Abstract
Background
Preclinical research findings suggest that exposure to stress and concomitant hypothalamus-pituitary-adrenal (HPA) axis activation during early development can have permanent and potentially deleterious effects. A history of early-life abuse or neglect appears to increase risk for mood and anxiety disorders. Abnormal HPA response to stress challenge has been reported in adult patients with Major Depressive Disorder and Post-Traumatic Stress Disorder.
Methods
Plasma adrenocorticotropin (ACTH) and cortisol reactivity to the Trier Social Stress Test were examined in healthy adults (N=50) without current psychopathology. Subjects with a self-reported history of moderate to severe childhood maltreatment (MAL; n=23) as measured by the Childhood Trauma Questionnaire were compared with subjects without such a history (CTL; n=27).
Results
Compared with CTLs, MAL subjects exhibited significantly lower cortisol and ACTH baseline-to-peak deltas. A significant group effect was seen in the (repeated measures) cortisol response to the stress challenge, reflecting lower concentrations among MAL subjects. A significant group × time effect characterized the relatively blunted ACTH response of the MAL group. Emotional Neglect (=−.34, p=.02) and Sexual Abuse (=+.31, p=.03) strongly predicted maximal cortisol release.
Conclusions
In adults without diagnosable psychopathology, childhood maltreatment is associated with diminished HPA axis response to a psychosocial stressor. Possible explanations for the finding are discussed.
Keywords: Trier Social Stress Test, HPA Axis, Childhood, Abuse, Cortisol, ACTH, Reactivity, Endophenotype
Introduction
In the past decade, both animal and human data have underscored the permanent and potentially deleterious neuroendocrine effects of exposure to stress during early infant or childhood development (1–4). In rodent and primate models, experimental conditions that induce stress in young subjects through disruption of usual mother-infant interactions can produce exaggerated or dampened stress system reactivity in the animal that persists to maturity. The direction of the experimentally-induced abnormality in pituitary-adrenal response appears to be determined in part by the age of the subject when exposed to the experimental stress paradigm (5, 6) as well by as the nature and chronicity of the stressful condition (7).
Cortisol dysregulation and deficient glucocorticoid feedback regulation have been identified as biological correlates of adult depression and anxiety disorders (8–10), and early life adversity is consistently associated with these disorders in epidemiological studies (11). A large body of clinical literature has characterized major depressive disorder (MDD) as a condition associated with excessive basal cortisol secretion and inadequate inhibitory feedback regulation of the hypothalamus-pituitary-adrenal (HPA) axis constituents (12). Conversely, relatively low basal cortisol concentrations (13–16), low awakening cortisol response (15–17), and enhanced cortisol suppression following low-dose dexamethasone administration (18) have been suggested as correlates of Post-Traumatic Stress Disorder (PTSD) (13–15, 19). Not all investigations have confirmed the apparent opposite pattern of hypo- and hypercortisolism (relative to healthy control cases) for PTSD (20) and MDD (21–23), respectively. The discrepancies may be due to diagnostic (24) or genetic (25) heterogeneity in study samples, comorbidity of the two disorders (26, 27), or other variables that significantly impact development and maintenance of HPA function (28–31).
Further elucidation of the determinants of the direction of HPA axis abnormalities may allow identification of abnormal patterns of adrenocorticotropic hormone (ACTH) and cortisol responses that can be used as biological markers, or endophenotypes, signaling elevated risk for these disorders (32). Detection of a biological risk marker before the onset of significant symptoms would provide an opportunity for interventions to prevent or otherwise alter the ensuing course of illness.
High trait anxiety, an established risk factor for depression (33), was found to be associated with suppressed ACTH and cortisol responses to a standardized laboratory stressor (i.e., the Trier Social Stress Test; 34) in a sample of healthy adult subjects (35), though a significant positive relationship between cortisol response and inhibited temperament has also been seen in healthy adults (36). Childhood maltreatment, another risk factor for depression, has recently been examined in nonclinical samples. Women with a history of sexual or physical abuse demonstrated increased ACTH but normal cortisol responses to the TSST when compared with female control subjects without abuse histories (37). However, the presence in some subjects of comorbid PTSD (38), which has independently been associated with HPA function abnormalities (15, 26, 39), may have confounded results.
We describe the ACTH and cortisol responses to a standardized laboratory stress test in healthy adults without MDD or PTSD. A group reporting a history of moderate to severe childhood maltreatment in the form of neglect or abuse was compared with a group reporting none. Based on our pilot work examining cortisol response to the dexamethasone/corticotropin releasing hormone (Dex/CRH) test as a function of perceived early life stress, we hypothesized that exaggerated cortisol response would be detected among healthy individuals reporting a history of childhood trauma.
Methods and Materials
Subjects
Fifty adults, ages 20 to 59, were selected for participation. Advertisements for “healthy adults with a history of early life stress” were posted in the community. We included only those who scored “moderate” to “severe” on at least one of the five subscales of the Childhood Trauma Questionnaire (CTQ; 40–42) and did not meet current DSM-IV criteria for MDD or PTSD (n=23). A second cohort of healthy volunteers (n=27) was recruited via advertisements for “healthy research subjects.” We selected only those who generated a categorical score of “none” on all five CTQ subscales and were similarly free of current MDD and PTSD. All subjects were free of pregnancy, significant medical illness and recreational drug use as evidenced by complete physical and neurological examination, standard laboratory tests, and electrocardiogram. Exclusion criteria included use of any psychotropic medication or other drug thought to influence HPA axis function, current or lifetime diagnosis of a primary psychotic disorder or bipolar disorder, current substance dependence or abuse, current major mood or anxiety disorder, and prominent personality pathology. Structured Clinical Interview for DSM-IV (SCID) for Axis I Disorders (43) was used for psychiatric diagnostic assessments. Written, informed consent was provided by all subjects on forms approved by the Institutional Review Board of Butler Hospital. Subjects were remunerated for their time and travel expenses.
Interview and Self-Report Assessment of Relevant History, Current Symptoms, and Personality
In addition to diagnostic interviews, subjects completed a battery of self-report assessments during their initial visit to the Mood Disorders Research Program at Butler Hospital. These included the Tridimensional Personality Questionnaire (TPQ; 44), the Inventory for Depressive Symptoms – Self-Rated (IDS-SR; 45), the State-Trait Anxiety Inventory (STAI; 46), the Perceived Stress Scale (PSS; 47), and the Childhood Trauma Questionnaire (CTQ).
The CTQ version 3 (41, 42, 48) is a 28-item, self-report instrument comprising 5 subscales (Emotional Abuse, Physical Abuse, Sexual Abuse, Emotional Neglect, Physical Neglect). Published guidelines for classification of CTQ subscale total scores were applied to determine threshold severity or absence of maltreatment on each subscale. Subjects generating scores for “low/minimal” levels of maltreatment were not included in this study.
The Psychosocial Stress Protocol
The TSST is a standardized laboratory psychosocial stress protocol that involves public speaking role-play and mental arithmetic tasks in front of a panel of confederate “judges” (34). The protocol consists of an anticipation period followed by a 10-minute test period during which the subject must deliver a monologue speech about his qualifications for a chosen vocation and perform mental calculation and recitation of serial subtraction by 13s. Blood samples and heart rate measurements are obtained before, during, and after the role-play/arithmetic stressor. An intravenous catheter was established at 11 a.m. to allow time for subjects to accommodate to the biological testing suite environment. Plasma samples were collected via intravenous access at time points 0 (1:45 p.m.; immediately before briefly meeting the judges and being told about the public-speech role play), 15 (2:00 pm; the end of the anticipation period during which the subject prepared his or her speech), 30 (2:15 pm; several minutes after completing the speaking/arithmetic test), 45, 60, 75, and 90 minutes. Subjects were debriefed after time 30 behavioral ratings were completed and remained awake in bed for the remainder of the protocol. The Profile of Mood States (POMS; 49) and standard Visual Analog Scales (scaled 0 to 100) for mood and somatic states were used for self-report of response to the TSST at times 0, 30, and 75.
Hormone Assays
Plasma ACTH was assayed in duplicate in 200 μl plasma samples using immunoradiometric assay (50) (Scantibodies Laboratory, Santee, CA). The minimum detectable ACTH concentration was 2 pg/ml, and intra- and inter-assay coefficients of variation were 4.6% and 5.3%, respectively. Plasma cortisol concentrations were assayed in duplicate using the GammaCoat cortisol I-125 coated-tube radioimmunoassay (RIA) kit (INCSTAR Corp., Stillwater, Minn.). Intra-assay and inter-assay CVs observed for quality assessment samples (3 and 20 μg/dl) were less than 5% and 10%, respectively.
Statistical Analyses
Analyses were conducted using SPSS 11.5.0. All analyses were two-tailed with alpha set to 0.05. Chi-square and t-tests were used to test for baseline group differences. For female subjects not taking daily hormone replacement or oral contraceptive pills, days from onset of menstrual period were used to approximate phase of menstrual cycle.
Pearson correlation coefficients (first bivariate and subsequently partial correlations controlling for age) were used to examine relatedness of baseline variables and hormone concentrations. Significant correlations were not observed between age and cortisol or ACTH values in this dataset, but in light of published reports of increasing HPA hormone concentrations with age (51) and a significant difference in mean age between our two groups, age was entered as a covariate in all hormone analyses. Analyses of covariance (ANCOVA) controlling for age were used to compare subject groups at baseline and on baseline-to-peak delta for cortisol and ACTH. Effects of gender, oral contraceptive use, and smoking were explored in post-hoc analyses.
Repeated-measures general linear models (GLM) with group as a fixed factor and age as a covariate were used to examine effects of time, group, and the interaction of group by time. Estimated marginal means were generated for the graphs in Figures 1 and 2. Post-hoc group comparisons at individual time points were made with GLM univariate analyses of covariance when significant main effects were detected. Mauchley’s Test of Sphericity was used and, when appropriate, the Huynh-Feldt adjustment was made.
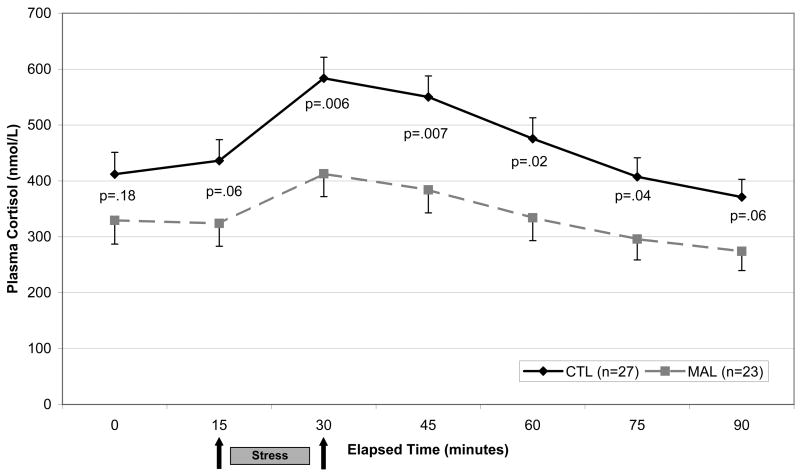
Figure 1.
Plasma cortisol response to Trier Social Stress Test in Healthy Adults with (n=23) and without (n=27) a history of childhood maltreatment. A significant main effect of group is present F=5.9[1], p=.02. P-values reported on the graph represent group differences at individual time points.
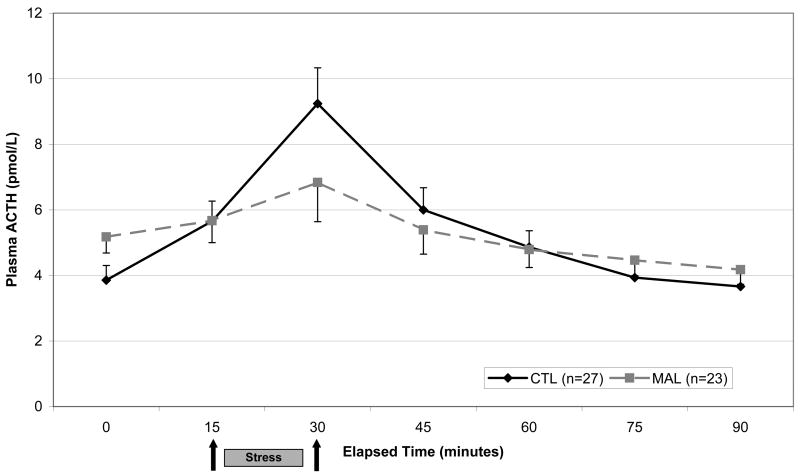
Figure 2.
Plasma ACTH response to Trier Social Stress Test. Repeated measures analysis showed a significant within-subjects interaction of Abuse × Time (F=4.3[1.6], p=.02). Analysis of individual time points revealed none with significant group difference.
To explore the hypothesis that adults with histories of early life abuse would experience more negative affect induction by the TSST than controls, we used repeated measures (time points 0, 30, and 75) GLMs for eight POMS subscales (Tension/Anxiety, Elation, Anger/Hostility, Fatigue, Depression, Confusion, Vigor, Arousal, Overall Mood) and 12 VAS scales (Angry, Anxious, Calm, Depressed, Drowsy, Energetic, Fearful, Happy, Irritable, Mellow, Nervous, Sad) assessing mood states.
Since subjects in the childhood maltreatment (MAL) group achieved threshold scores on any one or more of the five CTQ subscales, post-hoc testing was performed to determine if certain types of maltreatment were more likely than others to be related to stress-induced hormone responses. A partial correlation matrix was used to examine associations between each CTQ subscale score and Time 30 cortisol and ACTH concentrations. Since CTQ subscale scores were intercorrelated, multiple linear regression was conducted to determine whether they were unique predictors of Time 30 cortisol after controlling for effects of the other independent variables. A backward elimination model (removal based on preset criterion of F .100) was selected to identify the best set of unique predictors.
Due to malfunction of the cardiac monitor, heart rate data were only available for 15 subjects, so associations between heart rate and temperament factors were not tested due to the small N.
Results
Demographic and Clinical Characteristics
Clinical characteristics of the groups are displayed in Table 1. Subjects reporting moderate or severe childhood maltreatment (MAL; n=23) were on average older than the controls, but sex distribution was equivalent. The distribution of female subjects across three categories used to approximate relevant hormone status on the day of the TSST (i.e., [1] cycle days 1 to 14; [2] cycle days 15 – 28; [3] taking daily hormone therapy) did not differ statistically between groups, with 5 MAL and 4 CTL women undertaking the TSST during approximated luteal phases (days 15 – 28) of their cycles. Eleven subjects were taking oral contraceptives during the course of the study (MAL n=2; CTL n=9). Six sreported cigarette smoking (MAL n=2; CTL n=9). The MAL group scored significantly higher than CTLs on self-ratings of depressive symptomatology, state anxiety, trait anxiety, harm-avoidant personality characteristics, and current (past month) levels of perceived stress (Table 1). While no subject met diagnostic criteria for current PTSD or major depressive episode, past histories elicited during SCID interviews generated “probable” lifetime diagnoses of dysthymia in 2 (9%) subjects from the MAL group, lifetime PTSD in one MAL subject (9%), lifetime generalized anxiety disorder or panic disorder in 3 (11%) CTL and 4 (17%) MAL subjects, and lifetime MDD in 4 CTL (15%) and 3 MAL (13%) subjects. Lifetime diagnoses were also established for remote drug abuse/dependence in 2 subjects from each group and past alcohol abuse/dependence in one CTL and 6 MAL subjects, with the latter difference reaching statistical significance (4% vs, 26%, X2 =5.2, p=.03). All subjects meeting criteria for lifetime drug or alcohol dependence reported at least one year of sobriety or abstinence prior to participation.
Table 1.
Clinical and Demographic Characteristics for Control (CLT) and Maltreated (MAL) Subject Groups
| CTL (n=27) | MAL (n=23) | p-value | |
|---|---|---|---|
| Age (mean±SD years) | 24.1±6.6 | 35.0±12.9 | .001 |
| Sex (n [%] Women) | 16 (59%) | 17 (74%) | n.s. |
| Race ([n]%) | n.s. | ||
| Caucasian | 20 (74%) | 18 (78%) | |
| Black | 1 (4%) | 2 (9%) | |
| Asian | 3 (11%) | 1 (4%) | |
| Other | 3 (11%) | 2 (9%) | |
| CTQ Abuse Type (moderate to severe rating on subscale; a subject may meet criteria for more than one) | |||
| Physical Abuse | 0 | 9 (39%) | -- |
| Sexual Abuse | 0 | 8 (35%) | -- |
| Emotional Abuse | 0 | 13 (57%) | -- |
| Emotional Neglect | 0 | 12 (52%) | -- |
| Physical Neglect | 0 | 10 (43%) | -- |
| IDS-SR Total Symptom Score (mean±SD) | 5.8±5.1 | 11.9±6.1 | <.001 |
| STAI State Anxiety (mean±SD) | 26.3±4.7 | 32.0±8.0 | .005 |
| STAI Trait Anxiety (mean±SD) | 28.5±5.3 | 38.4±8.4 | <.001 |
| Perceived Stress Scale Total (mean±SD) | 17.0±4.2 | 21.9±5.4 | .001 |
| SCID “Probable” Lifetime Axis I Diagnoses: | |||
| Major Depressive Episode (n[%]) | 4 (15%) | 3 (13%) | n.s. |
| Dysthymic Disorder (n[%]) | 0 (0%) | 2 (9%) | n.s. |
| Alcohol Abuse/Dependence (n[%]) | 1 (4%) | 6 (26%) | .03 |
| Drug Abuse/Dependence (n[%]) | 2 (7%) | 2 (9%) | n.s. |
| Post-Traumatic Stress Disorder (n[%]) | 0 (0%) | 1 (4%) | n.s. |
| Generalized Anxiety or Panic Disorder (n%]) | 3 (11%) | 4 (17%) | n.s. |
| TPQ Novelty Seeking Scale | 19.1±4.5 | 18.5±5.5 | n.s. |
| TPQ Harm Avoidance Scale | 9.3±4.2 | 13.8±6.3 | .004 |
CTL = control subjects; MAL= subjects reporting childhood maltreatment; CTQ = Childhood Trauma Questionnaire; IDS-SR = Inventory of Depressive Symptomatology, Self-Rated; STAI = State-Trait Anxiety Inventory; SCID = Structured Clinical Interview for DSM-IV; TPQ = Tridimensional Personality Inventory
Subjective Response to TSST
Significant main effects of time seen on POMS scales of Tension/Anxiety (F=5.7[2], p=.006) and Arousal (F=3.7[2], p=.03) during the TSST confirmed that the protocol was successful in activating subjective stress response in study participants. Similarly, induction of stress by the TSST was evidenced by significant main effects of time on VAS ratings of “Anxious” (F=5.2[2], p=.01), “Fearful” (F=4.8[2], p=.01), and “Nervous” (F=5.0[2], p=.01). The absence of significant group effects on these same scales supports the notion that CTL and MAL subjects experienced equivalent levels of anxiety induction from the TSST. The only significant group effect observed in self-report data highlighted persistently higher self-rated levels of Confusion (F=9.7[1], p=.003), and somewhat lower levels of Elation (F=5.3[1], p=.03) and Vigor (F=4.6[1], p=.04) throughout the procedure in MAL compared to CTL subjects.
Relationships Between Hormones and Baseline Clinical Characteristics
Among CTL subjects, positive correlations between age and delta hormone responses were seen (r=.56, p=.002 for cortisol; r=.82, p<.001 for ACTH), but these relationships did not approach significance for the MAL group (p=.23 and p=.48, respectively). A partial correlation matrix controlling for age did not identify any significant relationships of Time 0 or Time 30 hormones with any of the following baseline variables: depressive symptoms (IDS-SR), perceived stress (PSS), state anxiety (STAI State Scale), trait anxiety (STAI Trait Scale), or inhibited personality style (TPQ Harm Avoidance and Novelty Seeking Scales).
Cortisol - Group Comparisons
Baseline (Time 0) plasma cortisol concentrations did not differ statistically between groups. Baseline-to-peak delta values were lower among MAL subjects than CTL subjects (F=5.1[1], p=.03) after controlling for significant age effects. Repeated measures analysis of cortisol response to the TSST revealed a significant within-subjects age × time interaction (F=3.3[2.3], p=.03), a nonsignificant within-subjects interaction of group × time (F=1.6[2.3], p=.19) and a significant overall effect of group (F=5.9[1], p=.02; Figure 1). The between-subjects effect of age was not significant (F=0.24[1], p=.63). Comparison of MAL and CTL groups at individual time points showed maximum mean separation (F=8.4[1], p=.006) at the 30-minute time point obtained after the role-play speech and mental arithmetic tasks. MAL subjects continued with a relatively suppressed cortisol response at the 45-minute time point (F=8.0[1], p=.007) and gradually returned to trend-level difference from CTL subjects at the end of the 90-minute recovery period (F=3.8[1], p=.06). Analyses were repeated with gender added as a covariate. No significant effects of gender were detected and p-values for the above findings remained significant.
ACTH – Group Comparisons
Baseline (Time 0) plasma ACTH concentrations trended toward higher values among MAL subjects (F=3.5[1], p=.07; Figure 2) relative to CTLs. Mean baseline-to-peak delta was lower for MAL than CTL subjects (F=6.2[1], p=.02). Repeated measures analysis of ACTH response to the TSST showed a significant within-subjects interaction of age × time (F=9.1[1.6], p=.001) and a significant within-subjects interaction of group × time (F=4.3[1.6] p=.023; Figure 2). Age-adjusted mean ACTH concentrations did not differ between MAL and CTL subjects at individual time points. The analyses were repeated with gender added as covariate and unlike the cortisol data, gender did emerge as a significant group effect for ACTH (F=7.9[1], p=.007). The ACTH analyses were subsequently conducted separately in male and female subgroups. Among men, a significant group × time effect (F=7.0[1.9}, p=.004) reflected a flattened response curve for the MAL group. For women the interaction effect was not significant, but a significant group effect (F=4.4[1], p=.044) emerged, reflecting decreased ACTH concentrations in the MAL group throughout the TSST.
Peak Hormone Concentrations and Type of Maltreatment
Partial correlations of Time 30 cortisol and CTQ subscale scores highlighted significant associations between cortisol and several types of maltreatment, with significant intercorrelation between subscales. A statistically significant linear regression model predicting Time 30 cortisol with forced entry of age and 5 CTQ subscales was generated (F=3.1[6,43], p=.01), accounting for 30% of the variance. Following automated backward elimination of two variables, a final model was produced (F=5.8[3,46], p=.002) which explained 27% of the variance and retained three predictors of cortisol: Emotional Neglect (=−.34, p=.02), Sexual Abuse (=+.31, p=.03), and Physical Abuse (=−.27, p=.08). Of note is the opposite direction of standardized beta coefficient for Emotional Neglect and Sexual Abuse. None of the CTQ subscales was significantly correlated with time 30 ACTH concentrations.
Individual Cortisol and ACTH Correlations
Within-subject correlations between cortisol and ACTH delta responses to the stress test were highly correlated for both the CTL (r=.79, p<.001) and MAL (r=.71, p<.001) groups.
Pot-hoc Analyses to Address Potential Confounds
The principal findings reported here remained consistent after repeating the main analyses without cigarette smokers and again after removing female subjects who were taking oral contraceptives during the course of the study. Despite the substantial loss of power, similar trends in the results were found when analyses were repeated without 24 individuals with a history of probable lifetime psychiatric disorders.
Discussion
The main finding of this study was suppression of cortisol response to a standardized laboratory psychosocial stressor among healthy adults reporting significant childhood maltreatment, relative to healthy controls reporting none. Corresponding with this dampened cortisol reactivity, our subjects with early life abuse or neglect had a blunted ACTH response curve when compared with the non-traumatized counterparts. Subjective ratings of emotional responses suggested both groups experienced the same level of anxiety induction, but maltreated subjects had more confusion and somewhat less vigor and elation throughout the stress test. Since none of the subjects participating in this investigation met criteria for current MDD or PTSD, the patterns of altered neuroendocrine response we observed cannot be considered biological correlates of those disorders per se. One interpretation, consistent with some findings from related animal and human research, implicates childhood maltreatment as a developmental determinant of enduring abnormality of HPA system function independent of the manifestation of diagnostic threshold-level symptomatology.
Assuming that self-report assessments of adverse childhood experiences retrospectively provided by a nonclinical sample of healthy adult volunteers can be regarded as a fairly accurate index of exposure to stress during early brain development, our data suggest that hyporeactive pituitary-adrenal response to an acute stressor represents a biological consequence of childhood maltreatment, especially in the form of emotional neglect. This finding is not in line with our original hypothesis and also is not in keeping with the majority of findings described for PTSD patients in cognitive challenge studies (52), where exaggerated cortisol response is typically seen. In a recent study utilizing both the TSST and the cold pressor test, relative to controls PTSD patients demonstrated adrenocortical hyporeactivity on the physical stressor but not on the psychosocial stressor (53). Our results do not, therefore, support the notion of a neuroendocrinopathy consistent with PTSD among individuals reporting moderate to severe childhood maltreatment but not meeting DSM-IV criteria for the disorder. The findings are complementary to the literature describing acute stress hyporeactivity in primates (54, 55) and rodents (56) exposed to chronic social stress, which may have some relevance for childhood maltreatment, especially emotional neglect/abuse. These animal models highlight the chronicity of past stress exposure as a key variable determining HPA hyporesponsiveness to an acute psychogenic stressor (57, 58). We lack sufficient data about our subjects to conclude that chronic rather than acute stress exposure accounts for the patterns we observed.
Our study was designed to examine acute, dynamic function of the HPA system in the context of a laboratory psychosocial stressor, rather than basal levels of stress hormones. The few published studies of adult subjects reporting early life maltreatment have confirmed significant relationships between self-reported early life stress exposure and “abnormal” pituitary-adrenal reactivity, as measured in adults without diagnosable psychiatric illness (37, 59), but the direction of findings is not fully consistent with the hypoactive stress response pattern we observed. The presence of diagnostic comorbidity, diverse methodology, and exposure to traumas or stressors of different types and durations at different points in development may all contribute to the lack of a clear signal when these investigations are considered in aggregate. As suggested by our finding of significant but opposite direction correlational relationships between stress-induced cortisol response and emotional neglect versus sexual abuse, the type of maltreatment reported by the subject may reflect various critical qualities of the environment which have differential long-term effects (60, 61).
The mechanism underlying diminished cortisol response to acute stress among healthy adults reporting childhood adversity is not known, but some researchers have hypothesized a trajectory of initial hyper-activation of the HPA system progressing to a state of chronic adrenal stress hyporeactivity (7, 62) as a type of counterregulative adaption following acute hyper-exposure to ACTH during stressful early development. Early deprivation led to impaired coping with aversive stimuli or events but not to frank anhedonia-like behavior in Fischer rats, a strain characterized by phenotypic high stress “sensitivity.” That animal model may be the best fit with the findings we have generated and also raises the question of how human genomic variation may affect TSST response (63) and perhaps provide resilience and protection from neuroendocrine and psychopathological consequences of interpersonally impoverished early environment.
One may challenge the assumption that retrospective self-ratings of childhood adversity necessarily reflect historically accurate events as would be recorded by an objective observer. Self-ratings of perceived and recalled past maltreatment by a healthy adult may be determined by the individual’s degree of sensitivity to environment, personality factors, or some other predisposition to exaggerate or inaccurately remember and/or report remote experiences. The diminished pituitary-adrenal response we observed to the TSST may be a function of some other primary determinant/s rather than reflect the biological sequelae of early childhood maltreatment per se. To address this possibility, we examined our data closely for the possible contributing roles of trait anxiety, inhibited personality, perceived stress, and other indices of subthreshold anxiety and depressive symptomatology, but none accounted for the statistically significant neuroendocrine patterns that were associated with CTQ childhood maltreatment scores. Published literature indicates that self-reports of early-life abuse (11), high levels of subjective stress-sensitivity (64), and ruminative coping cognitive styles (65) have each been associated with mood and anxiety psychopathology and are considered independent risk factors even though they may share a common etiology. Emerging data such as that demonstrating interactions between specific genetic polymorphisms, self-reported accounts of environmental stress, and presence of major depression (66), and between genetic polymorphisms and personality characteristics (67, 68), highlight how inter-related and overlapping these constructs are likely to be. Whether or not the data we collected from our subjects reflects actual or perceived childhood maltreatment does not invalidate the present findings, but a greater understanding of the determinants of reporting a history of childhood maltreatment and how these factors may influence HPA reactivity will certainly be an important step in this line of research.
While basal or awakening hypocortisolism has been associated with inflammatory conditions such as rheumatoid arthritis and fibromyalgia (69, 70), the physical and emotional health implications of hypocortisol acute stress responsivity have not been well elucidated. Attenuated HPA responses to a laboratory psychological stressor predicted early smoking relapse (71) in adults and were associated with atopic dermatitis in healthy children (72). Lower cortisol and ACTH responses to a Dex/CRH test retrospectively predicted recent suicide attempts among depressed inpatients (73). The complex modulation of memory by adrenal glucocorticoids during acute stress has been well documented (74), and there is some evidence that greater endogenous cortisol release during, as well as exogenous administration of cortisol prior to, acute stress exposure can buffer negative emotional responses (75, 76).
Results from relevant studies by Roelofs and colleagues (77, 78) demonstrated that healthy young adults with hypocortisol salivary response to the TSST had vigilant reactions to social threat cues while at rest, but markedly avoidant responses to social threat during an acute stress condition, relative to subjects with more robust cortisol response to the TSST. Additionally, low cortisol responders had relatively longer reaction times in executing affect-congruent behaviors during stress (79). Taken together, these investigations suggest that hypocortisol response to acute stress may compromise optimal cognitive performance and approach-avoidance behavior in situations where it may be important to function maximally, but whether and how such a consequence leads to psychopathology remains to be elucidated.
An alternative conceptualization of our findings involves the notion of dampened HPA stress responsivity reflecting resilience rather than risk for psychopathology. Follow-up studies which evaluate the extent to which individuals with low cortisol stress response develop future psychopathology and/or medical morbidity will be important to realize the potential of this proposed endophenotype.
Table 2.
Mean (SD) age-adjusted ACTH (pmol/L) and cortisol (nmol/L) values for CTL and MAL subjects in response to the TSST.
| CTL ACTH | MAL ACTH | CTL Cortisol | MAL Cortisol | |
|---|---|---|---|---|
| Baseline | 3.9 (0.5) | 5.2 (0.5) | 412.1 (38.9) | 329.5 (42.6) |
| Time 15 | 5.7 (0.6) | 5.7 (0.7) | 436.3 (37.4) | 324.0 (40.9) |
| Time 30 | 9.2 (1.1) | 6.8 (1.2) | 583.8 (37.4) | 412.8 (40.9) |
| Time 45 | 6.0 (0.7) | 5.4 (0.7) | 550.2 (37.5) | 383.9 (41.0) |
| Time 60 | 4.9 (0.5) | 4.8 (0.6) | 475.6 (37.3) | 334.0 (40.8) |
| Time 75 | 3.9 (0.4) | 4.5 (0.5) | 407.4 (34.1) | 296.0 (37.3) |
| Time 90 | 3.7 (0.4) | 4.2 (0.5) | 371.2 (31.4) | 274.0 (34.4) |
| Delta | 4.5 (5.8) | 3.1 (3.4) | 188.8 (167.3) | 143.3 (123.8) |
Acknowledgments
This research was funded in part by NARSAD Awards (LLC, ART), by a Pfizer/Society of Women’s Health Research Scholar Award (LLC), and by National Institutes of Health grant RO1 MH068767-01 (LLC). The data were presented in poster form at the December 2005 American College of Neuropsychopharmacology annual meeting.
Footnotes
Financial Disclosures
The authors report no disclosures relevant to this work. No pharmaceutical or therapeutic device products were utilized in this research protocol.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coplan JD, Smith EL, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, et al. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiatry. 2001;50:200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- 2.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 3.Meany MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glugogorticoid receptor binding in the rat. Neuroendocrinology. 1989;50 doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- 4.Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 5.Mathew SJ, Coplan JD, Smith EL, Scharf BA, Owens MJ, Nemeroff CB, et al. Cerebrospinal fluid concentrations of biogenic amines and corticotropin-releasing factor in adolescent non-human primates as a function of the timing of adverse early rearing. Stress. 2002;5:185–193. doi: 10.1080/1025389021000010521. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 7.Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, et al. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Holsboer F, Lauer CJ, Schreiber W, Krieg JC. Altered hypothalamic-pituitary-adrenocortical regulation in healthy subjects at high familial risk for affective disorders. Neuroendocrinology. 1995;62:340–347. doi: 10.1159/000127023. [DOI] [PubMed] [Google Scholar]
- 10.Yehuda R. Neuroendocrine aspects of PTSD. Handb Exp Pharmacol. 2005:371–403. doi: 10.1007/3-540-28082-0_13. [DOI] [PubMed] [Google Scholar]
- 11.Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- 12.Hansen-Grant SM, Pariante CM, Kalin NH, Miller AH. Neuroendocrine and Immune System Pathology in Psychiatric Disease. In: Schatzberg AF, Nemeroff CB, editors. Textbook of Psychopharmacology. 2. Washington, DC: American Psychiatric Press, Inc.; 1998. pp. 171–175. [Google Scholar]
- 13.Bierer LM, Tischler L, Labinsky E, Cahill S, Foa E, Yehuda R. Clinical correlates of 24-h cortisol and norepinephrine excretion among subjects seeking treatment following the world trade center attacks on 9/11. Ann N Y Acad Sci. 2006;1071:514–520. doi: 10.1196/annals.1364.055. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MG, Resick PA, Yehuda R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. Am J Psychiatry. 2005;162:1192–1199. doi: 10.1176/appi.ajp.162.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Yehuda R. Advances in Understanding Neuroendocrine Alterations in PTSD and Their Therapeutic Implications. Ann N Y Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 17.Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/s0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 19.Yehuda R, Yang RK, Buchsbaum MS, Golier JA. Alterations in cortisol negative feedback inhibition as examined using the ACTH response to cortisol administration in PTSD. Psychoneuroendocrinology. 2006;31:447–451. doi: 10.1016/j.psyneuen.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Inslicht SS, Marmar CR, Neylan TC, Metzler TJ, Hart SL, Otte C, et al. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:428–429. doi: 10.1196/annals.1364.035. [DOI] [PubMed] [Google Scholar]
- 21.Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, et al. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry. 2006;60:275–281. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Peeters F, Nicolson NA, Berkhof J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Res. 2004;126:1–13. doi: 10.1016/j.psychres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Young EA, Tolman R, Witkowski K, Kaplan G. Salivary cortisol and posttraumatic stress disorder in a low-income community sample of women. Biol Psychiatry. 2004;55:621–626. doi: 10.1016/j.biopsych.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154:1497–1503. doi: 10.1176/ajp.154.11.1497. [DOI] [PubMed] [Google Scholar]
- 25.Baghai TC, Schule C, Zwanzger P, Minov C, Zill P, Ella R, et al. Hypothalamic-pituitary-adrenocortical axis dysregulation in patients with major depression is influenced by the insertion/deletion polymorphism in the angiotensin I-converting enzyme gene. Neurosci Lett. 2002;328:299–303. doi: 10.1016/s0304-3940(02)00527-x. [DOI] [PubMed] [Google Scholar]
- 26.Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- 27.Yehuda R, Golier J, Wong C, Grossman R. Relationship among stessful life events in childhood vs. adulthood, HPA axis parameters, and Depression vs. PTSD (185) American College of Neuropsychopharmacology Scientific Abstracts. 2001:309. [Google Scholar]
- 28.Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depress Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- 29.Hellhammer J, Schlotz W, Stone AA, Pirke KM, Hellhammer D. Allostatic load, perceived stress, and health: a prospective study in two age groups. Ann N Y Acad Sci. 2004;1032:8–13. doi: 10.1196/annals.1314.002. [DOI] [PubMed] [Google Scholar]
- 30.Kusnecov AW, Goldfarb Y. Neural and behavioral responses to systemic immunologic stimuli: a consideration of bacterial T cell superantigens. Curr Pharm Des. 2005;11:1039–1046. doi: 10.2174/1381612053381602. [DOI] [PubMed] [Google Scholar]
- 31.Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, et al. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 33.Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 34.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’-A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiol. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 35.Jezova D, Makatsori A, Duncko R, Moncek F, Jakubek M. High trait anxiety in healthy subjects is associated with low neuroendocrine activity during psychosocial stress. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1331–1336. doi: 10.1016/j.pnpbp.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Tyrka AR, Mello AF, Mello MF, Gagne GG, Grover KE, Anderson GM, et al. Temperament and hypothalamic-pituitary-adrenal axis function in healthy adults. Psychoneuroendocrinology. 2006;31:1036–1045. doi: 10.1016/j.psyneuen.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-Adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 38.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 39.Santa Ana EJ, Saladin ME, Back SE, Waldrop AE, Spratt EG, McRae AL, et al. PTSD and the HPA axis: differences in response to the cold pressor task among individuals with child vs. adult trauma. Psychoneuroendocrinology. 2006;31:501–509. doi: 10.1016/j.psyneuen.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Baghai TC, Schule C, Zwanzger P, Minov C, Holme C, Padberg F, et al. Evaluation of a salivary based combined dexamethasone/CRH test in patients with major depression. Psychoneuroendocrinology. 2002;27:385–399. doi: 10.1016/s0306-4530(01)00060-9. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psych. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein DP, Fink LA. Childhood Trauma Questionnaire (CTQ) 1995. [Google Scholar]
- 43.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-V (SCID) Washington, D.C.: 1997. [DOI] [PubMed] [Google Scholar]
- 44.Cloninger CR, Przybeck TR, Svrakic DM. The Tridimensional Personality Questionnaire: U.S. normative data. Psychol Rep. 1991;69:1047–1057. doi: 10.2466/pr0.1991.69.3.1047. [DOI] [PubMed] [Google Scholar]
- 45.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 46.Spielberger CD. State-Trait Anxiety Inventory (Form Y) 1983. [Google Scholar]
- 47.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 48.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 49.Norcross JC, Guadagnoli E, Prochaska JO. Factor structure of the Profile of Mood States (POMS): two partial replications. J Clin Psychol. 1984;40:1270–1277. doi: 10.1002/1097-4679(198409)40:5<1270::aid-jclp2270400526>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 50.Wilkinson CW, Raff H. Comparative evaluation of a new immunoradiometric assay for corticotropin. Clin Chem Lab Med. 2006;44:669–671. doi: 10.1515/CCLM.2006.113. [DOI] [PubMed] [Google Scholar]
- 51.Sher L, Oquendo MA, Galfalvy HC, Cooper TB, Mann JJ. Age effects on cortisol levels in depressed patients with and without comorbid post-traumatic stress disorder, and healthy volunteers. J Affect Disord. 2004;82:53–59. doi: 10.1016/j.jad.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 52.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 53.McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: biological and subjective responses to the cold pressor and Trier Social stressors. Hum Psychopharmacol. 2006;21:377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- 54.Saltzman W, Hogan BK, Abbott DH. Diminished cortisol levels in subordinate female marmosets are associated with altered central drive to the hypothalamic-pituitary-adrenal axis. Biol Psychiatry. 2006;60:843–849. doi: 10.1016/j.biopsych.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Saltzman W, Prudom SL, Schultz-Darken NJ, Abbott DH. Reduced adrenocortical responsiveness to adrenocorticotropic hormone (ACTH) in socially subordinate female marmoset monkeys. Psychoneuroendocrinology. 2000;25:463–477. doi: 10.1016/s0306-4530(00)00003-2. [DOI] [PubMed] [Google Scholar]
- 56.Pohorecky LA, Baumann MH, Benjamin D. Effects of chronic social stress on neuroendocrine responsiveness to challenge with ethanol, dexamethasone and corticotropin-releasing hormone. Neuroendocrinology. 2004;80:332–342. doi: 10.1159/000083682. [DOI] [PubMed] [Google Scholar]
- 57.Ladd CO, Thrivikraman KV, Huot RL, Plotsky PM. Differential neuroendocrine responses to chronic variable stress in adult Long Evans rats exposed to handling-maternal separation as neonates. Psychoneuroendocrinology. 2005;30:520–533. doi: 10.1016/j.psyneuen.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP. Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology. 2006;147:2008–2017. doi: 10.1210/en.2005-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology. 2005;30:162–178. doi: 10.1016/j.psyneuen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- 61.Weissbecker I, Floyd A, Dedert E, Salmon P, Sephton S. Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology. 2006;31:312–324. doi: 10.1016/j.psyneuen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Derijk RH, Wust S, Meijer OC, Zennaro MC, Federenko IS, Hellhammer DH, et al. A common polymorphism in the mineralocorticoid receptor modulates stress responsiveness. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-0915. [DOI] [PubMed] [Google Scholar]
- 64.Bale TL. Stress sensitivity and the development of affective disorders. Horm Behav. 2006;50:529–533. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 65.Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109:504–511. [PubMed] [Google Scholar]
- 66.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 67.Jacobs N, Kenis G, Peeters F, Derom C, Vlietinck R, van Os J. Stress-related negative affectivity and genetically altered serotonin transporter function: evidence of synergism in shaping risk of depression. Arch Gen Psychiatry. 2006;63:989–996. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- 68.Tochigi M, Kato C, Otowa T, Hibino H, Marui T, Ohtani T, et al. Association between corticotropin-releasing hormone receptor 2 (CRHR2) gene polymorphism and personality traits. Psychiatry Clin Neurosci. 2006;60:524–526. doi: 10.1111/j.1440-1819.2006.01541.x. [DOI] [PubMed] [Google Scholar]
- 69.Catley D, Kaell AT, Kirschbaum C, Stone AA. A naturalistic evaluation of cortisol secretion in persons with fibromyalgia and rheumatoid arthritis. Arthritis Care Res. 2000;13:51–61. doi: 10.1002/1529-0131(200002)13:1<51::aid-art8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 70.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 71.al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- 72.Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- 73.Pfennig A, Kunzel HE, Kern N, Ising M, Majer M, Fuchs B, et al. Hypothalamus-pituitary-adrenal system regulation and suicidal behavior in depression. Biol Psychiatry. 2005;57:336–342. doi: 10.1016/j.biopsych.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 74.Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, et al. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A. 2006;103:5585–5590. doi: 10.1073/pnas.0509184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Het S, Wolf OT. Mood changes in response to psychosocial stress in healthy young women: effects of pretreatment with cortisol. Behav Neurosci. 2007;121:11–20. doi: 10.1037/0735-7044.121.1.11. [DOI] [PubMed] [Google Scholar]
- 77.Roelofs K, Bakvis P, Hermans EJ, van Pelt J, van Honk J. The effects of social stress and cortisol responses on the preconscious selective attention to social threat. Biol Psychol. 2006 doi: 10.1016/j.biopsycho.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Roelofs K, Elzinga BM. The effects of social stress and stress-induced cortisol on the selective attention to social threat cues. 36th Annual Conference International Society of Psychoneuroendocrinology; Montreal, Quebec, Canada. 2005. [Google Scholar]
- 79.Roelofs K, Elzinga BM, Rotteveel M. The effects of stress-induced cortisol responses on approach-avoidance behavior. Psychoneuroendocrinology. 2005;30:665–677. doi: 10.1016/j.psyneuen.2005.02.008. [DOI] [PubMed] [Google Scholar]