected cell morphology, actin organization, focal contact formation and cell motility in vitro, as well as metastatic efficiency in vivo [8], although it was not determined whether E-cadherin-dependent or independent mechanisms were involved in this case. Down-regulation of dysadherin in the pancreatic cells was associated with an enlarged, flattened and more adherent-looking morphology, with increased filamentous transverse actin stress fibres. There was also an increase in the formation of paxillin-containing focal adhesions. The authors proposed that dysadherin may facilitate cell movement by recruiting or maintaining actin filaments at the leading edge of the cell membrane, and by suppressing or disrupting formation of focal contacts.
A subsequent study confirmed that E-cadherin expression was also inversely correlated with dysadherin expression in a panel of breast cancer cell lines [9]. Interestingly however, experimental knockdown of dysadherin in two E-cadherin-positive breast cancer cell lines did not decrease levels of total E-cadherin protein, but rather suppressed interactions of E-cadherin with the actin cytoskeleton. Thus dysadherin can alter E-cadherin function independent of effects on expression in some systems (Fig. 1B). This observation may explain why there was no inverse correlation between E-cadherin and dysadherin expression in a large-scale breast cancer tissue microarray study (Nam et al, unpublished data), and in several of the published clinical studies (see later).
3.2. E-cadherin independent mechanisms
Unexpectedly, dysadherin was found to be capable of promoting invasion and metastasis of breast cancer cells that did not express any E-cadherin, suggesting that novel E-cadherin-independent mechanisms might also be important [9]. Using a globalgene expression analysis approach, the chemokine (C-C motif) ligand 2 (CCL2) was identified as the transcript most affected by dysadherin knockdown in MDA-MB-231 breast cancer cells [9]. CCL2 is a CC chemokine that is chemotactic for monocytes, memory T cells, and natural killer cells [12]. It is expressed by a wide variety of cancer types, and numerous studies have showed that CCL2 generally facilitates tumor progression [13-15], with contributing mechanisms likely to include chemoattraction of tumor-promoting leukocytes, and promotion of tumor cell migration and angiogenesis [16,17]. The ability of dysadherin to promote invasion of the MDA MB231 cells in vitro was dependent on the establishment of a CCL2 autocrine loop, and furthermore CCL2 secreted by the tumor cells could also promote endothelial cell migration in a paracrine fashion [9]. The functional importance of CCL2 was confirmed by the demonstration that experimental suppression of CCL2 in MDA-MB-231 cells reduced their ability to metastasize in vivo. Thus, dysadherin can have prometastatic effects that are independent of E-cadherin expression, through the upregulation of chemokines that influence both the tumor cell itself and the surrounding stroma (Fig 1B).
Global gene expression analysis in MDA-MB-231 cells showed that expression of several hundred genes was altered when dysadherin was knocked down using siRNA, so undoubtedly additional mechanisms of action may emerge in the future. It is provocative that a cDNA sequence almost identical to dysadherin was isolated from a library of human CD34+ hematopoietic stem/progenitor cells, suggesting a possible role for dysadherin in stem cell dynamics [1].
4. Clinical significance of dysadherin expression
4.1. Pathological studies
E-cadherin plays a critical role in establishing and maintaining the polarity and histological structure of cells, and dysfunction of the E-cadherin-mediated cell adhesion system results in progression of the relatively benign tumor to invasive, metastatic carcinoma [18]. In the original paper describing the discovery of dysadherin as a regulator of E-cadherin and promoter of metastasis, dysadherin expression correlated with poor survival in a small cohort of patients with stage II breast cancer [1]. Since then, the prognostic significance of dysadherin and E-cadherin expression has been investigated in many types of human cancer. Strong membranous staining was the typical staining pattern for dysadherin-positive tumors of all tumor types in the published studies. As expected, dysadherin tended to be expressed where the cell-cell contact was loose, or in dissociated cells [1]. In pancreatic ductal adenocarcinoma, nearly all cancer cells in infiltrative and poorly differentiated tumor nests overexpressed dysadherin, while only a small proportion of cells in well-differentiated nests did [19].
Overexpression of dysadherin was significantly associated with metastasis and/or poor prognosis in essentially all of the published studies, as summarized in Table 1. In some of these studies, there was a significant inverse correlation between dysadherin and E-cadherin expression (eg. tongue and thyroid carcinoma, and head and neck squamous carcinoma), suggesting that down-regulation of E-cadherin may be an important contributing mechanism. In other studies (eg. pancreatic, colorectal, and gastric carcinoma, non-small lung cancer and cutaneous malignant melanoma) dysadherin and E-cadherin were not inversely correlated, though dysadherin expression was still an independent indicator of poor prognosis. This suggests that E-cadherin-independent mechanisms of dysadherin action may dominate in these tumor types, or that dysadherin is affecting E-cadherin function rather than expression. In summary, dysadherin expression appears to be an independent biological predictor of metastatic spread and poor prognosis for many tumor types in human patients, and for some tumors, the prognostic power is increased by combining both dysadherin and E-cadherin status.
Table 1.
Immunohistochemical analysis of dysadherin expression in human cancer: summary of clinical studies
| Tumor type | Size of cohort | Inverse correlation with E-cadherin? | Independent indicator of poor prognosis? | Comments | Reference |
|---|---|---|---|---|---|
| Colorectal cancer | 78 | No* | ? | *Inverse correlation with E-cadherin in lymph node metastases, but no correlation in primary tumor | [20] |
| Head and neck squamous carcinoma | 108 | Yes | Yes | Dysadherin correlated with high clinical stage, presence of lymph node metastases, and intratumoral lymphatic density | [21] |
| Testicular tumors | 120 | In most | ? | Highly expressed in all germ cell tumors, except for some of the most terminally differentiated neoplasms | [22] |
| Non-small cell lung cancer | 131 | No | Yes | Prognostic power of elevated dysadherin increased when combined with low E-cadherin expression | [23] |
| Cutaneous malignant melanoma | 115 | No | Yes | Increased dysadherin correlated with multiple measures of aggressive disease including lymph node metastasis, high TMN classification and poor patient survival | [24] |
| Cervical squamous cell carcinoma | 206 | Not assessed | ? | High dysadherin was associated with shorter overall survival | [25] |
| Tongue cancer | 91 | Yes | Yes | High dysadherin correlated with infiltrative growth pattern, high TMN stage and poor survival | [26] |
| Esophageal squamous cell carcinoma | 117 | Yes | Combined analysis of dysadherin and E-cadherin improved prognostic power | [27] | |
| Gastric cancer | 276 | No | No | Addition of dysadherin status improved prognostic value of E-cadherin assessment. Patients with dysadherin positivity had higher risk of hematogenous metastasis compared with peritoneal dissemination | [28] |
| Thyroid cancer | 92 | Yes | ? | Dysadherin associated with poor prognosis, occurrence of secondary undifferentiated carcinomas, size of primary tumor and metastasis to lymph nodes | [29] |
| Pancreatic ductal adenocarcinoma | 125 | No | Yes | Dysadherin correlated with distant metastasis, high tumor grade and infiltrative growth pattern. Improved prognostic power when combined with low E-cadherin | [19] |
| Breast cancer (stage II) | 57 | Not assessed | ? | Dysadherin associated with shorter metastasis-free survival | [1] |
4.2. Cancer Microarray Data Mining
In silico data mining of results from large-scale clinical microarray studies (http://www.oncomine.org) yields further clues as to the possible importance of dysadherin in cancer progression. For example, dysadherin is much more highly expressed in the estrogen-receptor negative compared with estrogen-receptor positive breast cancers in multiple large-scale studies (see for example [30] and Fig. 2B), suggesting a particularly important role for dysadherin in this poor-prognosis breast cancer subtype [9]. It is also elevated in breast cancer patients with BRCA1 mutations, again a poor prognosis subgroup ([31] and Fig. 2C). In studies on brain cancer, dysadherin increases with increasing tumor grade in glioma [32], and is associated with poor survival in glioma [32], and with relapse in neuroblastoma [33]. Glioblastoma multiforme and astrocytoma both show higher levels of dysadherin message than oligodendroglioma [34]. Intriguingly, elevated expression of dysadherin was associated with sensitivity to combined chemotherapy with irinotecan and 5-fluorouracil in a study on colorectal cancer [35], suggesting that dysadherin status might be worth evaluating as a useful predictor of drug response for some tumors. While clearly not definitive, these types of analyses are useful for hypothesis generation, and may provide interesting leads for new studies.
5.0 Unsolved issues in dysadherin biology.
From this promising beginning, there is still much to be learned about the role of dysadherin in cancer progression. Currently we need more information on (1) what proteins interact with dysadherin, (2) whether dysadherin can propagate signals within the cell, (3) the detailed molecular mechanisms by which dysadherin regulates E-cadherin, CCL2 and other players that modify the metastatic phenotype, and (4) the regulation of dysadherin expression, including the possible role of post-translational modifications such as glycosylation and phosphorylation in modulating dysadherin localization and function.
One key question is whether the pro-metastatic effect of dysadherin is mediated by effects on the Na,K-ATPase, since all FXYD family members function as tissue-specific modulators of this ion pump [2]. Dysadherin has been shown to interact directly with the α subunit of the Na,K-ATPase, and high level expression of dysadherin in the normal kidney cortex suggests that like other FXYDs, dysadherin plays a role in regulating ion flux in normal homeostasis [2,36]. Interestingly, the Na,K-ATPase has been shown to act as a signal transducer, in addition to being an ion pump [37,38], so conceivably dysadherin might contribute to cancer metastasis through mechanisms involving changes in ion flux, leading to effects on the protein kinase C and extracellular signal-regulated kinase 1/2 pathways.
The possibility of additional mechanisms for dysadherin signaling should be considered. The short cytoplasmic tail of dysadherin makes it relatively unlikely that the intracellular domain signals directly. However, the unusually long extracellular domain of dysadherin may facilitate interactions with other membrane proteins or with extracellular matrix components, and affect signaling dynamics. A systematic analysis of the dysadherin protein interactome would be helpful to give insights into possible mechanisms. In the human breast cancer cell line MDA-MB-231, the presence of dysadherin was associated with activation of the NF-κB pathway, although the poor kinetic resolution of gene silencing experiments did not reveal whether NF-κB pathway activation was a primary or a secondary effect of dysadherin expression [9]. Tumor invasion and metastasis are known to be influenced by numerous NF-κB-regulated gene products, including matrix metalloproteinases, interleukin-8 and various chemokines [9]. It is provocative that chemokines have recently been implicated in the tumor-driven mobilization of bone marrow-derived cells to establish “premetastatic niches” at metastasis target sites [39].
Post-translational modification may also be important. Dysadherin in cancer cells is heavily glycosylated, and the O-linked glycosylation is necessary for stable expression of dysadherin, suppression of E-cadherin and disruption of cell adhesion [4]. Interestingly, the form of dysadherin present in normal mouse kidney carries very little carbohydrate, suggesting that the extensive O-linked glycosylation may be a feature of tumor rather than normal cells [2]. Other FXYD family members are subject to additional post-translational regulation that variously affects their localization, stability or function. For example, FXYD1 can be phosphorylated on serine residues by several different kinases, including protein kinases A and C [3]. It will be interesting to determine whether dysadherin is subject to similar regulation, and what cellular functions this might impact on. A model summarizing what is currently known about dysadherin function is given in Fig. 1B.
5. Conclusions and perspectives for the future
Collectively, both clinical and experimental data suggest that overexpression of dysadherin may contribute causally to tumor progression and metastasis, and dysadherin is an independent prognostic indicator of metastasis and/or poor survival in many different cancer types. The expression of dysadherin on normal tissues is somewhat broader than originally anticipated, with significant expression in the kidney, lung and duodenum, as well as in advanced cancers. Thus while dysadherin is attractive as a molecular target for development of novel therapeutics, it will be important to determine whether dysadherin plays essential and non-redundant roles in normal homeostasis, particularly in the regulation of ion flux. However, the higher level expression of dysadherin in tumor cells, or tumor-specific features such as heavy glycosylation, might generate an exploitable therapeutic window. Since it is a cell surface molecule, dysadherin could also potentially be exploited as a homing target for immunotoxins or cancer-directed imaging agents. Gaining a better insight into the regulation of dysadherin expression and its mechanisms of action might therefore lead to new therapeutic targets and better anticancer strategies.
Acknowledgements
We thank Ethan A. Kohn for critical reading of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci U S A. 2002;99:365–70. doi: 10.1073/pnas.012425299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, Maunsbach AB, Garty H. Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem. 2005;280:37717–24. doi: 10.1074/jbc.M506397200. [DOI] [PubMed] [Google Scholar]
- 3.Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol. 2006;290:F241–50. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- 4.Tsuiji H, Takasaki S, Sakamoto M, Irimura T, Hirohashi S. Aberrant O-glycosylation inhibits stable expression of dysadherin, a carcinoma-associated antigen, and facilitates cell-cell adhesion. Glycobiology. 2003;13:521–7. doi: 10.1093/glycob/cwg065. [DOI] [PubMed] [Google Scholar]
- 5.Fu X, Kamps MP. E2a-Pbx1 induces aberrant expression of tissue-specific and developmentally regulated genes when expressed in NIH 3T3 fibroblasts. Mol Cell Biol. 1997;17:1503–12. doi: 10.1128/mcb.17.3.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, Olson JA, Jr., Marks JR, Dressman HK, West M, Nevins JR. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–7. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 7.Robinson M, Jiang P, Cui J, Li J, Wang Y, Swaroop M, Madore S, Lawrence TS, Sun Y. Global genechip profiling to identify genes responsive to p53-induced growth arrest and apoptosis in human lung carcinoma cells. Cancer Biol Ther. 2003;2:406–15. doi: 10.4161/cbt.2.4.437. [DOI] [PubMed] [Google Scholar]
- 8.Shimamura T, Yasuda J, Ino Y, Gotoh M, Tsuchiya A, Nakajima A, Sakamoto M, Kanai Y, Hirohashi S. Dysadherin expression facilitates cell motility and metastatic potential of human pancreatic cancer cells. Cancer Res. 2004;64:6989–95. doi: 10.1158/0008-5472.CAN-04-1166. [DOI] [PubMed] [Google Scholar]
- 9.Nam JS, Kang MJ, Suchar AM, Shimamura T, Kohn EA, Michalowska AM, Jordan VC, Hirohashi S, Wakefield LM. Chemokine (C-C motif) ligand 2 mediates the prometastatic effect of dysadherin in human breast cancer cells. Cancer Res. 2006;66:7176–84. doi: 10.1158/0008-5472.CAN-06-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanagihara K, Tanaka H, Takigahira M, Ino Y, Yamaguchi Y, Toge T, Sugano K, Hirohashi S. Establishment of two cell lines from human gastric scirrhous carcinoma that possess the potential to metastasize spontaneously in nude mice. Cancer Sci. 2004;95:575–82. doi: 10.1111/j.1349-7006.2004.tb02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575–81. doi: 10.1111/j.1349-7006.2003.tb01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14:149–54. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- 14.Saji H, Koike M, Yamori T, Saji S, Seiki M, Matsushima K, Toi M. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer. 2001;92:1085–91. doi: 10.1002/1097-0142(20010901)92:5<1085::aid-cncr1424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol. 2003;22:773–8. [PubMed] [Google Scholar]
- 16.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 17.Youngs SJ, Ali SA, Taub DD, Rees RC. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer. 1997;71:257–66. doi: 10.1002/(sici)1097-0215(19970410)71:2<257::aid-ijc22>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–9. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimamura T, Sakamoto M, Ino Y, Sato Y, Shimada K, Kosuge T, Sekihara H, Hirohashi S. Dysadherin overexpression in pancreatic ductal adenocarcinoma reflects tumor aggressiveness: relationship to e-cadherin expression. J Clin Oncol. 2003;21:659–67. doi: 10.1200/JCO.2003.06.179. [DOI] [PubMed] [Google Scholar]
- 20.Batistatou A, Charalabopoulos AK, Scopa CD, Nakanishi Y, Kappas A, Hirohashi S, Agnantis NJ, Charalabopoulos K. Expression patterns of dysadherin and E-cadherin in lymph node metastases of colorectal carcinoma. Virchows Arch. 2006;448:763–7. doi: 10.1007/s00428-006-0183-8. [DOI] [PubMed] [Google Scholar]
- 21.Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ, Nakanishi Y, Hirohashi S, Charalabopoulos K. Dysadherin expression in head and neck squamous cell carcinoma: association with lymphangiogenesis and prognostic significance. Am J Surg Pathol. 2006;30:185–93. doi: 10.1097/01.pas.0000178090.54147.f8. [DOI] [PubMed] [Google Scholar]
- 22.Batistatou A, Scopa CD, Ravazoula P, Nakanishi Y, Peschos D, Agnantis NJ, Hirohashi S, Charalabopoulos KA. Involvement of dysadherin and E-cadherin in the development of testicular tumours. Br J Cancer. 2005;93:1382–7. doi: 10.1038/sj.bjc.6602880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura M, Ohta Y, Tsunezuka Y, Matsumoto I, Kawakami K, Oda M, Watanabe G. Prognostic significance of dysadherin expression in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130:740–5. doi: 10.1016/j.jtcvs.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 24.Nishizawa A, Nakanishi Y, Yoshimura K, Sasajima Y, Yamazaki N, Yamamoto A, Hanada K, Kanai Y, Hirohashi S. Clinicopathologic significance of dysadherin expression in cutaneous malignant melanoma: immunohistochemical analysis of 115 patients. Cancer. 2005;103:1693–700. doi: 10.1002/cncr.20984. [DOI] [PubMed] [Google Scholar]
- 25.Wu D, Qiao Y, Kristensen GB, Li S, Troen G, Holm R, Nesland JM, Suo Z. Prognostic significance of dysadherin expression in cervical squamous cell carcinoma. Pathol Oncol Res. 2004;10:212–8. doi: 10.1007/BF03033763. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi Y, Akimoto S, Sato Y, Kanai Y, Sakamoto M, Hirohashi S. Prognostic significance of dysadherin expression in tongue cancer: immunohistochemical analysis of 91 cases. Appl Immunohistochem Mol Morphol. 2004;12:323–8. doi: 10.1097/00129039-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Shimada Y, Hashimoto Y, Kan T, Kawamura J, Okumura T, Soma T, Kondo K, Teratani N, Watanabe G, Ino Y, Sakamoto M, Hirohashi S, Imamura M. Prognostic significance of dysadherin expression in esophageal squamous cell carcinoma. Oncology. 2004;67:73–80. doi: 10.1159/000080289. [DOI] [PubMed] [Google Scholar]
- 28.Shimada Y, Yamasaki S, Hashimoto Y, Ito T, Kawamura J, Soma T, Ino Y, Nakanishi Y, Sakamoto M, Hirohashi S, Imamura M. Clinical significance of dysadherin expression in gastric cancer patients. Clin Cancer Res. 2004;10:2818–23. doi: 10.1158/1078-0432.ccr-0633-03. [DOI] [PubMed] [Google Scholar]
- 29.Sato H, Ino Y, Miura A, Abe Y, Sakai H, Ito K, Hirohashi S. Dysadherin: expression and clinical significance in thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:4407–12. doi: 10.1210/jc.2002-021757. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–9. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 31.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 32.Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, Mischel PS, Nelson SF. Gene expression profiling of gliomas strongly predicts survival. Cancer Res. 2004;64:6503–10. doi: 10.1158/0008-5472.CAN-04-0452. [DOI] [PubMed] [Google Scholar]
- 33.Asgharzadeh S, Pique-Regi R, Sposto R, Wang H, Yang Y, Shimada H, Matthay K, Buckley J, Ortega A, Seeger RC. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J Natl Cancer Inst. 2006;98:1193–203. doi: 10.1093/jnci/djj330. [DOI] [PubMed] [Google Scholar]
- 34.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, Rosenblum M, Mikkelsen T, Fine HA. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Graudens E, Boulanger V, Mollard C, Mariage-Samson R, Barlet X, Gremy G, Couillault C, Lajemi M, Piatier-Tonneau D, Zaborski P, Eveno E, Auffray C, Imbeaud S. Deciphering cellular states of innate tumor drug responses. Genome Biol. 2006;7:R19. doi: 10.1186/gb-2006-7-3-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crambert G, Geering K. FXYD proteins: new tissue-specific regulators of the ubiquitous Na,K-ATPase. Sci STKE 2003. 2003:RE1. doi: 10.1126/stke.2003.166.re1. [DOI] [PubMed] [Google Scholar]
- 37.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem. 1998;273:15249–56. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 38.Mohammadi K, Kometiani P, Xie Z, Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem. 2001;276:42050–6. doi: 10.1074/jbc.M107892200. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan RN, Rafii S, Lyden D. Preparing the “Soil”: the pre-metastatic niche. Cancer Res. 2006;66:11089–93. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]