Abstract
Previous research suggests that target templates are stored visual working memory and used to guide attention during visual search. However, observers can search efficiently even if working memory is filled to capacity by a concurrent task. The idea that target templates are stored in working memory receives support primarily from studies of nonhuman primates in which the target varies from trial-to-trial, and it is possible that working memory templates are not necessary when target identity remains constant, as in most studies of visual search in humans. To test this hypothesis, we asked subjects to perform a visual search task during the delay interval of a visual working memory task. The two tasks were found to interfere with each other when the search targets changed from trial-to-trial, but not when target identity remained constant. Thus, a search template is stored in visual working memory only when the target varies from trial-to-trial. These findings suggest that the network of brain areas involved in shifting attention during visual search tasks may be able to operate essentially independently of the anatomical areas that perform visual working memory maintenance of objects, but only if the identity of the visual search target is stable across time.
Several influential theories of attention posit that attention is controlled during visual search by a target template1 that is maintained in visual working memory during search (e.g., Bundesen, 1990; Desimone & Duncan, 1995; Duncan & Humphreys, 1989). However, the strongest evidence comes from studies of monkey neurophysiology (e.g., Chelazzi, Duncan, Miller, & Desimone, 1998; Chelazzi, Miller, Duncan, & Desimone, 1993). For example, in the studies of Chelazzi and colleagues, neurons in posterior visual areas that are selective for the features of a given object exhibit an elevated baseline-firing rate when that object is currently the target of a visual search task, and this elevated firing is thought to be a neural manifestation of visual working memory. Presumably, neurons in the prefrontal cortex maintain target representations (Goldman-Rakic, 1996), and this sustained activity feeds back to neurons in posterior visual areas that perform primary perceptual analysis the target features (Fuster, 2004; Haenny & Schiller, 1988; Miller & Cohen, 2001; Miller & Desimone, 1991; Pasternak & Greenlee, 2005). In these single-unit studies, the identity of the search target was cued at the beginning of each trial and changed unpredictably from trial to trial, whereas most studies of visual search with human observers have used a constant target identity from trial to trial (e.g.,Wolfe, 1998).
The work of Goldman-Rakic and colleagues has been pivotal in describing the different working memory functions carried out in different regions of prefrontal cortex. She and her colleagues championed the idea that the dorsal and ventral streams of the posterior visual system converge on superior and inferior parts of the lateral prefrontal cortex, respectively (Wilson, Scalaidhe, & Goldman-Rakic, 1993). According to this view, the inferior portion of the lateral prefrontal cortex maintains working memory representations of objects. Because these object representations are essentially divorced from any specific spatial coordinates, they allow for flexible behavioral interactions with the other objects in our environments that vary widely across space (but see Rao, Rainer, & Miller, 1997). Visual search tasks appear to confront our cognitive apparatus with just such a situation. That is, we view an array of objects that are spread across the visual field, and we need some internal representation of what object we seek among the spatially distributed distractor objects. A natural assumption is that a target representation is maintained in visual working memory by prefrontal cortex neurons, and this representation serves as a target template with which to compare to incoming perceptual representations of objects. This is posited to allow the visual system to exhibit the flexibility necessary to find the task-relevant target in any location across the visual field (e.g., Desimone & Duncan, 1995) and to switch rapidly from searching for one kind of target to another (Vickery, King, & Jiang, 2005; Wolfe, Horowitz, Kenner, Hyle, & Vasan, 2004). However, it is possible that object representations need to be maintained in visual working memory during search only when flexibility of control is required across time and not across space.
Woodman, Vogel and Luck (2001) provided behavioral evidence from human observers that visual working memory for objects is not involved in visual search when target identity remains constant from trial to trial. Observers in this study performed a visual search task either alone or while visual working memory was filled to capacity by a concurrent change-detection task. Woodman et al. (2001) argued that, if visual search involves continuous access to working memory, then visual search should be less efficient (i.e., search slopes should be steeper) when visual working memory is filled to capacity with to-be-remembered objects by a concurrent task. However, Woodman et al. observed that search was just as efficient when performed in isolation as it was when performed while visual working memory was full of object representations. Moreover, memory performance was only slightly poorer when the memory and search tasks were performed together than when the memory task was performed alone; the small impairment observed when the two tasks were performed together was caused by the mere presentation of the search array and occurred even when subjects did not perform the search task. Thus, this study indicated that visual working memory for objects plays no significant role in attention demanding visual search2. However, the identity of the target remained constant across the entire experimental session. It is possible that the target template that guides shifts of attention can be stored in long-term memory under this condition. Long-term memory is a likely source of the representation that guides attention when target identity is stable across trials, because recordings from prefrontal attentional control areas such as the frontal eye fields clearly demonstrate that some kind of internal target object representation guides the deployment of attention to items containing target features (e.g., Bichot & Schall, 1999). The target template may be stored in visual working memory only when it changes frequently.
This hypothesis would be broadly consistent with previous research in both cognitive psychology and cognitive neuroscience. The cognitive psychological literature on automaticity in visual search indicates that the reliance on limited-capacity processing systems becomes reduced when the target remains constant over many trials (e.g., Schneider & Shiffrin, 1977; Shiffrin & Schneider, 1977). Similarly, Logan (1988) has proposed an instance theory of automaticity in which learning allows long-term memory representations to guide other cognitive operations, and Logan (1978) proposed that the visual search process itself might be a prepared reflex that could potentially become automatized. It is possible that, after many exposures to a search task with a constant target, long-term memory representations are activated by the onset of each search array and these representations guide attention to the target location. This would not be possible when the target changes frequently, and instead working memory representations would be necessary for guiding attention to the target. Indeed, Cowan (2001) suggested that the findings of Woodman et al. (2001) were due to the use of long-term memory representations to guide the search process. Such an explanation may help explain why many behavioral studies suggest a close relationship between visual working memory and visual attention (for a review see Awh & Jonides, 2001) while other studies, such as Woodman et al. (2001), do support such tight linkage.
Recent studies in cognitive neuroscience are also consistent with the notion that visual working memory representations of target object features only guide attention when the identity of the target changes frequently across time. In a lesion study, Rossi, Harris, Bichot, Desimone, and Ungerleider (2001) compared the efficiency of visual search in monkeys with and without intact prefrontal cortex. Specifically, monkeys were trained to search for a target that was cued on each trial while parametrically manipulating the frequency of target identity changes. With the prefrontal cortex removed, monkeys were severely impaired at search when the target identity changed on every trial. When the changes of target identity were less frequent, however, search performance increasingly came to resemble that observed for monkeys with an intact prefrontal cortex. This systematic decrease in search efficiency with increasing frequency of target identity change is consistent with our proposal that the object representations in visual working memory—maintained by cells in prefrontal cortex—play a minimal role in guiding attention during search when target identity remains constant across trials.
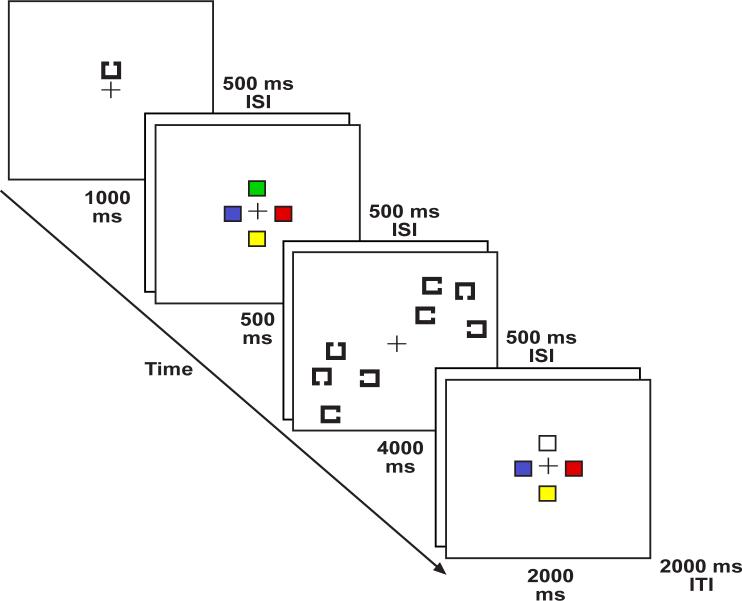
To assess the hypothesis that target templates are stored in visual working memory when the target changes frequently, but not when it is constant, we used the basic method of Woodman et al. (2001) with two groups of subjects, a constant-target group and a variable-target group. The search target was a square with a gap on one of its four sides, and the target for a given trial was cued at the beginning of the trials (see Figure 1). This target was the same on every trial for the constant-target group, whereas the position of the gap in the target varied randomly from trial-to-trial for the variable-target group. Both groups performed the search task by itself (search-alone condition), the memory task by itself (memory-alone condition), and the two tasks concurrently (search-and-memory condition).
Figure 1.
Example of the stimulus sequence on a single trial. The search array was replaced by an equivalent-duration blank interval in the memory-only condition. In the variable-target condition the cued target object changed identity every trial. In the constant-target the cue indicated the same target across all the trials.
The memory task was a change-detection task in which subjects were shown a sample array containing four colored squares, followed after a retention interval by a test array that was either identical or differed in the color of one item. At the end of the trial, subjects simply indicated whether the two arrays were identical or differed. This task is known to fill visual working memory to capacity (Woodman et al., 2001; Vogel et al. 2001). The search array was presented during the retention interval of this task, requiring subjects to search while visual working memory was already full.
If a template of the target object is stored in visual working memory, then it should be difficult to perform the search and memory tasks concurrently. Two types of interference might be expected. First, the target template may displace some of the information about the colored squares in visual working memory, leading to impaired memory performance in the search-and-memory condition compared to the memory-alone condition. Second, the target template may not always be stored properly in visual working memory, leading to a less efficient search process and steeper search slopes in the memory-and-search condition than in the search-alone condition. We predicted that one or both of these patterns of interference would be observed in the variable-target group, but not in the constant-target group.
Method
Subjects
Two separate groups of ten students from the University of Iowa with normal or corrected-to-normal visual acuity were paid to participate in this experiment.
Stimuli and Procedure
The stimuli and procedures used here were identical to those of Experiment 2 of Woodman et al. (2001) except where noted. We briefly review the experimental design and structure of the trials here. As illustrated in Figure 1, each trial began with a 1000-ms presentation of the search target for that trial, centered 1.0° above the fixation point. After a 500-ms delay, a sample array for the memory task was presented. This consisted of four colored squares, each subtending 0.45° × 0.45° and centered 0.68° above, below, to the right, or to the left of fixation. The colors of the items in the sample array were randomly selected without replacement from a set of seven highly-discriminable colors (red, green, blue, yellow, violet, black, and white -- see Vogel, Woodman, & Luck, 2001 for the color coordinates). The sample array was presented for 2000 ms, followed by a 500-ms delay, a 4000-ms search array, another 500-ms delay, a 2000-ms test array, and finally a 2000-ms blank intertrial interval. On 50% of trials the test array was identical to sample array, and on the other 50% the color of one randomly selected square was replaced by a color that was not present in the sample array. The search array was replaced by an equal-duration blank period in the memory-only condition.
Each visual search array contained 4, 8, or 12 items, and the target was present in half of the arrays. Each item was a black square subtending 0.45° × 0.45°, with a gap on the left, right, top, or bottom. In the constant-target condition, the target was chosen at random from this set of four alternatives at the beginning of the session and then remained constant. In the variable-target condition, the target varied randomly from trial to trial. The gap for each distractor was randomly selected (with replacement) from the three nontarget gap positions. To control for item density across set sizes, the search arrays consisted of clusters of 4 items, with one cluster per quadrant, and set size was manipulated by varying the number of quadrants that contained a cluster of items. Each quadrant extended 3° vertically and horizontally from the fixation point. The minimum distance between items was 0.6°, and the minimum distance from the fixation point was 1.0°.
The search-alone, memory-alone, and search-and-memory conditions were tested in separate, randomly ordered trial blocks. In the memory-alone block, subjects performed only the change-detection task; at the end of each trial, they made an unspeeded response on a game pad to indicate whether the sample and test arrays were the same (left middle finger response) or different (left index finger response). In the search-alone block, subjects made a speeded response to indicate whether the search target was absent (right middle finger response) or present (right index finger response). In the search-and-memory condition, subjects performed both tasks concurrently, making a speeded search response and then an unspeeded change-detection response on every trial. As in Woodman et al. (2001), subjects performed an articulatory suppression task in all conditions to minimize the use of verbal memory. Specifically, observers were shown four white letters or digits (“ABCD”, “1234”, “WXYZ”, or “6789”) at the beginning of each block of trials and were required to repeat these items aloud continuously during each trial.
Results
Visual Search Performance
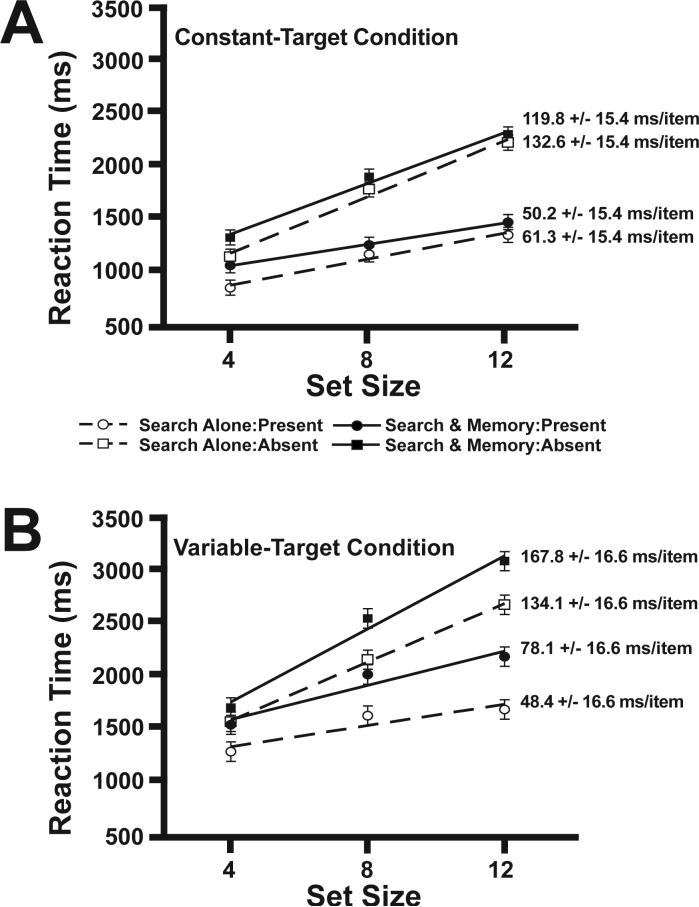
The visual search results are summarized in Figure 2. In all conditions, visual search RT increased as the number of distractors in the visual search arrays increased. For the constant-target group, the slopes of the functions relating mean RT to set size were similar when search was performed during the retention interval of the memory task or in isolation. Specifically, slopes on target-present trials were 61.3 ms/item and 50.2 ms/item in the search-alone and search-and-memory conditions, respectively, and the corresponding slopes for target-absent trials were 132.6 ms/item and 119.8 ms/item. Thus, the concurrent memory load led to no slowing of search in the constant-target group.
Figure 2.
Mean visual search RTs with linear regression shown. A) The visual search reaction times from the constant-target condition. B) The visual search RTs from the variable-target condition. Error bars represent 95% within-subjects confidence intervals for each group of subjects (Loftus & Loftus, 1988). To the right of each regression line is the mean slope and 95% within subjects confidence intervals.
In contrast, the variable-target group exhibited steeper slopes when the search task was performed during the retention interval of the working memory task than when it was performed alone. Specifically, slopes on target-present trials were 48.4 ms/item and 78.1 ms/item in the search-alone and search-and-memory conditions, respectively, and the corresponding slopes for target-absent trials were 134.1 ms/item and 167.8 ms/item.
RT slopes for the search task were analyzed using a mixed model ANOVA with a between-subjects factor of target variability (variable-target versus constant-target group) and within-subjects factors of memory load (search-alone or search-and-memory task) and target presence (present versus absent). The greater slopes observed for target-absent trials than for target-present trials led to a significant main effect of target presence, F(1, 18) = 47.75, p < .001. In addition, the steeper slopes observed when memory was loaded in the variable-target condition, but not in the constant-target condition, led to a significant interaction between target variability and memory load, F(1, 18) = 13.14, p < .002. Overall, the slopes were similar for the constant- and variable-target groups (p > .3 for the variability main effect), indicating that trial-to-trial variations in target identity do not make the search process itself more demanding.
Separate two-way ANOVAs were performed for the variable-target and constant-target groups. Both groups exhibited significantly greater slopes for target-absent trials than for target-present trials (ps < .01). The effect of memory load on the search slope led to a significant effect of memory load in the variable-target group, F(1,9) = 19.32, p < .01, but memory load did not have a significant effect in the constant-target group, p > .25.
Visual search accuracy is summarized in Table 1 and was analyzed just like the slope data, except that an additional factor of set size was included. Mean accuracy was lower in the variable-target group than in the constant-target group, F(1,18) = 16.29, p < .001. Accuracy was lower for target-present trials than for target-absent trials, F(1,18) = 15.79, p < .001, and decreased significantly as set size increased, F(2,36) = 12.40, p < .0001, especially on target-present trials, F(2,36) = 4.78, p < .05. Search accuracy did not differ significantly between the search-alone and search-and-memory conditions, nor were there any significant interactions involving the memory load factor. There were no signs of speed-accuracy tradeoffs.3
Table 1.
Accuracy of visual search responses (in percent correct +/− 95% within-subjects confidence interval) in the constant-target and variable-target conditions.
| Condition | Set Size | Search Alone | Search and Memory | |
|---|---|---|---|---|
| Constant Target | 4 | Present | 98.33 +/− 1.87 | 96.27 +/− 1.87 |
| Absent | 100 +/− 1.87 | 96.79 +/− 1.87 | ||
| 8 | Present | 93.78 +/− 1.87 | 97.13 +/− 1.87 | |
| Absent | 99.17 +/− 1.87 | 97.92 +/− 1.87 | ||
| 12 | Present | 92.53 +/− 1.87 | 90.16 +/− 1.87 | |
| Absent | 99.58 +/− 1.87 | 97.08 +/− 1.87 | ||
| Variable Target | 4 | Present | 92.18 +/− 3.12 | 93.37 +/− 3.12 |
| Absent | 96.63 +/− 3.12 | 93.02 +/− 3.12 | ||
| 8 | Present | 89.67 +/− 3.12 | 89.39 +/− 3.12 | |
| Absent | 97.08 +/− 3.12 | 96.14 +/− 3.12 | ||
| 12 | Present | 82.77 +/− 3.12 | 88.86 +/− 3.12 | |
| Absent | 92.98 +/− 3.12 | 91.33 +/− 3.12 |
Memory Performance
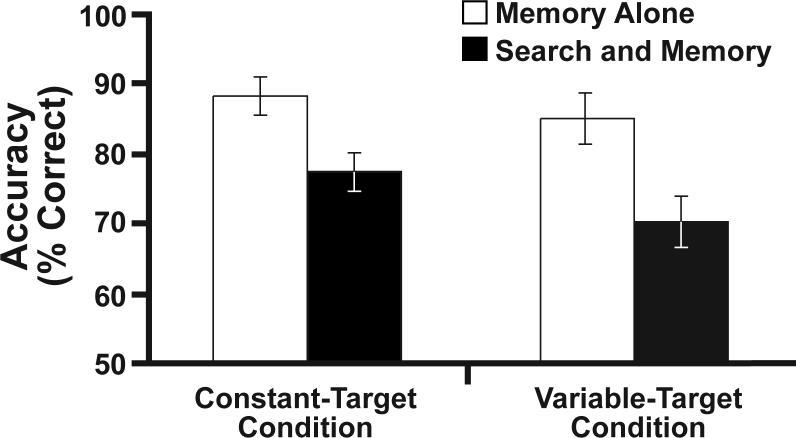
Accuracy (percent correct) in the memory task is shown in Figure 3. These data were analyzed using a mixed-model ANOVA with a between-subjects factor of target variability and a within-subjects factor of search load (memory-alone versus search-and-memory, collapsed across search set sizes). In both the constant-target and variable-target groups, memory performance was more accurate in the memory-alone condition than in the memory-and-search condition, leading to a significant main effect of search load, F(1, 18) = 53.01, p < .001. In addition, memory accuracy was somewhat lower in the variable-target condition than in the constant-target condition, leading to an effect of target variability that approached significance, F(1, 18) = 3.29, p < .10. The effect of search load on memory accuracy was approximately 3% in the constant-target condition and approximately 7% in the variable-target condition, but this interaction did not approach significance.
Figure 3.
Mean memory-task accuracy from the participants in the constant-target and variable-target conditions collapsed across target presence and set size.
Discussion
Observers in this study either searched for the same target on each trial or searched for a target that changed randomly from trial to trial. When the target remained constant over trials, we observed minimal interference between the search task and the concurrent visual working memory task. In contrast, when the identity of the search target changed from trial-to-trial, the processing of the visual search array was significantly impaired by the concurrent memory load.
These results are consistent with two interconnected hypotheses: a) a target template is stored in visual working memory when the target changes frequently, and b) a target template is not stored in visual working memory when the target remains constant. The data provide strong support for the second of these hypotheses, because filling visual working memory had no effect on search performance when the target remained constant. The data also support the first of these hypotheses, because the observed interference in the variable-target group is exactly what would be expected if a template were stored in visual working memory when the target changes from trial to trial. However, the support for this first hypothesis is somewhat indirect, because behavioral methods do not provide a direct means of observing the storage of the template in working memory.
More direct evidence for the storage of target templates in visual working memory under variable-target conditions has been observed in single-unit studies in which the target was specified by a sample stimulus at the beginning of each trial (Chelazzi et al., 1998; Chelazzi et al., 1993). In these studies, inferotemporal neurons that are selective for a given target stimulus become active when that item is presented as the sample and remain active over a brief delay period until the search array is presented. This delay activity is thought to be a neural signature of visual working memory representations, and this pattern of results suggests that a template of the target is stored in visual working memory. The variable-target condition of the present study also used a target cueing procedure that signaled the target with a sample stimulus at the beginning of each trial, and we found that search was significantly slowed by a concurrent visual working memory task, consistent with the use of working memory to perform the search task. Thus, these results provide converging evidence for the hypothesis that visual working memory is sometimes used to store a template of a visual search target.
In contrast, search was not slowed by the concurrent working memory task when the target remained constant from trial to trial. Memory performance for this group was somewhat less accurate when the search task was performed during the delay period of the memory task compared to when the memory task was performed alone, but this effect was quite small (3%). Moreover, Woodman et al. (2001) showed that this drop in accuracy most likely reflects some sort of nonspecific interference caused by the mere appearance of the search array, because the appearance of a search array causes a reduction in memory performance even if subjects do not perform any task with the search array. Thus, the present results indicate that it is not necessary to store a search template in visual working memory when the target is consistent from trial to trial.
Before accepting this conclusion, it is necessary to consider an alternative explanation for the constant-target results, namely that a target template is stored in working memory but requires very little in the way of working memory resources when it is repeated throughout a trial block. This is probably an unfalsifiable explanation, because it is possible to postulate that the amount of resources required by the target template is so small that the template is undetectable. However, all existing evidence indicates that highly familiar stimuli require no fewer resources in visual working memory than unfamiliar stimuli (Olson & Jiang, 2004; Pashler, 1988). Moreover, even the least resource-demanding visual stimuli appear to require 20−25% of the available visual working memory resources (Alvarez & Cavanagh, 2004). Thus, it is very unlikely that the lack of significant interference in the constant-target group reflected the use of minimal resources to store the target template in visual working memory.
What, then, is the nature of the memory representation that guides visual search when the target remains constant across trials? There are three main possibilities. First, subjects may store a template of the target in long-term memory and use this template to guide attention. This would be similar to Logan's (1988) proposal that automaticity occurs when the presentation of a stimulus leads to the rapid retrieval of a stimulus-response association from long-term memory. In the present case, the presentation of a search array would lead to the rapid retrieval of a set of search parameters that would in turn guide attention to the target (i.e., search would operate as a prepared reflex, as suggested by Logan, 1978). A second possibility is that the consistent repetition of the target may lead to priming within the visual system that effectively guides attention to the target without an explicit template of the target. Previous studies have shown that substantial priming of this nature occurs during visual search (e.g., Found & Mueller, 1996; Kristjánsson, Wang, & Nakayama, 2002), making this explanation plausible. A third possibility is that a representation of the target is stored in an amodal, prefrontal working memory subsystem that stores abstract task descriptions rather than visual representations (see Logan, 2004).
One piece of evidence regarding these possibilities comes from monkey neurophysiology studies in which the identity of the target remained constant for 10−30 trials (Chelazzi et al., 1998; Chelazzi et al., 1993). Under these conditions, delay activity was observed in inferotemporal neurons but was already present at the beginning of each trial. It is possible that this delay activity reflected the maintenance of a template in visual working memory, perhaps because the target's identity still switched relatively frequently (especially given the more limited cognitive abilities of the monkey subjects compared to the human subjects studied here). However, the presence of target-related pretrial activity also accords well with a priming account, in which the frequent occurrence of a given target on previous trials leads to persisting neural activity that guides attention to that target on future trials.
Other supporting evidence comes from the previously discussed study of monkeys with prefrontal cortex lesions (Rossi et al., 2001), which should impair the implementation of working memory control processes. As discussed above, monkeys in this study performed a visual search task in which the identity of the target changed frequently (as often as every trial) or infrequently (after an entire day of testing). When the identity of the search target remained constant, search efficiency was not influenced by the prefrontal cortex lesions. In contrast, when target identity changed frequently, the lesions led to a severe impairment in search efficiency. These findings are consistent with the hypothesis that a memory representation other than one actively maintained by prefrontal cortex neurons is used to control the search process when the target remains constant across trials. In contrast, more complex visual working memory mechanisms—which rely on prefrontal cortex—are necessary when the target changes frequently. Thus, it is unlikely that visual or amodal working memory representations stored in prefrontal cortex are used to control attention when the target remains constant from trial to trial.
Additional evidence from single-unit recordings in an area of the network that controls deployments of attention during visual search shows that such neurons are also sensitive to the frequency of changes of search target identity. Specifically, a large body of evidence indicates that FEF, the posterior parietal cortex, and subcortical regions such as the superior colliculus, form a network that enables both shifts of covert attention and shifts of gaze during visual search tasks (e.g., Ipata, Gee, Goldberg, & Bisley, 2006; Krauzlis & Dill, 2002; McPeek & Keller, 2002; Thompson, Hanes, Bichot, & Schall, 1996). Interestingly, different cell types within these areas appear to perform different functions (for a review see Schall & Thompson, 1999). For example, in the FEF, the neurons in one subset exhibit visual responses and appear to participate in the visual selection of task-relevant targets during visual search tasks. These visual cells take longer to unambiguously signal the spatial location of the target item in the search array when monkeys view search arrays that are more attention demanding, based on theories of visual attention (Duncan & Humphreys, 1989; T. Sato, Murthy, Thompson, & Schall, 2001). A partially overlapping subset of cells in FEF control eye movements and the direction of gaze, and the activity of these cells can be used to determine when an eye-movement response will be made (Hanes & Schall, 1996).
By focusing on the activity of the visual activity in this network, studies have found that the efficiency of attentional deployment during search is significantly influenced by the recency of a change in target identity. Specifically, in single-unit recording studies of FEF neurons, Bichot, Schall, and colleagues (Bichot & Schall, 1999; Bichot, Schall, & Thompson, 1996) have shown that the efficiency of visual selection of task-relevant targets is modulated by the frequency with which the identity of the target changes. When the target of the visual search task has just changed, FEF neurons take longer to select the target object. This occurs whether the target is defined by the presence of a simple feature attribute (Bichot & Schall, 2002) or is a complex multifeature object (Bichot & Schall, 1999). Finally, Sato et al., (2003) showed that even when a distractor was in the receptive field that the firing rate of FEF neurons was higher when item was more similar to the searched for target. This indicates that the neural representation of the distractors that are more similar to the target is greater than when distractors that are less similar to the target are in the cell's receptive field. These findings could be accounted for either by low-level priming of a target's features or by the increased involvement of long-term memory representations guiding the visual attention network, as the cognitive models of automaticity discussed above suggest. As the visual attention network repetitively selects targets defined by the same features, the degree of controlled processing diminishes. The dominant theory of automaticity proposes that the accrual of long-term memory representations of encounters with specific search arrays leads to automatic retrieval of the appropriate task set from the long-term memory store (Logan, 1988).
Currently, we favor the proposal that, as long-term memory becomes dominant in guiding attention to a constant-identity target object, the importance of visual working memory representations of target objects in prefrontal cortex diminishes. Not only is this explanation consistent with single-unit studies and studies of monkeys with prefrontal lesions, it is also grounded in theories of cognitive processing that account for a large body of data from other tasks (Logan, 1988). It should also be emphasized that repetition priming and automaticity have common underlying assumptions, both based on the strength of long-term memory representations (Logan, 1990), so the priming-based and automaticity-based accounts of the present findings may be functionally equivalent, but described in different terms by different researchers. For example, it may seem as though these hypotheses could be distinguished by parametric manipulations of the frequency of the target change. An automaticity-based account would predict that the interference caused by a change of target identity should decrease and asymptote within 20−30 trials, fitting a power function (Logan, 1990). However, such results are also generally compatible with a priming account (Kirsner & Speelman). This indicates that new insights and the use of neuroscience techniques in further research will be necessary to distinguish between these alternatives which are close theoretical cousins.
Finally, we consider an explanation for the present results which appeals to the idea that different levels of executive control were required in the constant- and variable-target conditions and that it is the limited –capacity of these executive mechanisms which lead to the observed effects. Such an interpretation is a more general description of the particular hypothesis we tested. That is, in the variable-target condition executive control over search performance is implement by maintaining a target template in visual working memory, as proposed by the biased competition theory (Desimone & Duncan, 1995). Maintaining such a representation helps to suppress attentional deployment to targets from previous trials, which are now distractors, and to enable accurate performance of the search task in the face of concurrently maintenance of the other information in working memory. Theories of executive control have already identified the need to account for dual-task performance (Logan & Gordon, 2001) and the present findings serve to further constrain such models by showing specific task requirements which influence the degree to which concurrent tasks interfere with one another.
Acknowledgements
We would like to thank Gordon Logan, Andrew Rossi, Shaun Vecera, and Edward Vogel for valuable discussions regarding this work and the anonymous reviewers for their comments. This study was made possible by individual National Research Service Awards to G.F.W. (F31 MH12995 and F32 NEI015043), grants from the National Institute of Mental Health (R01 MH63001 and R01 MH65034) to S.J.L., and grants from the National Institute of Health (RO1-EY08890, P30-EY08126, P30-HD015052), and Robin and Richard Patton through the E. Bronson Ingram Chair to J.D.S. This work also appeared in a portion of the unpublished dissertation of G.F.W.
Footnotes
As is common in this area of research, we use the term target template to refer to some kind of representation of the target, but with no assumptions about the nature of this representation. In particular, we do not assume that it is an array format, picture-like representation.
Woodman and Luck (2004) found significant interference when a spatial working memory task was used instead of an object working memory task, indicating that spatial working memory plays an important role in search (see also Oh & Kim, 2004). In the present study, however, we focus solely on object working memory, which would be the likely system for storing target templates in visual search tasks.
The RTs shown in Figure 2 are from all trials in which the search target discrimination was correct. In an additional analysis, trials were further divided based on whether or not the concurrent memory task was accurately performed in the search and memory conditions. Neither mean RTs nor search slopes showed a significant effects of memory task response accuracy (ps > .30). However, because the experiment was not designed to examine this comparison only 20−30% of the total number of search trials (i.e., approximately 10−14 trials per subject) contributed to the mean search performance when the memory task response was incorrect. Thus, our sensitivity to potential effects of forgetting the memory load was low and we cannot be confident that search performance might also exhibit an effect of memory task accuracy if a much longer experimental design were possible. An interesting hypothesis that such an experiment could test is whether forgetting the memory load improves search performance on that trial, particularly in the variable-target condition.
References
- Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological Science. 2004;15:106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends in Cognitive Sciences. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nature Neuroscience. 1999;2(6):549–554. doi: 10.1038/9205. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Priming in macaque frontal cortex during popout visual search: Feature-based facilitation and location-based inhibition of return. Journal of Neuroscience. 2002;22:4675–4685. doi: 10.1523/JNEUROSCI.22-11-04675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Schall JD, Thompson KG. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature. 1996;381(6584):697–699. doi: 10.1038/381697a0. [DOI] [PubMed] [Google Scholar]
- Bundesen C. A theory of visual attention. Psychological Review. 1990;97:523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. Journal of Neurophysiology. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Miller EK, Duncan J, Desimone R. A neural basis for visual search in inferior temporal cortex. Nature. 1993;363:345–347. doi: 10.1038/363345a0. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Duncan J, Humphreys GW. Visual search and stimulus similarity. Psychological Review. 1989;96(3):433–458. doi: 10.1037/0033-295x.96.3.433. [DOI] [PubMed] [Google Scholar]
- Found A, Mueller HJ. Searching for unknown feature targets on more than one dimension: Investigating a ”dimension-weighting” account. Perception & Psychophysics. 1996;58:88–101. doi: 10.3758/bf03205479. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Upper processing stages of the perception action cycle. Trends in Cognitive Sciences. 2004;8:143–145. doi: 10.1016/j.tics.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenny PE, Schiller PH. State dependent activity in monkey visual cortex. I. Single cell activity in V1 and V4 on visual tasks. Experimental Brain Research. 1988;69:225–244. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274(5286):427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. Journal of Neuroscience. 2006;26:3656–3661. doi: 10.1523/JNEUROSCI.5074-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis R, Dill N. Neural correlates of target choice for pursuit and saccades in primate superior colliculus. Neuron. 2002;35:355–363. doi: 10.1016/s0896-6273(02)00756-0. [DOI] [PubMed] [Google Scholar]
- Kristjánsson Á, Wang D, Nakayama K. The role of priming in conjunctive visual search. Cognition. 2002;85:37–52. doi: 10.1016/s0010-0277(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Loftus EF. Essence of Statistics. 2nd ed. Random House; New York: 1988. [Google Scholar]
- Logan GD. Attention in character classification tasks: Evidence for the automaticity of component stages. Journal of Experimental Psychology: General. 1978;107:32–63. [Google Scholar]
- Logan GD. Toward an instance theory of automatization. Psychological Review. 1988;95:492–527. [Google Scholar]
- Logan GD. Repetition priming and automaticity: Common underlying assumptions. Cognitive Psychology. 1990;22:1–35. [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychological Review. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. Journal of Neurophysiology. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller EK, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991;254:1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Oh SH, Kim MS. The role of spatial working memory in visual search efficiency. Psychonomic Bulletin and Review. 2004;11(2):275–281. doi: 10.3758/bf03196570. [DOI] [PubMed] [Google Scholar]
- Olson IR, Jiang Y. Visual short-term memory is not improved by training. Memory & Cognition. 2004;32:1326–1332. doi: 10.3758/bf03206323. [DOI] [PubMed] [Google Scholar]
- Pashler H. Familiarity and visual change detection. Perception & Psychophysics. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nature Reviews Neuroscience. 2005;6:97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK. Integration of what and where in the primate prefrontal cortex. Science. 1997;276:821–824. doi: 10.1126/science.276.5313.821. [DOI] [PubMed] [Google Scholar]
- Rossi AF, Harris BJ, Bichot NP, Desimone R, Ungerleider LG. Deficits in target selection in monkeys with prefrontal lesions. Society for Neuroscience Abstracts. 2001:574–579. [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron. 2001;30:583–591. doi: 10.1016/s0896-6273(01)00304-x. [DOI] [PubMed] [Google Scholar]
- Sato TR, Watanabe K, Thompson KG, Schall JD. Effect of target-distractor similarity on FEF visual selection in the absence of the target. Experimental Brain Research. 2003;151(3):356–363. doi: 10.1007/s00221-003-1461-1. [DOI] [PubMed] [Google Scholar]
- Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Annual Review of Neuroscience. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing. I: Detection, search and attention. Psychology Review. 1977;84:1–66. [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing. II: Perceptual learning, automatic attending, and a general theory. Psychological Review. 1977;84:127–190. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. Journal of Neurophysiology. 1996;76(6):4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Vickery TJ, King L-W, Jiang Y. Setting up the target template in visual search. Journal of Vision. 2005;5:81–92. doi: 10.1167/5.1.8. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993;260(5116):1955–1958. doi: 10.1126/science.8316836. see comments. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Visual search. In: Pashler H, editor. Attention. Psychology Press/Erlbaum (Uk) Taylor & Francis; Hove, England UK: 1998. pp. 13–73. [Google Scholar]
- Wolfe JM, Horowitz TS, Kenner N, Hyle M, Vasan N. How fast can you change your mind? The speed of top-down guidance in visual search. Vision Research. 2004;44:1411–1426. doi: 10.1016/j.visres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Visual search is slowed when visuospatial working memory is occupied. Psychonomic Bulletin & Review. 2004;11:269–274. doi: 10.3758/bf03196569. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Vogel EK, Luck SJ. Visual search remains efficient when visual working memory is full. Psychological Science. 2001;12:219–224. doi: 10.1111/1467-9280.00339. [DOI] [PubMed] [Google Scholar]