Abstract
Purpose
To evaluate the effectiveness of a liver-targeted dextran prodrug (DMP) of methylprednisolone (MP) in cold preservation-warm reperfusion injury associated with liver transplantation.
Methods
The effects of donor pretreatment with single 5-mg/kg doses of MP or DMP on ischemia-reperfusion damage to the liver were studied after 8 or 24 h of cold preservation in both isolated perfused rat liver (IPRL) and syngeneic orthotopic rat liver transplantation (OLT) models.
Results
In IPRL studies, donor pretreatment with DMP, and to a lesser degree MP, significantly improved the uptake of hyaluronic acid (HA), a marker of endothelial cell function, following 8 h of cold preservation. However, neither pretreatment was protective after 24 h of preservation. In the OLT model using 24 h-preserved livers, the seven-day survival of untreated grafts was 50%. DMP pretreatment of donors significantly improved graft survival to 100%, whereas MP pretreatment was ineffective. Additionally, only DMP significantly increased the blood glucose concentrations and decreased the plasma concentrations of tumor necrosis factor-α after OLT. Other measured markers of liver injury were not affected by either pretreatment.
Conclusions
Selective delivery of methylprednisolone to the liver as a donor pretreatment strategy improves 24-h preserved graft survival in the OLT model.
Keywords: macromolecular prodrug, ischemia-reperfusion injury, isolated perfused rat liver, hepatic disposition, rat liver transplantation
INTRODUCTION
Despite improvements in organ preservation solutions, ischemia-reperfusion (IR) damage during the preservation and implantation of the liver is still a major problem in liver transplantation (1). Although reducing the metabolic state of the liver, the cold ischemic period, which happens after the explantation of the liver from the donor, by itself causes damage to the liver (1, 2). This damage is mostly due to lack of oxygen that alters energy metabolism, consequently resulting in a decrease in adenosine triphosphate concentrations, an increase in cellular edema, and accumulation of intracellular sodium (3). Ironically, provision of oxygen by reperfusion of the liver after implantation in the recipient causes production of reactive oxygen species and a number of proinflammatory cytokines, further damaging the organ (1). The extent of IR damage is proportional to the ischemic period, and prolonged preservation and subsequent reperfusion causes hepatocellular injury, which may result in primary graft nonfunction, requiring retransplantation (2). Given the multi-factorial etiology of IR damage, therapeutic regimens aimed at one or more levels are required for its successful prevention and/or treatment (1). However, no such treatment is currently available (1).
We have previously proposed the use of a liver-targeted dextran prodrug of methylprednisolone (MP) as a donor pretreatment strategy aimed at prevention and/or attenuation of cold IR injury (4, 5). This dextran prodrug of MP (DMP) was shown to selectively accumulate and release the parent drug MP in the liver and spleen following systemic administration (6). In addition, pretreatment of donors with DMP, compared with equimolar doses of MP, resulted in superior attenuation of Kupffer cell (KC) activation (4) and improvement in hepatocyte function (5) in an ex vivo isolated perfused rat liver (IPRL) model. In addition to KCs and hepatocytes, sinusoidal endothelial cells (ECs) of the liver are also known to play a critical role in development of cold IR injury (2). Therefore, the first aim of this study was to assess the effects of MP and DMP on the function of ECs in an IPRL model of cold ischemia-warm reperfusion injury. Additionally, IPRL, although a suitable model for relatively short-term mechanistic studies, cannot replace in vivo studies, which can determine both the short- and long-term effects of hepatic IR on the overall health of the animal. Therefore, the second aim of the study was to investigate the effects of pretreatment of donors with DMP or its parent drug MP on the preservation-induced injury in an in vivo orthotopic rat liver transplantation (OLT) model. Based on therapeutic potential of DMP, demonstrated in our previous ex vivo experiments, we hypothesized that DMP pretreatment would decrease hepatocellular damage and improve graft survival in animals transplanted with livers subjected to cold preservation damage.
MATERIALS AND METHODS
Chemicals
Dextran, with an average molecular weight of 73 kDa and a polydispersity of <2, hyaluronic acid (HA) from rooster comb, and Purpald (4-amino-3-hydrazino-5-mercapto-1,2,4-triazole) were obtained from Sigma Chemical Co. (St. Louis, MO). Belzer's University of Wisconsin (UW) solution (Viaspan) was obtained from Dupont Pharma (Wilmington, DE). 6α-Methylprednisolone 21-hemisuccinate sodium was obtained from Steraloids, Inc. (Newport, RI). An enzyme linked binding protein assay kit for measurement of HA was obtained from Corgenix, Inc (Westminster, CO). Enzyme-linked immunosorbent assay (ELISA) kit for measurement of tumor necrosis factor (TNF)-α was purchased from Biosource International, Inc. (Camarillo, CA). Kits for determination of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were purchased from Teco Diagnostics (Anaheim, CA). Automated blood glucose measurement kit (Onetouch Ultra®) was obtained from Lifescan, Inc (Milpitas, CA).
Dextran–methylprednisolone succinate (DMP) was synthesized, purified, and characterized as described before (7). The MP and MPS impurities in the conjugate powder were less than 0.1% (w/w), and the degree of substitution of the powder was 8 mg of MP per 100 mg of powder.
The MP (as its sodium succinate salt) and DMP dosing solutions were prepared in saline immediately before drug administration.
Animals
A total of 115 male Sprague-Dawley rats were used in this study. Animals were purchased from a commercial source and housed in a humidity- and temperature-controlled room under a 12 h-light/dark cycle. Rats were provided with food and water ad libitum throughout the period of the study. All the procedures involving animals in this study were consistent with the “Principles of Laboratory Animal Care” (NIH publication #85−23, revised 1985) and approved by the Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee.
Effect of MP or DMP Pretreatment on Endothelial Cell Function in an IPRL Model
Twenty five Sprague-Dawley rats weighing 225−250 g were used as liver donors for these studies and divided into 7 groups (n=3−4/group). Two groups were used for testing the effect of MP following 8 (MP-8) or 24 (MP-24) h of cold preservation. Similarly, two groups were used to assess the effect of DMP following 8 (DMP-8) or 24 (DMP-24) h of preservation. Corresponding untreated groups (Control-8 and Control-24) were included as references. Additionally, a second control group with no cold preservation (Unpreserved) was included in this study to determine the disposition of HA in fresh livers. Rats were given a single 5-mg/kg (MP equivalent) dose of MP or DMP or an equal volume of saline (Control) via their tail vein. Two hours following drug or saline administration, livers were perfused and stored in UW solution for 8 or 24 h at 4°C. The techniques used for isolation, cannulation and harvest of livers were reported by us before (4, 8). Following 8 or 24 h of cold preservation, the livers were washed with 20 ml of lactated Ringer's solution to remove the UW solution. Then, the livers were reperfused in a water-jacketed, all-glass perfusion system (Radnoti Glass Technology Inc., Monrovia, CA) kept at 37°C. The perfusate was Krebs-Henseleit bicarbonate buffer (pH 7.4) fortified with 1.2 g/l glucose and 4.75 mg/l of sodium taurocholate, which was oxygenated with a 95:5 oxygen:carbon dioxide mixture. The perfusate flow rate was maintained at 30 ml/min (3−4 ml/min/g of liver weight). Immediately following reperfusion, the livers were allowed to stabilize for 10 min before start of the experiment. Subsequently, HA was added to the perfusate to attain an initial concentration of 50−60 ng/ml, and the perfusion was continued in a recirculating manner (reservoir volume = 150 ml) for 70 min. Samples (∼ 1 ml) were collected periodically from the perfusate reservoir and stored at −80°C until analysis. Bile was collected in preweighed micro-centrifuge tubes.
Effect of MP or DMP on Cold Preservation-Reperfusion Injury following OLT
Ninety rats resulting in 45 transplanted animals were used in these studies. Sprague-Dawley rats were used as both donors (240−280 g) and recipients (280−320 g) for the transplantation procedure. This syngeneic model, which is devoid of immunologic rejection, has been used before by other investigators to study IR damage (9, 10). Donor rats were administered a single intravenous 5-mg/kg dose of MP or DMP via the penile vein 2−3 h before liver isolation. Control rats received an equal volume of saline. A non-arterialized model of liver transplantation was performed by modification of Kamada's technique (11), as reported by us recently (12). Briefly, after removal of the livers from the donors, grafts were perfused with 10 ml of cold (4°C) UW solution and cuffs were mounted on the portal vein, infra-hepatic vena cava, and supra-hepatic vena cava. Liver grafts were stored in 40 ml of UW solution maintained at 4°C for 24 h. Following the hepatectomy of the recipient liver, the graft was placed in an orthotopic position and anastomoses of all the three veins were completed by the cuff technique. Given that the post-operative survival following OLT of preserved livers is largely dependent on the portal venous clamping time (13), the anhepatic period was fixed to 19 min in all the experiments.
Twenty four transplanted rats were used for the graft survival study, resulting in 8 transplants in each of the control, MP, and DMP groups. Animals surviving greater than 7 days were considered as long-term survivors. Additional 21 transplanted animals were used to monitor biochemical parameters. In these studies, transplanted rats receiving MP-, DMP-, or vehicle-treated livers were euthanized at either 1 or 6 h following OLT (n=3−4 transplants/group/time point). Blood was collected and centrifuged to separate the plasma and stored at −80 °C until further analysis.
Sample Analysis
Blood glucose was measured following completion of transplantation procedure with an automated analyzer. Plasma concentrations of TNF-α, HA, ALT, and AST were estimated spectrophotometrically using commercially available kits, according to the instructions of the manufacturers.
Data Analysis
For the isolated perfused rat liver studies, the area under the perfusate HA concentration-time curve (AUC) was calculated using trapezoidal rule (0−70 min), with extrapolation to infinity using the terminal first-order rate constant (k). The terminal half life (t1/2), hepatic clearance (Clh), and extraction ratio (E) of HA were calculated using the following equations:
| (1) |
| (2) |
| (3) |
where Dose is the total dose of hyaluronic acid in the recirculating system and Q is the perfusate flow rate.
Statistical Analysis
The effects of drug pretreatments (Control, MP, or DMP) on the kinetics of HA following isolated liver perfusion and on the blood glucose concentrations immediately following OLT were assessed using a one-way ANOVA with a Fisher's post-hoc test. The statistical evaluation of differences in the graft survival was performed using the log rank test applied to Kaplan-Meier plots. The effects of drug treatment and reperfusion time on the plasma concentrations of TNF-α, HA, ALT, and AST were evaluated by a two-way ANOVA with Fisher's post hoc analysis. All tests were performed at a significance level (α) of 0.05. Data are presented as mean ± S.D.
RESULTS
Effect of MP or DMP Pretreatment on Endothelial Cell Function in an IPRL Model
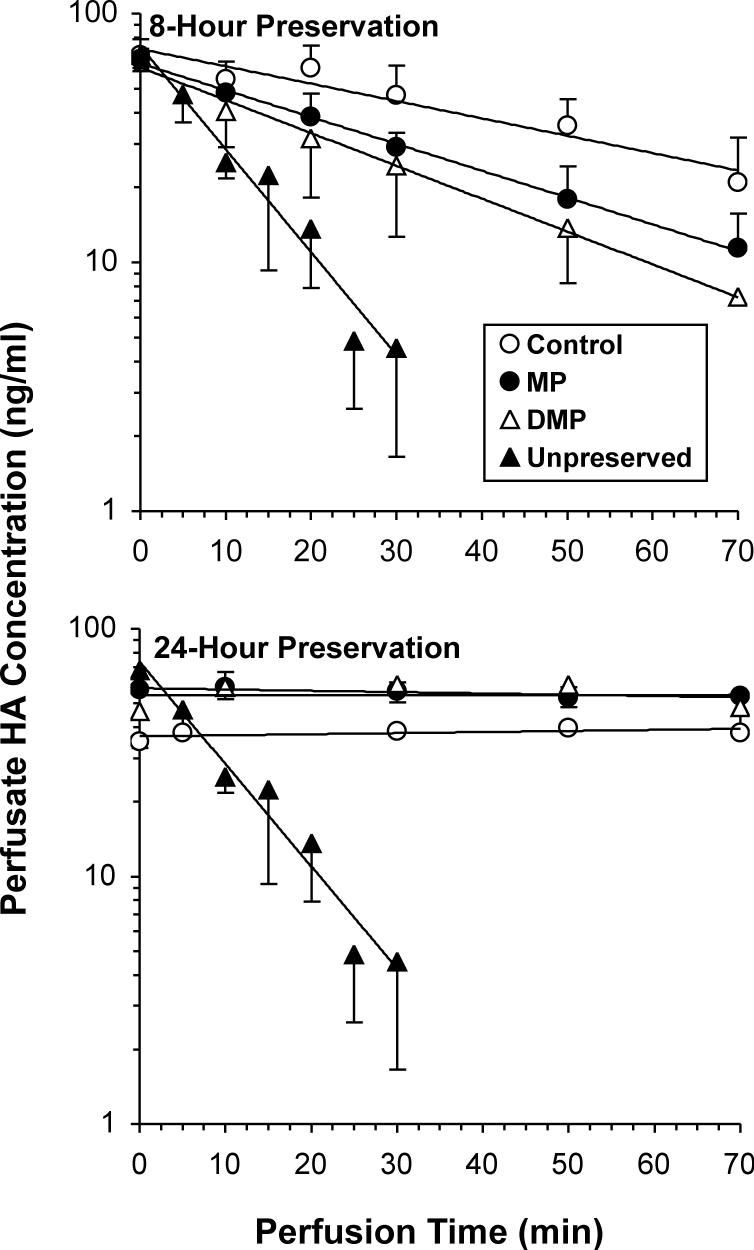
The plots of perfusate concentrations of HA against perfusion time in the control and treated groups are shown Fig. 1. Hyaluronic acid concentrations in the perfusate followed a mono-exponential decline in the Unpreserved and the 8-h preserved groups (Fig. 1, top). HA was rapidly taken up by Unpreserved livers, and its concentrations could not be measured after 30 min of perfusion. Compared with Unpreserved livers, preservation of livers for 8 h resulted in a slower decline in HA concentrations, indicating decreased function/viability of the ECs (Fig. 1, top panel). Both MP (MP-8) and DMP (DMP-8) pretreatment resulted in faster declines in the concentrations of HA in the perfusate, compared with vehicle pretreatment (Control-8) (Fig. 1, top panel). In contrast to the 8-h preservation groups (Fig. 1, top), untreated livers preserved for 24 h (Control-24) showed relatively constant perfusate concentrations of HA during 70 min of reperfusion, indicating an almost complete lack of function/viability of ECs (Fig. 1, bottom panel). Pretreatment of donors with MP or DMP after 24 h of preservation did not cause any improvements in the liver uptake of HA (Fig. 1, bottom).
Figure 1.
The perfusate concentration-time courses of hyaluronic acid (HA) in isolated livers subjected to 8 (top panel) or 24 h (bottom panel) of cold ischemia followed by 70 min of warm reperfusion. Similar profiles in Unpreserved controls are provided for reference. Rats were pretreated intravenously, 2 h prior to liver harvest, with a single 5-mg/kg dose (MP equivalent) of MP or DMP or with saline (Control) (n=3−4/group). Symbols and bars represent the average and standard deviation values, respectively.
The kinetic parameters of HA in the Unpreserved and 8-h preservation groups are presented in Table I. Cold preservation of livers from untreated rats for 8 h caused a significant (p<0.05, ANOVA followed by Fisher's post-hoc) decrease (∼80%) in the E and Clh of HA, associated with a substantial increase in the marker half life, when compared with Unpreserved livers (Table I). Although pretreatment of donors with MP caused a significant decrease (36%) in the half life of HA, the MP-induced increase in the E and Clh of HA did not reach statistical significance (Table I). In contrast, pretreatment of donors with DMP (DMP-8) significantly increased (by ∼ 140%) both E and Clh of HA, resulting in a 60% decrease in its half life, compared with untreated livers (Control-8) (Table I).
Table I.
Kinetic Parameters (Mean ± SD) of Hyaluronic Acid in Isolated Perfused Rat Livers Pretreated with Vehicle (Control), Methylprednisolone (MP), or Dextran-Methylprednisolone (DMP) after 0 (Unpreserved) or 8 h of Cold Preservation and 70 min of Warm Reperfusion
| Parameter | Preservation Time (h) | |||
|---|---|---|---|---|
| 0 | 8 | |||
| Unpreserved | Control | MP | DMP | |
| Clearance (ml/min) | 12 ± 2 | 2.2 ± 0.8a | 3.5 ± 0.6a | 5.1 ± 2.2a,b |
| Extraction Ratio | 0.40 ± 0.07 | 0.072 ± 0.025a | 0.12 ± 0.02a | 0.17 ± 0.07a,b |
| Terminal Half-life (min) | 6.9 ± 0.6 | 45 ± 14a | 29 ± 8a,b | 20 ± 10b |
Significantly different from 0 h preserved group: p<0.05, ANOVA, followed by Fisher's post-hoc test.
Significantly different from saline-treated preserved group: p<0.05, ANOVA, followed by Fisher's post-hoc test.
Due to the lack of an appreciable decline in the perfusate HA concentrations after 24 h of preservation (Fig. 1, bottom), the HA kinetic parameters were not calculated for these groups.
The bile flow rates of IPRLs in Control, MP and DMP groups following 8 and 24 h of cold preservation along with the flow rate in Unpreserved livers are shown in Figure 2. The average bile flow rate in the Unpreserved livers was 0.36 ± 0.05 ml/h. Neither preservation time nor pretreatment with MP or DMP resulted in altered bile flow rates, when compared with Unpreserved livers (Fig. 2).
Figure 2.
The bile flow rates in isolated livers subjected to 0, 8, or 24 h of cold ischemia followed by 70 min of warm reperfusion. Rats were pretreated intravenously, 2 h prior to liver harvest, with a single 5-mg/kg dose (MP equivalent) of MP or DMP or with saline (Control) (n=3−4/group). Symbols and bars represent the average and standard deviation values, respectively.
Effect of MP or DMP on Cold Preservation-Reperfusion Injury following OLT
The plots of percent survival as a function of post-transplantation time for Control, MP, and DMP groups are shown in Fig. 3. The seven-day survival of transplant recipients grafted with 24-h preserved livers harvested from untreated (Control) donors was 50%. Treatment of donors with MP did not significantly alter the survival of animals (60% in the MP group). In contrast, treatment of donors with DMP significantly (p<0.05, log rank test applied to Kaplan Meier survival curves) increased survival to 100% (Fig. 3).
Figure 3.
Percent survival curves of transplanted animals receiving livers preserved for 24 h in UW solution. Donor rats were pretreated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) 2−3 h prior to graft harvest (n=8/group).
The blood glucose concentration measured immediately after the completion of transplantation surgery was 122 ± 52 mg/dl in the untreated Control group. Treatment of donors with MP did not significantly alter the blood glucose concentrations (174 ± 18 mg/dl). In contrast, DMP pretreatment significantly (p<0.05, one-way ANOVA, with Fisher's post-hoc) increased the blood glucose levels by a factor of two to 236 ± 41 mg/dl.
The plasma concentrations of TNF-α and HA at 1 and 6 h following transplantation are shown in Fig. 4. Generally, an increase in the perfusion time caused a significant increase in the plasma concentrations of both TNF-α (Fig. 4, top) and HA (Fig. 4, bottom). Treatment of donors with MP did not significantly alter the plasma TNF-α concentrations. In contrast, DMP pretreatment significantly (p<0.05, two-way ANOVA, with Fisher's post-hoc) decreased the plasma levels of TNF-α, compared with untreated controls (Fig. 4, top). As for HA, consistent with the IPRL data for 24 h of cold preservation (Fig. 1, bottom), no effects of MP or DMP pretreatment was observed in vivo either at 1 or 6 h post-transplantation (Fig. 4, bottom).
Figure 4.
Plasma concentrations of TNF-α (top) and hyaluronic acid (HA) (bottom) in OLT recipients at 1 and 6 h after implantation of livers preserved for 24 h. Donor rats were pretreated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) 2−3 h prior to graft harvest. Columns and bars represent mean and standard deviation values, respectively (n=3−4/group).
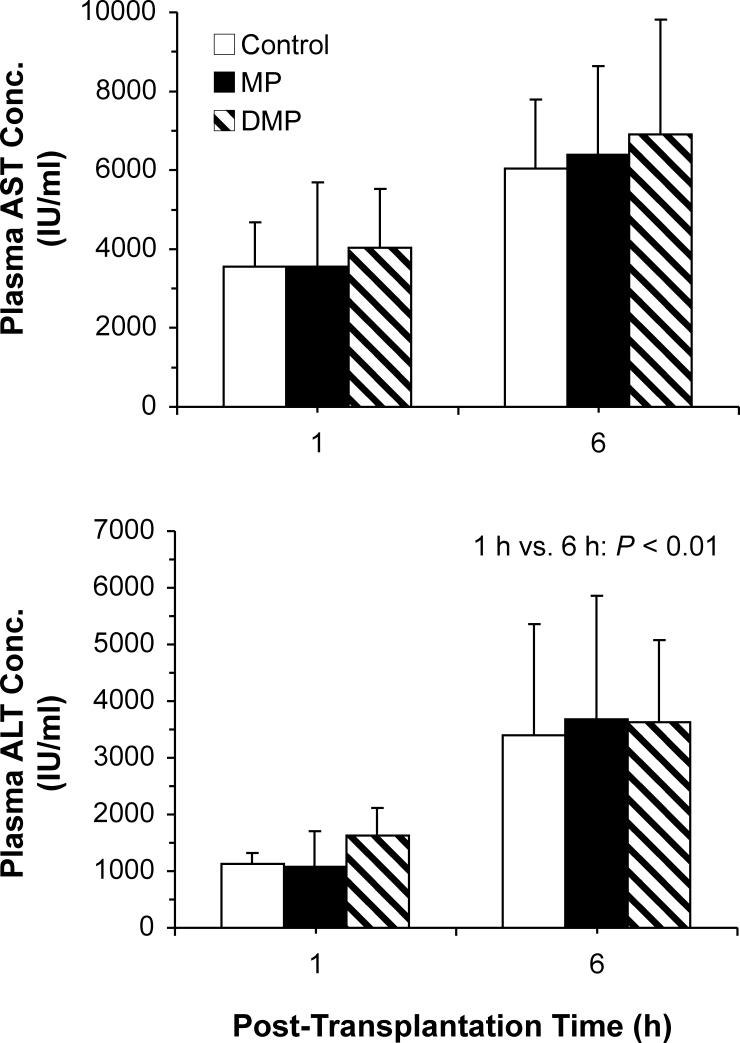
The plasma concentrations of AST and ALT in transplanted animals are presented in Fig. 5. Following 1 h of reperfusion, the plasma concentrations of AST (Fig. 5, top) and ALT (Fig. 5, bottom) in untreated controls were 3560 ± 1110 and 1130 ± 195 IU/ml, respectively. Reperfusion for 6 h further increased the ALT concentrations by factor of 3 (p<0.01). However, a two-fold increase in the AST concentrations after 6 h of reperfusion did not reach statistical significance. Treatment of donors with either MP or DMP did not significantly alter the plasma ALT or AST concentrations (Fig. 5).
Figure 5.
Plasma concentrations of AST (top) and ALT (bottom) in OLT recipients at 1 and 6 h after implantation of livers preserved for 24 h. Donor rats were pretreated with a single 5-mg/kg i.v. dose of MP or DMP or with saline (Control) 2−3 h prior to graft harvest. Columns and bars represent mean and standard deviation values, respectively (n=3−4/group).
DISCUSSION
Methylprednisolone, a glucocorticoid, is currently used as an anti-inflammatory and immunosuppressive drug (14). Most of the long-term effects of corticosteroids are probably mediated by their action on the intracellular glucocorticoid receptors (14). Activation of glucocorticoid receptors initiates alterations in nuclear factor (NF)-κB activation, which is critical for the expression of various proinflammatory cytokines following ischemic insults (14). Therefore, glucocorticoids, such as MP, may be of therapeutic value in reducing the inflammatory processes associated with ischemia-reperfusion injury.
While MP has a potent anti-inflammatory activity, it distributes rapidly to multiple organs (6, 15), resulting in the lack of tissue specificity in its effects. Tissue specificity in action is critical in conditions wherein only a specific tissue/organ undergoes ischemic injury, like the liver following cold preservation prior to transplantation. Therefore, development of strategies aimed at selectively delivering corticosteroids to the liver may help in attenuation of cold IR injury. Indeed, recent studies from our laboratory (4, 5) have shown that pretreatment of donors with a liver-targeted prodrug of MP (DMP) results in selective accumulation of DMP in the liver and improvement in cold preservation injury in an IPRL model.
Previous reports have suggested that all three major liver cells, namely KCs (1, 2), ECs (1, 2), and hepatocytes (16) contribute to the preservation-induced IR injury. In our recent studies using IPRL models (4, 5), we demonstrated the superiority of DMP over equimolar doses of the parent drug MP as a donor pretreatment in reducing cold preservation-induced KC activation (reduction of cytokine release into the perfusate) and improvement of hepatocyte functions (increased biliary excretion of indocyanine green). However, the potential effects of MP or DMP on preservation-induced damage to ECs are not known. Our present IPRL studies demonstrating the positive effects of DMP on IR-induced EC damage (Fig. 1 and Table I) are in agreement with the superior protective effects of the prodrug, in comparison with the parent drug, on KC (4) and hepatocytes (5), observed before in an IPRL model of preservation-induced injury. However, in contrast to the effects of DMP on KCs and hepatocytes, the protective effects of the prodrug on ECs, observed after 8 h of preservation, were lost by an extension of the preservation time to 24 h (Fig. 1). Indeed, after 24 h of cold preservation, no uptake of HA into the preserved livers was observed, regardless of the donor pretreatment (Fig. 1, bottom), indicating a total loss of viability of ECs during 24 h of cold preservation. Cold IR-induced rapid and complete loss of EC function, observed in our study after 24 h of preservation (Fig. 1), has also been reported by others (2, 17, 18). Nevertheless, our current IPRL studies (Fig. 1) suggest that MP or DMP pretreatment may be protective of ECs only following shorter durations of cold preservation when these cells are injured to a lesser extent.
The protective effect of DMP on EC function, as assessed by HA clearance in the IPRL studies (Fig. 1 and Table I), may be due to its direct action on ECs and/or an indirect action via KC inactivation. Studies (19) conducted on corneal ECs have demonstrated that corticosteroids inhibit the decrease in viability of these cells during preservation. Therefore, the enhanced EC function observed in our current study (Fig. 1 and Table I) may be, at least in part, due to the direct protective actions of regenerated MP on ECs. Secondly, it has been demonstrated (1, 2, 18) that cytokines like TNF-α and reactive oxygen radicals, released following cold IR-mediated KC activation, are cytotoxic to ECs. Consequently, agents that inactivate KCs also cause a decrease in EC damage (17, 20). Given that DMP decreases activation of KCs as shown elsewhere by us (4) and also in this study (Fig. 4, top), the enhancement of EC viability may also be due to DMP-induced decrease in KC activation in those livers. Further studies are required to elucidate the exact mechanisms involved in EC protection by DMP.
Overall, our present studies on EC function (Fig. 1 and Table I) and previous studies on the functions of KCs (4) and hepatocytes (5) in an IPRL model suggest that DMP and, to a lesser degree, MP may be effective as donor pretreatment to reduce cold preservation-induced liver damage in transplantation. Therefore, we also conducted in vivo studies in a syngeneic rat liver transplantation model. Previous studies have shown that cold preservation in UW for ≤ 24 h along with anhepatic times of ≤ 14 min does not significantly affect graft survival in rat liver transplantation models, whereas a 24 h preservation time coupled with an anhepatic time of 18−21 min significantly reduces graft survival (13). Therefore, we selected a preservation time of 24 h and an anhepatic time of 19 min in these studies to induce sufficient IR injury in vivo. Consistent with our hypothesis, donor pretreatment with DMP significantly increased survival of the transplanted grafts from 50% to 100 on day 7 post-transplantation, whereas MP pretreatment was not effective (Fig. 3).
The DMP-induced improvement in survival of animals after transplantation (Fig. 3) was associated with a decrease in the plasma TNF-α concentrations (Fig. 4, top), suggestive of a reduction in KC activation. This is in agreement with our previous IPRL studies, which demonstrated that DMP and MP pretreatments reduced TNF-α concentrations in the perfusate by 75% and 34%, respectively, after 24 h of cold preservation (4). Additionally, the significantly higher concentrations of glucose immediately after OLT using DMP-pretreated livers, compared with vehicle- or MP-pretreated livers (See Results), is in agreement with the increased survival in this group (Fig. 3). Cold IR injury depresses the high-energy nucleotide content and increases oxidant stress in the hepatocytes, leading to imbalances in energy metabolism and subsequent hepatocellular dysfunction (16, 21, 22). Indeed, studies have demonstrated a direct relationship between glycogen or adenosine triphosphate concentrations in liver allografts and post operative graft function or survival (23, 24). Therefore, the protective effects of DMP on graft survival (Fig. 3) may be due, at least in part, to the improved hepatocellular energy metabolism as evidenced by the increased post-operative blood glucose concentrations in this group (See Results). Additionally, although not studied here, our previous IPRL studies (5) clearly showed that DMP pretreatment increased the biliary excretion of ICG, a marker of graft hepatocytes function, by > 12 fold in livers preserved in cold for 24 h, whereas MP was ineffective. Overall, these data suggest that the improvement in graft survival observed in our in vivo study after DMP pretreatment (Fig. 3) is most likely due to inactivation of KCs and possibly improvement of hepatocytes functions by the prodrug.
In contrast to the TNF-α and glucose concentrations, the plasma concentrations of HA (Fig. 4, bottom), AST (Fig. 5, top), and ALT (Fig. 5, bottom) were not significantly affected by pretreatment with either MP or DMP in our transplanted rats. The OLT results for HA (Fig. 4, bottom) were expected because our IPRL studies clearly showed an almost total loss of EC function after 24 h of cold preservation, regardless of donor pretreatment (Fig. 1, bottom). Additionally, the lack of effects of pretreatments on the liver injury markers (AST and ALT) suggests involvement of extra-hepatic factors in the observed improved survival (Fig. 3).
Following cold IR, in addition to the hepatic injury by preservation and subsequent reperfusion, extrahepatic factors like pulmonary injury and systemic endotoxin-like shock syndrome may also play a major role in the mortality of transplant recipients (25-29). Prolonged intestinal congestion during the anhepatic time followed by reperfusion results in translocation of intestinal endotoxin into the portal circulation. Subsequently, exposure of liver KCs to endotoxin causes KC activation, resulting in the release of cytokines like TNF-α into the systemic circulation (25-27, 30). TNF-α, along with other cytokines, may damage extrahepatic organs like lung, resulting in mortality observed in transplant recipients (28, 29, 31). Therefore, improvement in survival despite a lack of improvement in liver injury markers may be due to decreased extra-hepatic organ toxicity caused by reduced TNF-α in the DMP group (Fig. 4, top). Indeed, Urata et al. in a rat OLT model with an identical portal vein clamping time, demonstrated that decreased TNF-α production, caused by pretreatment with KC depleting or inactivating agents resulted in improved 10-day survival despite a lack of improvement in hepatic biochemical (AST and lactate dehydrogenase) indices (32). In addition, they also reported significant decreases in pulmonary injury in the KC depleted livers, further confirming that pulmonary and other extrahepatic organ injuries have a major influence in survival following 24-h preservation in a rat OLT model (32). Therefore, it is likely that the DMP-induced improved survival rate observed in our study (Fig. 3) is, in part, a result of decreased damage to the lungs and/or other extrahepatic tissues.
Although not determined in this study, the time course of the liver exposure to DMP or MP after administration of a single 5-mg/kg dose of the prodrug or the parent drug to rats has been reported in several studies before (4-6, 33). Based on one of these studies (6), 2 h after the in vivo injection of a single 5-mg/kg dose of MP, the liver concentration of the drug was ∼0.4 μg/g. However, after the administration of an equivalent dose of the prodrug, DMP achieved a liver concentration (∼40 μg/g) that was almost 100 fold higher than that after the MP injection. Additionally, the concentration of the regenerated MP after DMP injection at this time point (2 h) was ∼0.3 μg/g. Further studies (4, 5) in the livers isolated 2 h after a single 5-mg/kg dose of MP or DMP and subjected to 24 h of ex vivo preservation (similar to the current study) showed no detectable concentration of MP after the parent drug injection. However, after DMP injection, relatively high concentrations of DMP persisted in the preserved livers, slowly regenerating the active drug. Therefore, the difference between MP and DMP in their effects on graft survival (Fig. 3) is most likely due to persistence of the drug in the liver after the prodrug injection.
CONCLUSION
Pretreatment of liver donors with a liver-targeted dextran prodrug of MP significantly increased graft survival following transplantation of livers subjected to cold preservation for 24 h. However, an equivalent dose of the parent drug was ineffective. The protective effect of DMP on preservation-induced injury appears to be due to a reduction in the systemic concentrations of TNF-α and an increase in the glucose production by the graft. Pretreatment of donors with DMP may decrease cold IR damage and favorably influence the outcome of liver transplantation.
ACKNOWLEDGEMENT
This work was partially supported by a grant from the National Institute of General Medical Sciences of NIH (R01 GM069869). The authors would like to acknowledge Imam H. Shaik for his technical assistance.
REFERENCES
- 1.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp. Mol. Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 2.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu. Rev. Pharmacol. Toxicol. 1997;37:327–38. doi: 10.1146/annurev.pharmtox.37.1.327. [DOI] [PubMed] [Google Scholar]
- 3.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–6. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Chimalakonda AP, Mehvar R. Attenuation of Kupffer cell activation in cold-preserved livers after pretreatment of rats with methylprednisolone or its macromolecular prodrug. Pharm. Res. 2003;20:1001–8. doi: 10.1023/a:1024402121053. [DOI] [PubMed] [Google Scholar]
- 5.Chimalakonda AP, Mehvar R. Effects of duration of ischemia and donor pretreatment with methylprednisolone or its macromolecular prodrug on the disposition of indocyanine green in cold-preserved rat livers. Pharm. Res. 2004;21:1000–8. doi: 10.1023/b:pham.0000029290.54167.7c. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Mehvar R. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: plasma and tissue disposition. J. Pharm. Sci. 2001;90:2078–87. doi: 10.1002/jps.1158. [DOI] [PubMed] [Google Scholar]
- 7.Mehvar R. Simultaneous analysis of dextran-methylprednisolone succinate, methylprednisolone succinate, and methylprednisolone by size-exclusion chromatography. J. Pharm. Biomed. Anal. 1999;19:785–92. doi: 10.1016/s0731-7085(98)00308-2. [DOI] [PubMed] [Google Scholar]
- 8.Mehvar R, Zhang X. Development and application of an isolated perfused rat liver model to study the stimulation and inhibition of tumor necrosis factor-alpha production ex vivo. Pharm. Res. 2002;19:47–53. doi: 10.1023/a:1013603331899. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchihashi S, Kaldas F, Chida N, Sudo Y, Tamura K, Zhai Y, Qiao B, Busuttil RW, Kupiec-Weglinski JW. FK330, a novel inducible nitric oxide synthase inhibitor, prevents ischemia and reperfusion injury in rat liver transplantation. Am. J. Transplant. 2006;6:2013–2022. doi: 10.1111/j.1600-6143.2006.01435.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez L, Heredia N, Peralta C, Xaus C, RoselloCatafau J, Rimola A, Marco A, Serafin A, Deulofeu R, Gelpi E, Grande L. Role of ischemic preconditioning and the portosystemic shunt in the prevention of liver and lung damage after rat liver transplantation. Transplantation. 2003;76:282–289. doi: 10.1097/01.TP.0000067529.82245.4E. [DOI] [PubMed] [Google Scholar]
- 11.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 12.Chimalakonda AP, Montgomery DL, Weidanz JA, Shaik IH, Nguyen JH, Lemasters JJ, Kobayashi E, Mehvar R. Attenuation of acute rejection in a rat liver transplantation model by a liver-targeted dextran prodrug of methylprednisolone. Transplantation. 2006;81:678–85. doi: 10.1097/01.tp.0000177654.48112.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urata K, Nguyen B, Brault A, Lavoie J, Rocheleau B, Huet PM. Decreased survival in rat liver transplantation with extended cold preservation: role of portal vein clamping time. Hepatology. 1998;28:366–73. doi: 10.1002/hep.510280211. [DOI] [PubMed] [Google Scholar]
- 14.Krensky AM, Strom TB, Bluestone JA. Immunomodulators: immunosuppressive agents, tolerogens, and immunostimulants. In: Hardmanand JG, Limbird LE, Hardmanand JG, Limbird LE, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2001. pp. 1463–1484. [Google Scholar]
- 15.Mishina EV, Jusko WJ. Selected tissue distribution of liposomal methylprednisolone in rats. Res. Commun. Chem. Pathol. Pharmacol. 1994;84:47–52. [PubMed] [Google Scholar]
- 16.Kukan M, Haddad PS. Role of hepatocytes and bile duct cells in preservation-reperfusion injury of liver grafts. Liver Transpl. 2001;7:381–400. doi: 10.1053/jlts.2001.23913. [DOI] [PubMed] [Google Scholar]
- 17.Niwano M, Arii S, Monden K, Ishiguro S, Nakamura T, Mizumoto M, Takeda Y, Fujioka M, Imamura M. Amelioration of sinusoidal endothelial cell damage by Kupffer cell blockade during cold preservation of rat liver. J. Surg. Res. 1997;72:36–48. doi: 10.1006/jsre.1997.5162. [DOI] [PubMed] [Google Scholar]
- 18.Clavien PA. Sinusoidal endothelial cell injury during hepatic preservation and reperfusion. Hepatology. 1998;28:281–5. doi: 10.1002/hep.510280201. [DOI] [PubMed] [Google Scholar]
- 19.Panda A, Kumar G, Basak SK, Mohan M, Gupta SK. The effects of corticosteroid and reduced glutathione on the endothelial viability during culture in MK medium. A light microscopy study. Acta Ophthalmol. (Copenh) 1994;72:731–6. doi: 10.1111/j.1755-3768.1994.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 20.Deaciuc IV, Bagby GJ, Niesman MR, Skrepnik N, Spitzer JJ. Modulation of hepatic sinusoidal endothelial cell function by Kupffer cells: an example of intercellular communication in the liver. Hepatology. 1994;19:464–70. [PubMed] [Google Scholar]
- 21.Palombo JD, Hirschberg Y, Pomposelli JJ, Blackburn GL, Zeisel SH, Bistrian BR. Decreased loss of liver adenosine triphosphate during hypothermic preservation in rats pretreated with glucose: implications for organ donor management. Gastroenterology. 1988;95:1043–9. doi: 10.1016/0016-5085(88)90181-3. [DOI] [PubMed] [Google Scholar]
- 22.Quintana AB, Guibert EE, Rodriguez JV. Effect of cold preservation/reperfusion on glycogen content of liver. Concise review. Ann. Hepatol. 2005;4:25–31. [PubMed] [Google Scholar]
- 23.Adam R, Astarcioglu I, Gigou M, Isaac J, Bismuth H. The influence of the glycogen content of the donor liver on subsequent graft function and survival in rat liver transplantation. Transplantation. 1992;54:753–6. [PubMed] [Google Scholar]
- 24.Lanir A, Jenkins RL, Caldwell C, Lee RG, Khettry U, Clouse ME. Hepatic transplantation survival: correlation with adenine nucleotide level in donor liver. Hepatology. 1988;8:471–5. doi: 10.1002/hep.1840080306. [DOI] [PubMed] [Google Scholar]
- 25.Pillay SP, Wynter C, Lynch S, Wall D, Balderson G, Strong R. Endotoxin levels in donors and recipients during orthotopic liver transplantation. Aust. N. Z. J. Surg. 1997;67:187–91. doi: 10.1111/j.1445-2197.1997.tb01938.x. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama I, Todo S, Miyata T, Selby R, Tzakis AG, Starzl TE. Endotoxemia and human liver transplantation. Transplant. Proc. 1989;21:3833–41. [PMC free article] [PubMed] [Google Scholar]
- 27.Miyata T, Yokoyama I, Todo S, Tzakis A, Selby R, Starzl TE. Endotoxaemia, pulmonary complications, and thrombocytopenia in liver transplantation. Lancet. 1989;2:189–91. doi: 10.1016/s0140-6736(89)90373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu H, Kataoka M, Ohtsuka M, Ito H, Kimura F, Togawa A, Yoshidome H, Kato A, Miyazaki M. Extended cold preservation of the graft liver enhances neutrophil-mediated pulmonary injury after liver transplantation. Hepatogastroenterology. 2005;52:1172–5. [PubMed] [Google Scholar]
- 29.Liu DL, Jeppsson B, Hakansson CH, Odselius R. Multiple-system organ damage resulting from prolonged hepatic inflow interruption. Arch. Surg. 1996;131:442–7. doi: 10.1001/archsurg.1996.01430160100022. [DOI] [PubMed] [Google Scholar]
- 30.Maring JK, Klompmaker IJ, Zwaveling JH, van Der Meer J, Limburg PC, Slooff MJ. Endotoxins and cytokines during liver transplantation: changes in plasma levels and effects on clinical outcome. Liver Transpl. 2000;6:480–8. doi: 10.1053/jlts.2000.8311. [DOI] [PubMed] [Google Scholar]
- 31.Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957–78. doi: 10.1097/00007890-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Urata K, Brault A, Rocheleau B, Huet PM. Role of Kupffer cells in the survival after rat liver transplantation with long portal vein clamping times. Transpl. Int. 2000;13:420–7. doi: 10.1007/s001470050724. [DOI] [PubMed] [Google Scholar]
- 33.Chimalakonda AP, Mehvar R. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: local immunosuppressive effects in liver after systemic administration to rats. Pharm. Res. 2003;20:198–204. doi: 10.1023/a:1022358702643. [DOI] [PubMed] [Google Scholar]