Recent prospective studies indicate that long-term glycemic control of diabetes is an important predictor not only of microvascular disease but also of macrovascular complications, including coronary heart disease (1). Possible links between glucose and cardiovascular events in the diabetic patient include modifications of important vascular functions of the endothelium with a switch from a quiescent, relaxant, antithrombotic, antioxidant, and antiadhesive state to an activated state displaying a more atherogenetic risk profile (2). Generation of reactive oxygen species could be a common downstream mechanism by means of which multiple byproducts of glucose are exerting their adverse effects on blood vessels (3). Indeed, in April 2001, Pennathur et al. (4) reported in the JCI that hyperglycemia favors oxidative reactions in the microenvironment of the artery wall in vivo.
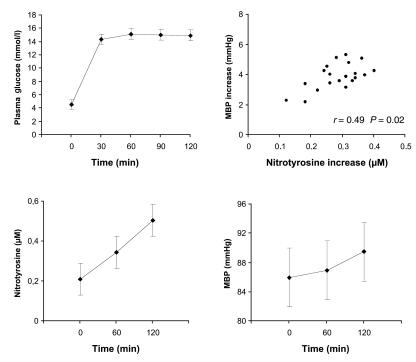
We studied the effect of acute elevations of plasma glucose levels on plasma nitrotyrosine, a marker of oxidative stress, in 20 healthy subjects (11 men, 9 women). Their age was 34 ± 4 years (mean ± SD), and the body mass index was 24 ± 1 kg/m2. None used any medication. After giving informed written consent to participate in the study, each subject underwent a hyperglycemic glucose clamp test in which plasma glucose concentrations were acutely raised at about 15 mmol/l for 120 minutes (Figure 1). Mean blood pressure and nitrotyrosine (5) rose significantly during the clamp; there was a positive correlation (r = 0.49) between nitrotyrosine and mean blood pressure increases during hyperglycemia. In control studies (n = 6), in which plasma glucose was maintained at normal concentrations (5 mmol/l for 120 minutes), we could detect no variation in nitrotyrosine plasma levels from baseline (baseline: 0.15 ± 0.05 μmol/l; 120-minute values: 0.13 ± 0.05 μmol/l, P = not significant).
Figure 1.
Twenty healthy subjects were submitted to a hyperglycemic glucose clamp study in which plasma glucose levels were acutely raised to 15 mmol/l (0.33 g/kg as intravenous bolus injection followed by a variable 30% glucose infusion). Mean blood pressure (MBP) was calculated as diastolic plus one-third pulse pressure. Nitrotyrosine was assayed according to Ter Steege et al. (5): the standard curve was constructed with serial dilution of a nitrated protein solution; glucose interference was excluded by performing the ELISA assay of standard solution in the presence of various glucose concentrations (from 10 to 100 mmol/l); the limit of detection of the assay was 10 nmol/l, with intra- and interassay coefficient of variations of 4.5% and 8%, respectively. Nitrotyrosine levels rose steadily during hyperglycemia and remained significantly above baseline at the end of the study. The correlation between nitrotyrosine and MBP increases during the clamp suggests that the two phenomena are related and strictly dependent upon ongoing hyperglycemia. Data are mean ± SD.
We show here that acute hyperglycemia in normal subjects causes an oxidative stress as evidenced by the raised circulating nitrotyrosine levels during the hyperglycemic clamp. However, we cannot exclude the possibility that some nitrotyrosine can be generated via a peroxynitrite-independent mechanism, or that a reduced nitrotyrosine clearance during hyperglycemia could also contribute to its raised plasma concentrations. Since nitrotyrosine is considered a good marker of peroxynitrite formation (6), and since peroxynitrite may account for a considerable portion of the toxic effects previously attributed to nitric oxide or the superoxide anion (7), it is possible that some of the toxic effects of hyperglycemia on the vascular tree may be modulated by peroxynitrite. Acute hyperglycemia in normal subjects may in fact induce vasoconstriction, activate thrombosis, increase the circulating levels of soluble adhesion molecules, and prolong the QT interval (8, 9). The recent demonstration (10) that apoptosis of myocytes, endothelial cells, and fibroblasts in heart biopsies taken from diabetic patients is selectively associated with intracellular levels of nitrotyrosine supports a role for high-energy oxidants (such as peroxynitrite) as mediators of the vascular damage brought about by hyperglycemia.
References
- 1.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 2.Giugliano D, et al. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95:1783–1790. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa T, et al. Normalizing mithocondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 4.Pennathur S, Wagner JD, Leeuwenburgh C, Litwak KN, Heinecke JW. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J Clin Invest. 2001;107:853–860. doi: 10.1172/JCI11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ter Steege JCA, Koster-Kamphuis L, Vas Straaten E, Forget P, Buurman WA. Nitrotyrosine in plasma of celiac patients as detected by a new sandwich ELISA. Free Radical Biol Med. 1998;25:953–963. doi: 10.1016/s0891-5849(98)00184-1. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 7.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 8.Marfella R, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101:2247–2251. doi: 10.1161/01.cir.101.19.2247. [DOI] [PubMed] [Google Scholar]
- 9.Marfella R, et al. The effect of acute hyperglycaemia on QTc duration in healthy man. Diabetologia. 2000;43:571–575. doi: 10.1007/s001250051345. [DOI] [PubMed] [Google Scholar]
- 10.Frustaci A, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1129. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]