Abstract
Both N,N′-(2,3-dihydroxybenzyl)-N,N,N′,N′-tetramethyl-1,6-hexanediamine dibromide (DTH, 6) and N,N′-(2,3-dihydroxybenzyl)-N,N,N′,N′-tetramethyl-1,10-decanediamine dibromide (DTD, 7), which are symmetrical bis-catechol substituted hexamethonium and decamethonium analogues, respectively, were found to inhibit high affinity choline transport in mouse brain synaptosomes. Inhibitory properties were evaluated using an extraordinarily sensitive capillary electrophoresis method employing electrochemical detection at an enzyme-modified microelectrode. Dose-response curves were generated for each inhibitor and IC50 values were determined to be 76 μM for 6 and 21 μM for 7. Lineweaver-Burk analysis revealed that both molecules inhibit high affinity choline uptake by a mixed inhibition mechanism. The KI values for 6 and 7 were determined to be 73 ± 1 and 31 ± 2 μM, respectively. The inhibition properties were further compared to a series of mono-catechol analogues, 3-[(trimethylammonio)methyl]catechol (1), N,N-dimethylepinephrine (4) and 6-hydroxy-N,N-dimethylepinephrine (5), as well as the well-characterized hemicholinium inhibitors, hemicholinium-15 (HC-15, 8) and hemicholinum-3 (HC-3, 9).
Keywords: high-affinity choline transport, cholinomimetic inhibitors, neuronal degradation, capillary electrophoresis with electrochemical detection, enzyme microelectrode
Introduction
Cholinergic neurons play a vital role in cognition and memory. Changes in the cholinergic system are linked to the development of neurodegenerative disorders such as Alzheimer’s disease.1–3 One significant challenge for the diagnosis of neuronal degradation is that loss of cognitive function is gradual, resulting from subtle, yet progressive changes in the cholinergic system. Therefore, it is important to understand both the mechanism of selective cholinergic degradation and the changes in the levels of critical neurochemicals that serve as biomarkers of the cholinergic system.
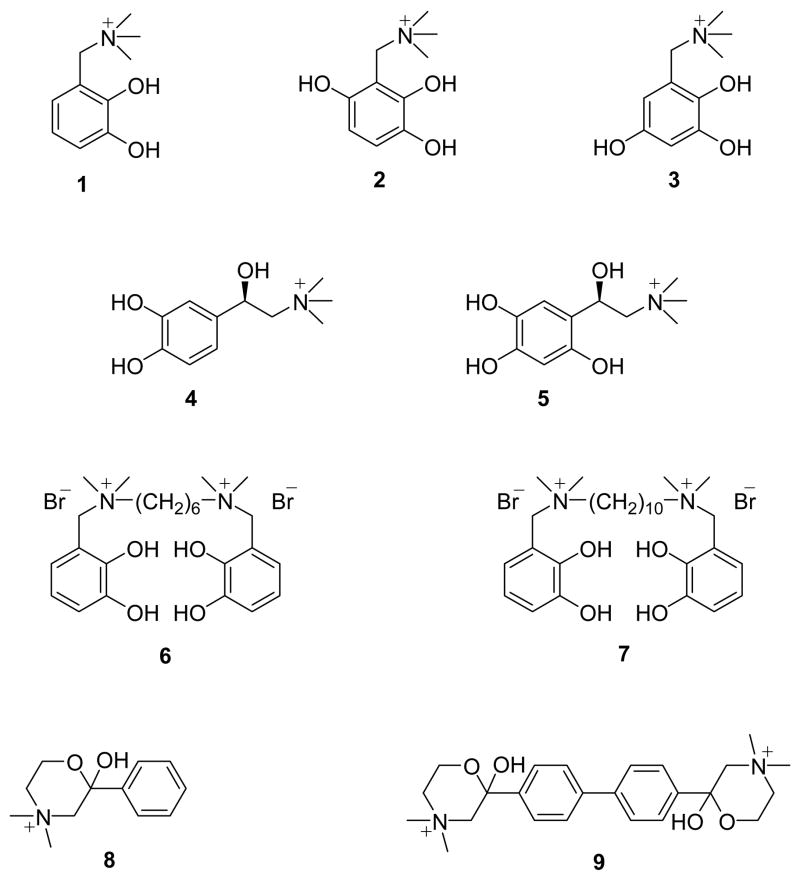
Structure-activity relationships suggest that the high-affinity choline transporter system (CHT) requires several points of interaction for which the presence of a quaternary nitrogen atom with a neighboring hydroxyl group or another hydrogen-bond acceptor ligand is an essential prerequisite.4 In order to probe selective cholinergic degradation, we have synthesized a unique series of cholinomimetic quaternary ammonium catechol-based molecules (Figure 1).5–10 The defining structural features of this series include a quaternary ammonium group and a neighboring hydroxyl that produces a choline (Ch) analogue type affinity for cholinergic sites and a bulky, redox-active catechol moiety. While an extended structure-activity series may be of eventual value, our purpose was to incorporate the catechol moiety into a relatively simple carbon framework possessing the elements of Ch, acetylcholine (ACh) or other elements of known classical inhibitors of cholinergic activity. Inhibitor functionality was selected based on their cholinomimetic activity at external macromolecular sites regulating cholinergic function, e.g., the nicotinic ACh receptor (nAcChR), acetylcholine esterase (AChE), or CHT. It was postulated that one or more of these novel agents would be selectively effective in inhibiting either post-synaptically or presynaptically, but not simultaneously at both sites.
Figure 1.
Chemical structures of 3-[(trimethylammonio)methyl]catechol (1), 4-hydroxy-3-[(trimethylammonio)methyl]catechol (2), 5-hydroxy-3-[(trimethylammonio)methyl]catechol (3), N,N-dimethylepinephrine (4), 6-hydroxy-N,N-dimethylepinephrine (5), N,N′-(2,3-dihydroxybenzyl)-N,N,N′,N′-tetramethyl-1,6-hexanediamine dibromide (DTH, 6), N,N′-(2,3-dihydroxybenzyl)-N,N,N′,N′-tetramethyl-1,10-decanediamine dibromide (DTD, 7), hemicholinium-15 (HC-15, 8) and hemicholinium-3 (HC-3, 9).
Two mono-catechol derivatives, 3-[(trimethylammonio)methyl]catechol (1) and N,N-dimethylepinephrine (4), were found to inhibit choline acetyltransferase,6,7 CHT,7 and half of all neurotoxin sites of nAcChR.5,8 Selective inhibition studies of 1 suggested that the catechol group was instrumental for inactivation of ACh receptor sites through a redox-dependent mechanism. Slow auto-oxidation of 1 was proposed to lead to the formation of 4- and 5-hydroxy-3-[(trimethylammonio)methyl]catechol (2 and 3) intermediates, which were found to be even more reactive than 1 toward the nAcChR.8 Comparative electrochemical measurements of the redox potentials for 1-3 demonstrated that 2 and 3 were more easily oxidized than 1 and thus, further supported the proposed redox-inactivation mechanism. In light of these findings, 6-hydroxy-N,N-dimethylepinephrine (5) was also synthesized for comparison to 4.10 Similar to the observation for 2 and 3, the tri-hydroxy derivative, 5, was determined to be a more potent inhibitor.11
Previous studies on a wide variety of inhibitors also indicated that an increase in hydrophobicity of these reagents increases the affinity for cholinergic sites.12 for this reason two additional reagents, N,N′-(2,3-dihydroxybenzyl)-N,N,N′,N′-tetramethyl-1,6-hexanediamine dibromide (DTH, 6) and N,N′-(2,3-dihydroxybenzyl)-N,N,N′,N′-tetramethyl-1,10-decanediamine dibromide (DTD, 7) were designed and synthesized incorporating a bridging hydrophobic domain along with the cholinomimetic and redox active properties of the corresponding mono-catechol molecules.9 6 and 7 were based on the well-known hexamethonium and decamethonium reagents13,14 and were also previously shown to be highly effective for the inactivation of the nAcChR.9 Furthermore, 6 and 7 possessed a symmetrical bis-catechol feature in their structures. It was well-established that inhibition of CHT by hemicholinium-15 (HC-15, 8) is 100 to 300 times less effective than the corresponding bis-analogue hemicholinum-3 (HC-3, 9), the efficient inhibitor of CHT.13–16 Thus, this investigation focused on the quantitative evaluation of the inhibition parameters of the bis-catechol methonium analogues 6 and 7 for CHT and examined their efficacy relative to the mono-catechol agents 1, 4 and 5. The hypothesis was that the activity of 6 and 7 toward nAcChR might substantially eclipse the activities observed toward CHT.
Variations in the structure of these reagents selectively tuned their ability to inhibit CHT. Utilizing these molecules we developed a new assay using mouse brain synaptosomes as a model for CHT to determine whether the measurement of small, time-dependent changes in Ch transport were possible.11,19 The assay combined capillary electrophoresis (CE) with electrochemical detection (EC) at an enzyme-modified microelectrode to achieve a mass detection limit for Ch of 100 amol.17,18 The sensitivity of detection clearly established the unique inhibition parameters for these molecules, as well as the ability to evaluate subtle fluctuations in neurochemical concentrations under a variety of conditions during selective neuronal degradation.11,19 CE also offered the analytical advantages of nL-sized sample volumes and the efficient separation of components from complex sample matrices without tedious sample preparation or pre-concentration steps, making a wide range of in vitro and in vivo studies possible.
Experimental
Materials and Methods
Choline oxidase (ChO) (EC1.1.3.17, Alcaligenes species), AChE (EC 3.1.1.7, Type III from electric eel), choline chloride (>98%), glutaraldehyde (grade I, 25% aqueous solution) and butyrylcholine (BuCh) chloride (>98%) were purchased from Sigma (St. Louis, MO, USA) and stored in a desiccator at −10 °C. N-Tris(hydroxymethyl)methyl–2-aminoethanesulfonic acid (TES) (>99%), bovine albumin (>98%) and Bradford reagent were also purchased from Sigma and refrigerated at 4 °C. Compounds 6 and 7 were previously synthesized and characterized.9 All other chemicals were of reagent grade and used as received. Hard tempered 25 μm diameter platinum wire (99.95%) was obtained from Goodfellow (Berwyn, PA, USA). Solutions were prepared in distilled and deionized water purified to a resistivity of 17.5 MΩ cm by a Barnstead B-pure water purification system (Dubuque, IA).
Instrumentation
CE-EC experiments were performed on a laboratory-built instrument as described previously with minor modifications.20 The modifications included the use of an on-column bare fracture decoupler to isolate the detection cell from the separation voltage.21 The electrochemical detection cell was a three electrode system consisting of a Model RE-4 Ag/AgCl reference electrode, a platinum auxiliary electrode and an enzyme modified microelectrode as the working electrode. The electrochemical cell was controlled with a BAS LC-4C amperometric detector, which was modified for use with CE. The preparation of the enzyme modified microelectrode was previously described in detail.17 The enzyme microelectrode tip was carefully aligned with the capillary outlet by placing both the electrode and the capillary inside a custom made detection cell (Allied Plastics, Toledo, OH, USA).22 Alignment in this manner optimized physical contact with the flowing liquid at the end of the capillary and minimized disruption of the enzyme layer. The distance from the decoupler to the capillary outlet was ~2.5 cm. Separation was achieved on an 80 cm polyimide-coated fused-silica capillary with an i.d. of 50 μm and an o.d. of 300 μm (Polymicro Technologies, Phoenix, AZ, USA). Electropherograms were generated by applying 17 kV separation voltage with a Spellman CZ100R high-voltage power supply (Spellman, Plainview, NY). The separation current during operation ranged from 4 to 20 μA. Data were collected by an IBM P166 MHz computer through an A/D converter. P/ACE MDQ Capillary Electrophoresis System software (Beckman Scientific Instruments, Fullerton, CA) was used for data analysis.
Methods
TES (50 mM, pH 8) was used as the run buffer for all CE separations. New capillary was conditioned with HCl (10 min, 25 psi) to suppress electroosmotic flow, followed by H2O (10 min, 25 psi) and finally rinsed with TES (30 min, 25 psi) prior to use. Samples were injected by pressure injection using high purity argon at 5 psi for 2 s corresponding to an injection volume of 12.5 nL. When not in use the capillary was rinsed and filled with water. Standard stock solutions of Ch and BuCh were prepared daily and stored in ice. Ch concentrations were analyzed using BuCh as an internal standard.17,18
Evaluation of the inhibition properties of 6 and 7 utilized the Ch transport assay methods developed by Barkhimer et al.11,19 using mouse synaptosomes as the CHT model. Synaptosome suspensions were prepared from C57BL6 adult male mice (Harlan Sprague Dawley, Indianapolis, IN) following the general procedure of Gray and Whittaker,23 as modified by Patel.7 Incubation of synaptosomes was performed at 37 °C using an Isotemp Model 125D Digital Dry Bath Incubator from Fisher Scientific. A range of standard Ch solutions from 2 to 10 μM was used for the study. Standard solution concentrations of 6 varied from 10 to 3000 μM, which corresponded to a final concentration range of 2.7 to 815.6 μM. Standard concentrations used for 7 varied from 5 to 1000 μM, which corresponded to final concentrations of 1.4 to 271.8 μM. A typical incubation consisted of 300 μL of the synaptosome suspension and 112 μL of the stock Ch solution containing either 6 or 7. The mixture was equilibrated at 37 °C with 75 μL aliquots removed beginning at t = 0 min and continuing every minute for four minutes. Data collection was limited to the first four minutes of the incubation to minimize the effect of aging of the synaptosomes.17,19 Samples were immediately placed on ice and then centrifuged at 5000 rpm for 8 minutes. The resulting supernatant was stored at −20 °C until analysis.
Before analysis, BuCh was added to the centrifuged supernatant solution in a 2:1 ratio, v/v, to give a final BuCh concentration of 25 μM. The initial rate of Ch uptake at each inhibitor concentration was obtained by plotting the ratio of the peak areas of Ch/BuCh versus the incubation time (n = 3) and calculating the slope. The analysis for each inhibitor concentration was repeated in triplicate using different synaptosome preparations with the measured rate normalized for differences in protein concentration from the different synaptosome preparations. Protein concentrations were determined by the Bradford dye assay.24
IC50 values for 6 and 7 were evaluated from their dose response curves using Delta Graph version 5 software (Red Rock Software). The dose response curve was fitted to an equation: rate = A − m(10x) / (10x + 10K ), where x = log (inhibitor), K = log(IC50), A = extrapolated rate of Ch uptake in the absence of inhibitor, and m = A − (lowest rate observed in the presence of inhibitor).
Results and Discussion
The cholinomimetic derivatives, 6 and 7, were evaluated for their ability to selectively inhibit CHT. Concentration-dependent inhibition of Ch transport by 6 and 7 was established from their dose-response curves. Plots of the initial rate of Ch uptake versus log [6] and log [7] are shown in Figure 2. Table 1 summarizes the IC50 values for 6 and 7 and provides a comparison to the values for 1, 4, 5, 8 and 9.
Figure 2.
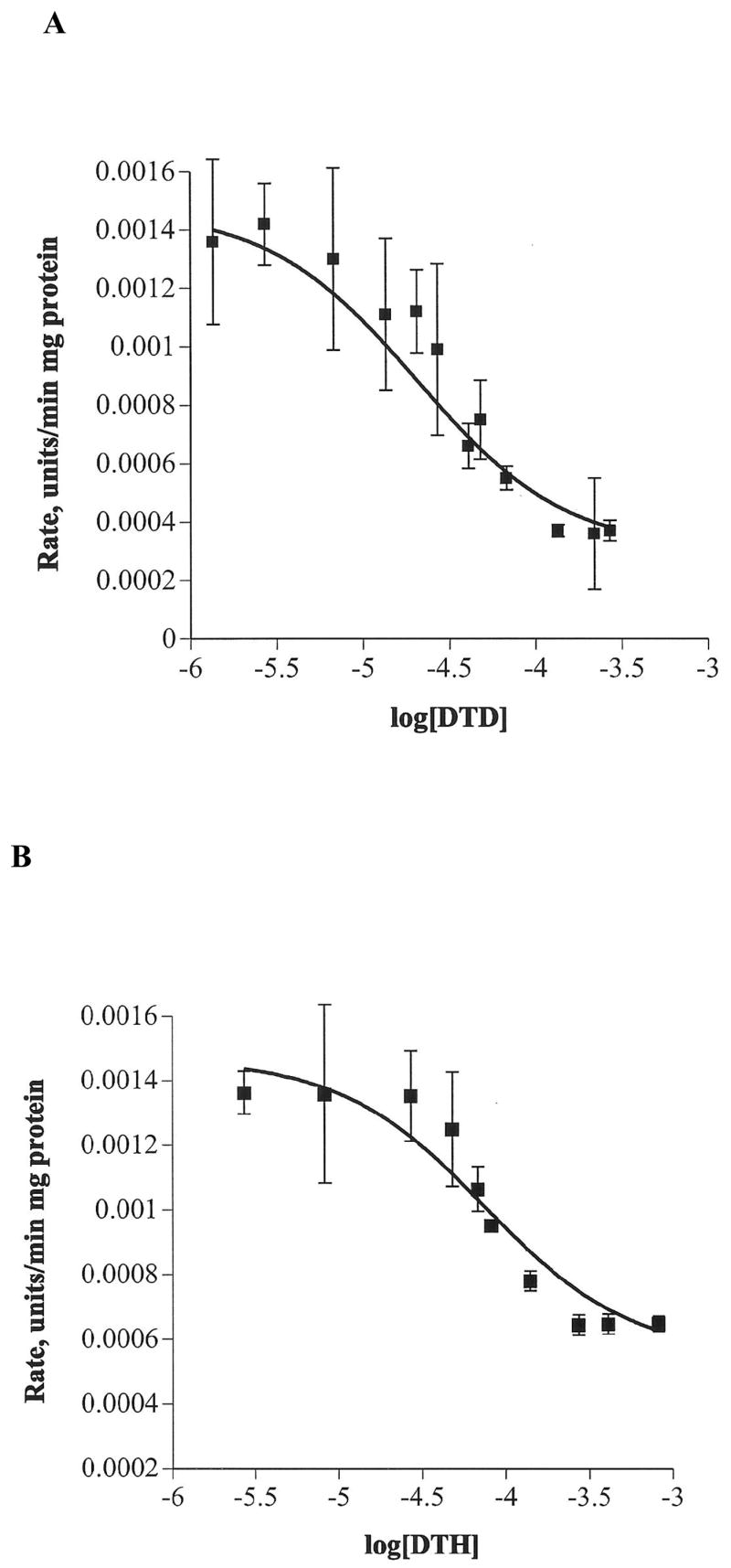
Plot of the initial rate of Ch uptake normalized for protein concentration versus log[inhibitor] at 37 °C for concentrations of (A) 7 from 1.4 to 271.8 μM; R2 = 0.9311, and (B) 6 from 2.7 to 815.6 μM; R2 = 0.9294. The Ch concentration was held constant at 2 μM. Each data point on the curve is the average rate of Ch uptake obtained from three different synaptosome preparations, each of which was analyzed in triplicate.
Table 1.
Comparison of the Inhibition Properties for the Quaternary Ammonium Catechol Based Inhibitors.
IC50 values for 6 and 7 were determined to be 76 and 21 μM, respectively. These values are similar in magnitude to the mono-catechol derivatives and did not reveal any obvious trends with respect to their structures. Interestingly, the IC50 value for the bis-quaternary ammonium compound hexamethonium (130 μM) was similar in magnitude to 6 and 4 for inhibition of CHT, while the decamethonium (1.5 μM) analogue was at least an order of magnitude better inhibitor than the best catechol-based inhibitors 1, 5 and 7.13 Hexamethonium and decamethonium also exhibited similar inhibition efficiency relative to their mono-quaternary ammonium analogues.13,16 In contrast, there was a dramatic reduction in the IC50 value observed for 9 compared to 8 for the hemicholinium analogues.13,15,16 Such a significant change was not evident from the values obtained for the mono- to bis-catechols structures, indicating additional effects were likely responsible for the exceptional inhibition properties of 9.
It was important to note that the rate of Ch uptake in our experiments did not decrease to zero even at high inhibitor concentrations, suggesting that nonspecific transport of Ch into synaptosomes occurred to a small degree. Control experiments have shown two important points with respect to the CE-EC assay performance: 1) minimum Ch efflux was observed within the first 8 minutes of analysis indicating a negligible effect on the initial rate data collected during the time frame defined in the experimental section, and 2) non-specific transport was limited to at most 12% of the total transport observed.19 The dose-response curve also offers the convenience of comparing the relative inhibitor potencies of multiple inhibitors for CHT provided the Ch substrate concentration remains constant for all experiments.25 for our experiments, the Ch concentration was fixed at 2 μM for ease of comparison to the mono-catechol reagents.
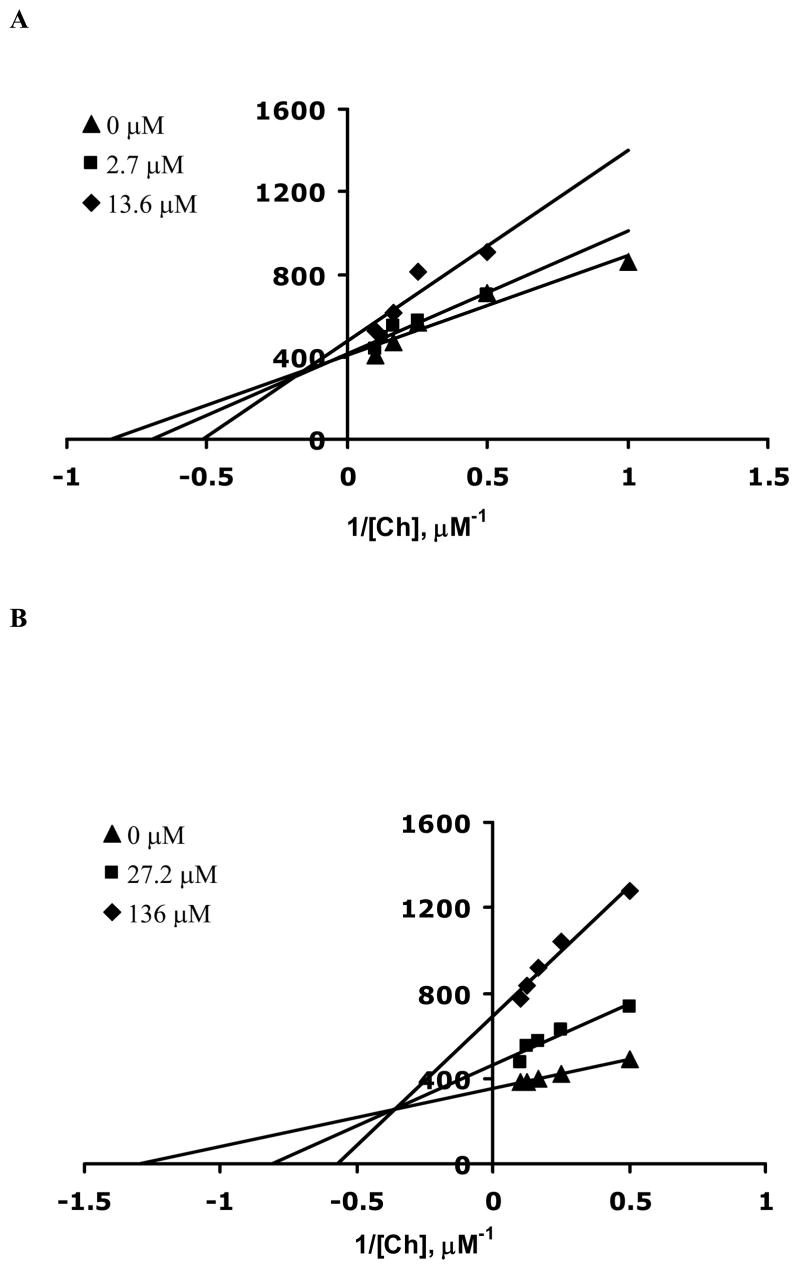
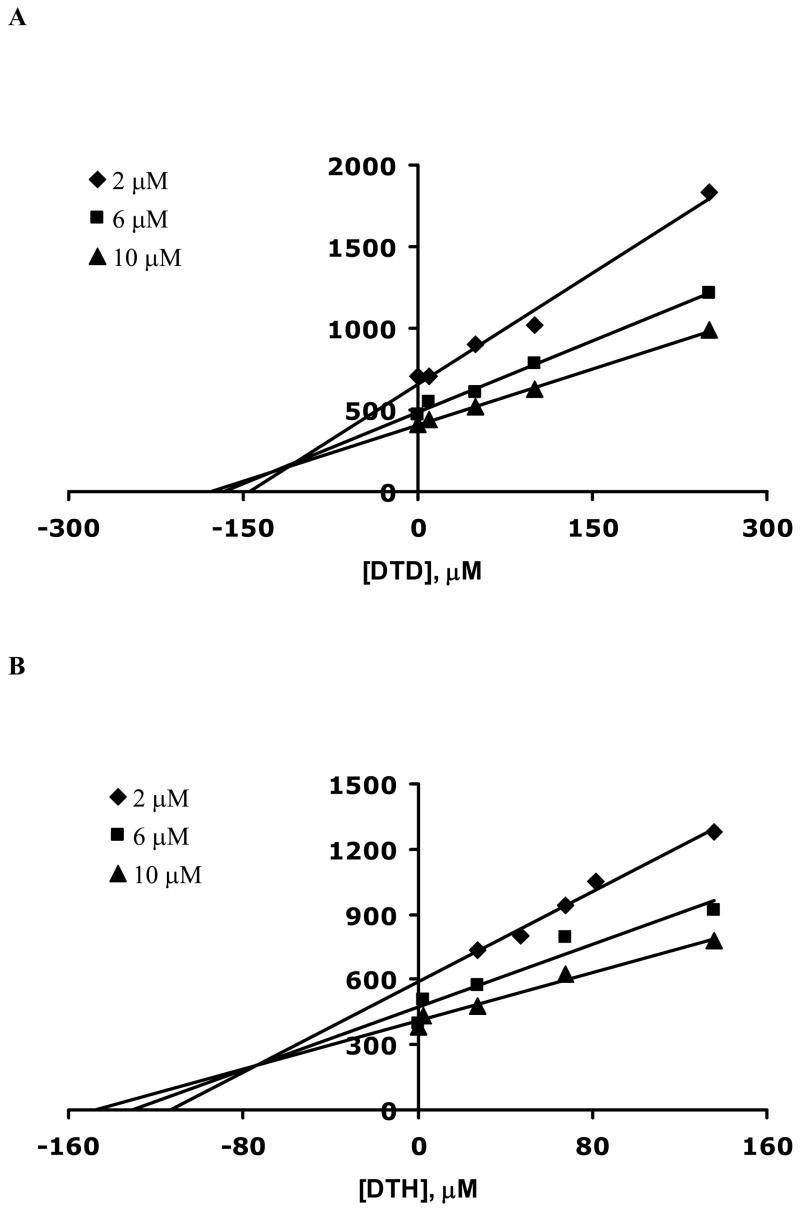
Once the IC50 values for 6 and 7 were determined, the mode of inhibition and KI values were also evaluated. These values are summarized in Table 1. The assay was performed in a similar manner and multiple Lineweaver-Burk plots were generated at several fixed inhibitor concentrations and Ch concentrations ranging from 2 to 10 μM (Figure 3). Addition of both 6 and 7 caused a change in both KM and Vmax consistent with a mixed inhibition mechanism. Since both 6 and 7 have two quaternary nitrogen atoms with neighboring hydroxyl groups in addition to the hydrophobic bridging alkyl group, it is reasonable that multiple modes of interaction are possible with the Ch binding site, resulting in mixed inhibition. KI values for 6 and 7 were evaluated by the method of Dixon26 by holding the Ch concentrations constant at several fixed inhibitor concentrations. Dixon plots were generated by plotting 1/rate of Ch uptake as a function of inhibitor concentrations as shown in Figure 4. The points of intersection of the three lines in Figures 4A and 4B yield KI values for 6 (73 ± 1 μM) and 7 (31 ± 2 μM).
Figure 3.
Double reciprocal plots for the rate of Ch uptake as a function of Ch for three different inhibitor concentrations. (A) 7 concentrations were 0 (▲), 2.7 (■), and 13.6 (◆) μM and (B) 6 concentrations were 0 (▲), 27.2 (■), and 136 (◆) μM. Each data point is the average rate of Ch uptake determined from three different synaptosome preparations analyzed in triplicate. Error bars were removed for clarity of presentation. The average error for 6 and 7 was determined to be 9 and 12%, respectively.
Figure 4.
Dixon plots for the rate of Ch uptake as a function of (A) 7 and (B) 6 concentrations for three different Ch concentrations, 2 (◆), 6 (■) and 10 (▲) μM. Each data point on the curve is the average rate of Ch uptake determined from three different synaptosome solutions, each analyzed in triplicate. Error bars were removed for clarity of presentation. The average error for 6 and 7 were calculated to be 7% and 10%, respectively.
The relative IC50 and KI values indicate that 7 was a more potent inhibitor than 6. Each molecule has a similar molecular structure except for the bridging alkyl link between the two catechol moieties. This difference in structure clearly increases the hydrophobic nature of 7 relative to 6. The increase in inhibition for 7 was similar to the results observed for hexamethonium and decamethonium13 and therefore consistent with the contribution of the hydrophobic domain playing a role in the enhancement of the inhibition of CHT in mouse synaptosomes.12 These results also suggested that 9, which also has two quaternary nitrogen atoms separated by twelve carbon atoms through two hydrophobic aromatic rings, has other constraints that dominate its improved binding to the transporter.4 An increase in distance between the two quaternary nitrogen atoms also results in an increasing affinity for inhibition of CHT.27
The relatively low binding affinity of 6 and 7 toward CHT was important as both molecules have some structural analogy with 9, but apparently not enough to approach the activity of the latter. However, both 6 and 7 possessed high levels of activity toward nAcChR at concentrations substantially lower than those necessary to inhibit CHT.9 There was also a selective metal-induced reactivity toward nAcChR that compromised receptor activity at still lower concentrations than those observed for 6 and 7 toward CHT.9 Thus, the bis-catechol molecules studied here would be expected to be predominantly effective in compromising cholinergic synapses through a post-synaptic mechanism. This was also the case in the compromise of cholinergic systems in Alzheimer’s disease. The use of 6 and/or 7 under the proper conditions using experimental animals or cholinergic cells in culture would be expected to simulate a neurotoxic response similar to that seen in the early to intermediate onset of Alzheimer’s dementia.1
Conclusions
The inhibition of rate of Ch uptake in mice synaptosomes was studied using an in vitro non-radioactive assay based on capillary electrophoresis coupled with electrochemical detection. The assay was used to evaluate the inhibitory properties of the cholinomimetic compounds 6 and 7. IC50 and KI values were determined from dose-response curves and Dixon plots, respectively. Compounds 6 and 7 both interacted with CHT via a mixed inhibition mechanism. The results also demonstrated the importance of the hydrophobic alkyl chain length between the two quaternary nitrogen atoms, which provided some enhancement in the degree of inhibition but was otherwise not consistent with a more substantial improvement in binding observed for 9 compared to 8. A further distinction was that the mode of inhibition for the cholinomimetic quaternary ammonium catechol-based molecules was either a mixed or non-competitive process rather than a competitive process as observed in the hemicholinium molecules.
Acknowledgments
Funding for this work was provided by the National Institutes of Health (R15 NS35305). Additional financial support from The University of Toledo and the Ohio Board of Regents for the Development of a microanalytical laboratory is also gratefully acknowledged. Jhindan Mukherjee was the recipient of a University of Toledo Graduate Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarter M, Parikh V. Nat Rev Neurosci. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- 2.Araujo JA, Studzinski CM, Milgram NW. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:411–422. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Francis PT, Palmer AM, Snape M, Wilcock GK. J Neurol Neurosurg Psychiatr. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterling GH, Doukas PH, Ricciardi FJ, Jr, Biedrzycka DW, O’Neill JJ. J Neurochem. 1986;46:1170–1175. doi: 10.1111/j.1471-4159.1986.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 5.Nickoloff BJ, Grimes M, Wohlfeil E, Hudson RA. Biochemistry. 1985;24:999–1007. doi: 10.1021/bi00325a029. [DOI] [PubMed] [Google Scholar]
- 6.Patel PJ, Wohfeil ER, Stahl SS, McLaughlin KA, Hudson RA. Biochem BioPhys Res Commun. 1991;175:407–413. doi: 10.1016/0006-291x(91)91579-2. [DOI] [PubMed] [Google Scholar]
- 7.Patel PJ, Messer WS, Jr, Hudson RA. J Med Chem. 1993;36:1893–1901. doi: 10.1021/jm00065a012. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Lee H, Kirchhoff JR, Manzey L, Hudson RA. Biochemistry. 1994;33:8486–8494. doi: 10.1021/bi00194a013. [DOI] [PubMed] [Google Scholar]
- 9.Gu Y, Lee H, Hudson RA. J Med Chem. 1994;37:4417–4420. doi: 10.1021/jm00051a021. [DOI] [PubMed] [Google Scholar]
- 10.Shen R, Tillekeratne LMV, Kirchhoff JR, Hudson RA. Biochem Biophys Res Commun. 1996;228:187–192. doi: 10.1006/bbrc.1996.1637. [DOI] [PubMed] [Google Scholar]
- 11.Barkhimer TV, Kirchhoff JR, Hudson RA, Messer WS., Jr Anal Biochem. 2005;339:216–222. doi: 10.1016/j.ab.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 12.Lockman PR, Allen DD. Drug Dev Ind Pharm. 2002;28:749–771. doi: 10.1081/ddc-120005622. [DOI] [PubMed] [Google Scholar]
- 13.Tamaru M, Roberts E. Brain Res. 1988;473:205–226. doi: 10.1016/0006-8993(88)90850-5. [DOI] [PubMed] [Google Scholar]
- 14.Holden JT, Rossier J, Beaujouan JC, Guyenet P, Glowinski J. Mol Pharmacol. 1975;11:19–27. [PubMed] [Google Scholar]
- 15.Chatterjee TK, Long JP, Cannon JG, Bhatnagar RK. Eur J Pharmacol. 1988;149:241–248. doi: 10.1016/0014-2999(88)90654-1. [DOI] [PubMed] [Google Scholar]
- 16.Simon JR, Mittag TW, Kuhar MJ. Biochem Pharmacol. 1975;24:1139–1142. doi: 10.1016/0006-2952(75)90208-7. [DOI] [PubMed] [Google Scholar]
- 17.Wise DD, Barkhimer TV, Brault PA, Kirchhoff JR, Messer WS, Jr, Hudson RA. J Chromatogr, B. 2002;775:49–56. doi: 10.1016/s1570-0232(02)00269-6. [DOI] [PubMed] [Google Scholar]
- 18.By decreasing the internal standard concentration to 25 μM, the mass detection limit for Ch was reduced to 100 amol.
- 19.Barkhimer TV, Kirchhoff JR, Hudson RA, Messer WS., Jr Electrophoresis. 2002;23:3699–3704. doi: 10.1002/1522-2683(200211)23:21<3699::AID-ELPS3699>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Smith AR, Kirchhoff JR, Tillekeratne LMV, Hudson RA. Anal Commun. 1999;36:371–374. [Google Scholar]
- 21.Linhares MC, Kissinger PT. Anal Chem. 1991;63:2076–2078. [Google Scholar]
- 22.Fermier AM, Gostkowski ML, Colon LA. Anal Chem. 1998;68:1661–1664. doi: 10.1021/ac9510346. [DOI] [PubMed] [Google Scholar]
- 23.Gray EG, Whittaker VP. J Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Copeland RA. Enzymes, A Practical Introduction to Structure Mechanism and Data Analysis. VCH Publishers; New York: 1996. [Google Scholar]
- 26.Dixon M, Webb EC, Thorne JCR, Tipton KF. Enzymes. 3. Academic Press; New York: 1979. [Google Scholar]
- 27.Martin K. Br J Pharmacol. 1969;36:458–469. doi: 10.1111/j.1476-5381.1969.tb08002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchi M, Bergaglia F, Pedrini A, Raiteri M. J Neurosci Res. 2000;61:533–540. doi: 10.1002/1097-4547(20000901)61:5<533::AID-JNR8>3.0.CO. [DOI] [PubMed] [Google Scholar]
- 29.Barker LA, Mittag TW. J Pharmacol Exp Ther. 1975;192:86–94. [PubMed] [Google Scholar]
- 30.Yamamura HI, Snyder SH. J Neurochem. 1973;21:1355–1374. doi: 10.1111/j.1471-4159.1973.tb06022.x. [DOI] [PubMed] [Google Scholar]