Abstract
This study was performed to determine the effectiveness of the Rho kinase inhibitor and NF-κB inhibitor in renal injury of ANG II-infused hypertensive rats. Male Sprague-Dawley rats, maintained on a normal diet, received either a sham operation (n = 7) or continuous ANG II infusion (120 ng/min) subcutaneously via minipumps. The ANG II-infused rats were further subdivided into three subgroups (n = 7 each) to receive one of the following treatments during the entire period: vehicle, Rho kinase inhibitor (fasudil; 3 mg·kg−1 ·day−1 ip), or NF-κB inhibitor (parthenolide; 1 mg·kg−1 ·day−1 ip). After 12 days of ANG II infusion, systolic blood pressure (BP; 208 ± 7 vs. 136 ± 3 mmHg), Rho kinase activity, NF-κB activity, renal ANG II contents (160 ± 25 vs. 84 ± 14 pg/g), monocytic chemotactic protein (MCP) 1 mRNA, interstitial macrophage infiltration, transforming growth factor-β1 (TGF-β1) mRNA, interstitial collagen-positive area, urinary protein excretion (43 ± 6 vs. 11 ± 2 mg/day), and urinary albumin excretion were significantly enhanced compared with the Sham group. While fasudil or parthenolide did not alter systolic BP (222 ± and 190 ± 21, respectively), both treatments completely blocked ANG II-induced enhancement of NF-κB activity, renal ANG II contents (103 ± 11 and 116 ± 21 pg/g, respectively), MCP1 mRNA, interstitial macrophage infiltration, TGF-β1 mRNA, interstitial collagen-positive area, urinary protein excretion (28 ± 6 and 23 ± 3 mg/day, respectively), and urinary albumin excretion. Importantly, parthenolide did not alter ANG II-induced Rho kinase activation although fasudil abolished ANG II-induced Rho kinase activation. These data indicate that the Rho-NF-κB axis plays crucial roles in the development of ANG II-induced renal injury independently from BP regulation.
Keywords: hypertension
Recent findings related to the intrarenal renin-angiotensin system (RAS), which is one of the most important regulatory mechanisms for blood pressure (BP) homeostasis, have provided us with an improved understanding of the pathophysiology of hypertension (22, 50-52, 71). However, these findings have also led to unique concepts and questions that need to be investigated in more depth. More detailed investigations can now be performed to characterize the mechanisms responsible for these alterations which lead to the development of hypertension and, either directly or indirectly, to progressive renal damage.
Increased peripheral vascular resistance plays an important role in the pathogenesis of hypertension. Recent studies report that ANG II and a small GTPase, Rho, are involved in these mechanisms (47, 67, 79). Rho kinase is activated by Rho, and it induces vascular contractions via two mechanisms. First, Rho kinase phosphorylates myosin light chain, which causes vascular smooth muscle cell contraction. Second, Rho kinase inhibits myosin phosphatase activity, and this inhibits dephosphorylation of myosin light chain, which sustains vascular smooth muscle cell contraction. A Rho kinase inhibitor is now clinically developed and used for the treatment of brain ischemia as a vasodilator and is being evaluated for the treatment of angina pectoris. In signaling pathways of ANG II, Rho kinase has a potent role to regulate effects of ANG II. Yamakawa et al. (79) demonstrated that ANG II-induced hypertrophy is regulated by the Rho kinase pathway in rat aortic smooth muscle cells. In skeletal dynamics, Shome et al. (67) demonstrated that small GTPases modulate the ANG II-induced activation of phospholipase D in cultured vascular smooth muscle cells. In renal microvasculature, Nakamura et al. (47) demonstrated that the Rho kinase pathway mediates the basal tone and its inhibitor diminishes ANG II-induced vasoconstriction in a hydronephrotic kidney model. It is suggested that Rho kinase can be one of the important modulators in ANG II-dependent hypertension.
Although many kinds of transcriptional factors are involved in the ANG II signaling pathway, NF-κB has pivotal roles in the ANG II-induced inflammatous change (43, 58). Muller et al. (43) demonstrated that inhibition of NF-κB ameliorates ANG II-induced cardiac and renal damages. Ruiz-Ortega et al. (58) demonstrated that NF-κB is activated by ANG II in rat thoracic aorta vascular smooth muscle cells. Interestingly, Perona et al. (55) demonstrated that Rho induces the transcriptional activity of NF-κB by stimulating the phosphorylation of the IκBα in Ser-32 and Ser-36 residues. Thus the Rho-NF-κB pathway may have the important role in ANG II-induced inflammatous change in ANG II-dependent hypertensive nephropathy.
We have recently reported that NF-κB-dependent upregulation of monocytic chemotactic protein (MCP) 1 and transforming growth factor-β1 (TGF-β1) plays an important role in the development of renal injury in ANG II-dependent hypertension (54). However, it has not been established whether a Rho kinase inhibitor and/or NF-κB inhibitor exerts renoprotective effects in ANG II-dependent hypertension. Therefore, this study was performed to determine the effectiveness of the Rho kinase inhibitor fasudil and the NF-κB inhibitor parthenolide in renal injury of ANG II-infused hypertensive rats.
MATERIALS AND METHODS
Preparation of animals
The experimental protocol was approved by the Animal Care and Use Committees of Tulane University. Male Sprague-Dawley rats (∼250 g, Charles River), maintained on a normal diet, received either a sham operation (n = 7) or continuous ANG II infusion (120 ng/min) subcutaneously via minipumps (Alzet). The ANG II-infused rats were further subdivided into three subgroups (n = 7 each) to receive one of the following treatments during the entire period: vehicle, Rho kinase inhibitor (fasudil; 3 mg·kg−1 ·day−1 ip, Asahi Kasei), or NF-κB inhibitor (parthenolide; 1 mg·kg−1 ·day−1 ip, Biomol). All rats were monitored up to 12 days of ANG II infusion with free access to a regular diet and water. Systolic BP was measured in conscious rats using tail-cuff plethysmography (Visitech) every 3 days as previously described (28-30, 33, 36). Twenty-four-hour urine samples were collected the day before the tissue harvesting, and the protein concentration and albumin concentration in urine samples were measured as previously described (28-30, 33, 36).
Sample collection
Kidney samples were harvested by decapitation after 12 days of ANG II infusion. Immediately after removal, one kidney was homogenized in cold methanol and renal ANG II was measured as previously described (28-30, 33, 36).
The contralateral kidneys were separated into four pieces. The first piece was immersed in RNAlater (Ambion) for total RNA extraction. The second piece was immersed in zinc-saturated formalin (Anatech) for tissue fixation. The third piece and the last piece were immersed in liquid nitrogen in Cryotubes (Nalgene) for protein extraction and nuclear protein extraction, respectively.
Quantitative real-time RT-PCR
Total RNA extraction from rat kidneys and quantitative real-time RT-PCR for RelA, MCP1, and TGF-β1 mRNA were performed as previously described (34, 44, 54, 56, 57). Data of quantitative real-time RT-PCR were normalized by GAPDH mRNA expression. The sequence information of the primers and the probes for real-time RT-PCR are summarized in Table 1.
Table 1.
Sequence information for primers and probes for quantitative real-time PCR
| Gene | Sense Primer | Antisense Primer | Probe |
|---|---|---|---|
| GAPDH | CAGAACATCATCCCTGCATC | CTGCTTCACCACCTTCTTGA | CCTGGAGAAACCTGCCAAGTATGATGA |
| RelA | CATCAAGATCAATGGCTACA | CACAAGTTCATGTGGATGAG | AACAGTTCGAATCTCCCTGGTCAC |
| MCP1 | AGCACCTTTGAATGTGAACT | AGAAGTGCTTGAGGTGGTT | CCCATAAATCTGAAGCTAATGCATCC |
| TGF-β1 | TACCATGCCAACTTCTGTC | AAGGACCTTGCTGTACTGTGT | CCCTACATTTGGAGCCTGGAC |
MCP, monocyte chemotactic protein; TGF, transforming growth factor.
Interstitial macrophage/monocyte infiltration
Using zinc-saturated formalin-fixed paraffin-embedded renal sections, the numbers of macrophages/monocytes were examined by immunohistochemistry with a commercially available antibody against CD68 (Serotec) as previously described (26, 34, 54). Immunohistochemistry was performed by a robotic system (Dako) as previously described (34, 36, 54, 56) and counterstained with hematoxylin-eosin. Twenty consecutive microscopic fields were examined for each rat, and CD68-positive cells (brown) were counted in the interstitium in each microscopic field. The averaged numbers of macrophages/monocytes in the interstitium were then obtained for each rat.
Interstitial collagen-positive area
Using zinc-saturated, formalinfixed, paraffin-embedded renal sections, the extent of interstitial collagen-positive areas was quantitatively evaluated by an automatic image analysis occupied by interstitial tissue staining positively for collagen in picro-Sirius red-stained sections (Mass Histology) as previously described (15, 34, 54). The fraction of renal cortex occupied by interstitial tissue was performed using Image-Pro plus software (Media Cybernetics). For each microscopic field, the collagen-positive area (pink) was automatically calculated by the software, and this affected area was, in turn, divided by the total area of the microscopic field. Twenty consecutive microscopic fields were examined for each rat, and the averaged percentages of the collagen-positive lesions were obtained for each rat.
The above-mentioned histological analyses were performed by an outsourcing company (Mass Histology) or a robotic system (Dako) with automatic image-analysis software (Media Cybernetics) in a blind manner to avoid any biases.
Rho kinase activity assay
Protein extraction, sample purification, and Rho kinase activity assay were performed with rat kidneys using a commercially available kit (CycLex) according to the manufacturer's instructions.
Electromobility shift assay
Nuclear protein extraction and electromobility shift assay for NF-κB were done with rat kidneys using a commercially available kit (Panomics) according to the manufacturer's instructions as previously described (31, 54).
Western blot analysis
Protein extraction and Western blot analysis were performed with rat kidneys as previously described (32, 34-36, 54, 70) using an infrared imaging system (LI-COR Biosciences). Polyclonal primary antibodies against phosphorylated IκBα, phosphorylated IκBKα, and phosphorylated IκBKβ were purchased from Cell Signaling Technology. Polyclonal primary antibody against phosphorylated NF-κB-inducing kinase was purchased from Santa Cruz Biotechnology. Polyclonal primary antibody against β-actin was purchased from Abcam. Appropriate secondary antibodies were purchased from LI-COR Biosciences.
Statistical analysis
Statistical analysis was performed using a one-way factorial ANOVA with post hoc Scheffé's F-test. All data are presented as means ± SE. P < 0.05 was considered significant.
RESULTS
Body weight
Body weight was similar among the four groups before the treatments. As previously described, chronic ANG II infusion in rats significantly suppressed the increase in body weight may be due to increased peripheral metabolism that is independent of elevations in BP (18). However, fasudil or parthenolide treatment did not show any additional effect on body weight in ANG II-infused rats (Table 2).
Table 2.
Body weight of different groups at day 12
Ri, Rho kinase inhibitor; Ni, NF-κB inhibition.
P < 0.05 compared with Sham group.
Systolic BP
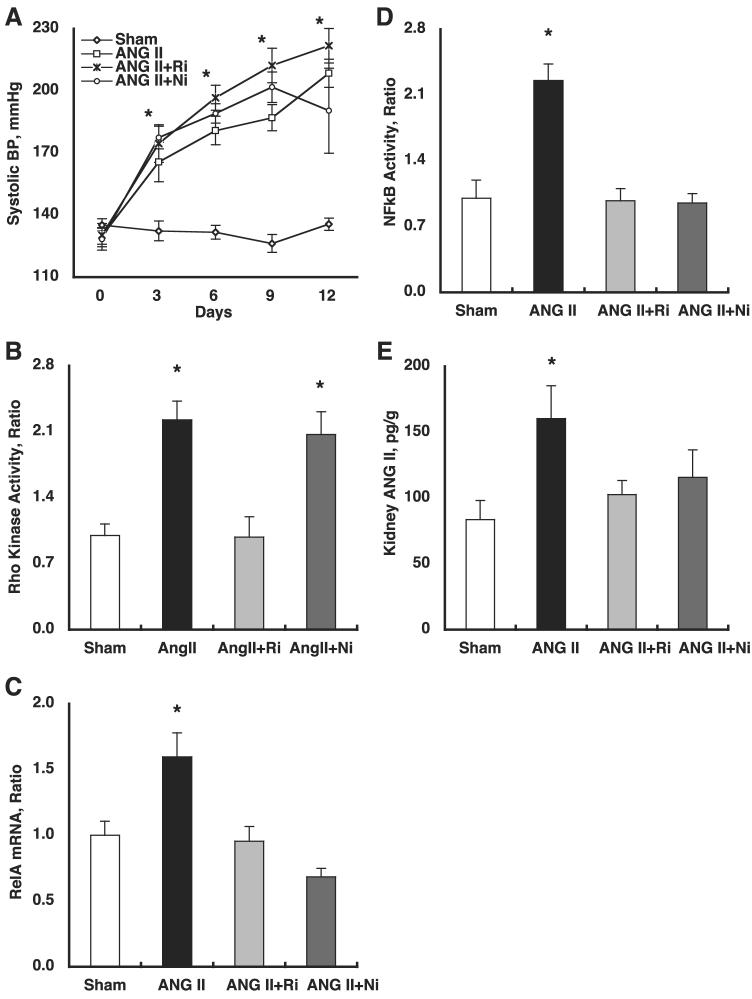
Systolic BP (Fig. 1A) was similar among the four groups before the treatments. However, systolic BP progressively and significantly increased (208 ± 7 for ANG II vs. 136 ± 3 mmHg for Sham at day 12). Fasudil or parthenolide did not alter systolic BP (222 ± 8 and 190 ± 21 at day 12, respectively).
Fig. 1.
A: temporal profile of systolic blood pressure (BP). Systolic BP was similar among the 4 groups before the treatments. However, systolic BP progressively and significantly increased (208 ± 7 for ANG II vs. 136 ± 3 mmHg for Sham at day 12). Fasudil (Ri) or parthenolide (Ni) did not alter systolic BP (222 ± 8 and 190 ± 21 mmHg at day 12, respectively). *P < 0.05 compared with the corresponding Sham group at that time period and P < 0.05 compared with the corresponding group at day 0. B: chronic ANG II infusion significantly increased Rho kinase activity (2.23 ± 0.20 for ANG II vs. 1.00 ± 0.12 arbitrary units for Sham). Importantly, while fasudil abolished ANG II-induced Rho kinase activation, parthenolide did not alter ANG II-induced Rho kinase activation (0.98 ± 0.22 for ANG II+fasudil and 2.07 ± 0.25 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group. C: for evaluation of NF-κB expression, mRNA levels of RelA (p65), a part of the NF-κB complex, were measured by real-time-PCR. Chronic ANG II infusion significantly increased RelA mRNA levels (1.60 ± 0.18 for ANG II vs. 1.00 ± 0.11 arbitrary units for Sham). Both treatments completely blocked ANG II-induced enhancement of RelA mRNA levels (0.95 ± 0.11 for ANG II+fasudil and 0.68 ± 0.06 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group. D: chronic ANG II infusion significantly increased NF-κB activity (2.25 ± 0.18 for ANG II vs. 1.00 ± 0.19 arbitrary units for Sham). Both treatments completely blocked ANG II-induced enhancement of NF-κB activity (0.98 ± 0.13 for ANG II+fasudil and 0.95 ± 0.10 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group. E: chronic ANG II infusion significantly increased kidney ANG II levels (160 ± 25 for ANG II vs. 84 ± 14 pg/g for Sham). Both treatments completely blocked ANG II-induced enhancement of kidney ANG II levels (103 ± 11 for ANG II+fasudil and 116 ± 21 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group.
Rho kinase activity
As shown in Fig. 1B, chronic ANG II infusion significantly increased Rho kinase activity (2.23 ± 0.20 for ANG II vs. 1.00 ± 0.12 arbitrary units for Sham). Importantly, while fasudil abolished ANG II-induced Rho kinase activation, parthenolide did not alter ANG II-induced Rho kinase activation (0.98 ± 0.22 for ANG II+fasudil and 2.07 ± 0.25 arbitrary units for ANG II+parthenolide, respectively).
RelA mRNA
For the evaluation of NF-κB expression, mRNA levels of RelA (p65), a part of the NF-κB complex, were measured by real-time RT-PCR. As shown in Fig. 1C, chronic ANG II infusion significantly increased RelA mRNA levels (1.60 ± 0.18 for ANG II vs. 1.00 ± 0.11 arbitrary units for Sham). Both treatments completely blocked ANG II-induced enhancement of RelA mRNA levels (0.95 ± 0.11 for ANG II+fasudil and 0.68 ± 0.06 arbitrary units for ANG II+parthenolide, respectively).
Electromobility shift assay
NF-κB activity was evaluated by electromobility shift assay. As shown in Fig. 1D, chronic ANG II infusion significantly increased NF-κB activity (2.25 ± 0.18 for ANG II vs. 1.00 ± 0.19 arbitrary units for Sham). Both treatments completely blocked ANG II-induced enhancement of NF-κB activity (0.98 ± 0.13 for ANG II+fasudil and 0.95 ± 0.10 arbitrary units for ANG II+parthenolide, respectively).
Kidney ANG II levels
As demonstrated in Fig. 1E, chronic ANG II infusion significantly increased kidney ANG II levels (160 ± 25 for ANG II vs. 84 ± 14 pg/g for Sham). Both treatments completely blocked ANG II-induced enhancement of kidney ANG II levels (103 ± 11 for ANG II+fasudil and 116 ± 21 pg/g for ANG II+parthenolide, respectively).
MCP1 mRNA
As demonstrated in Fig. 2A, chronic ANG II infusion significantly increased intrarenal MCP1 mRNA levels (1.55 ± 0.15 for ANG II vs. 1.00 ± 0.13 arbitrary units for Sham). Both treatments completely blocked ANG II-induced enhancement of intrarenal MCP1 mRNA levels (1.16 ± 0.13 for ANG II+fasudil and 0.90 ± 0.05 arbitrary units for ANG II+parthenolide, respectively).
Fig. 2.
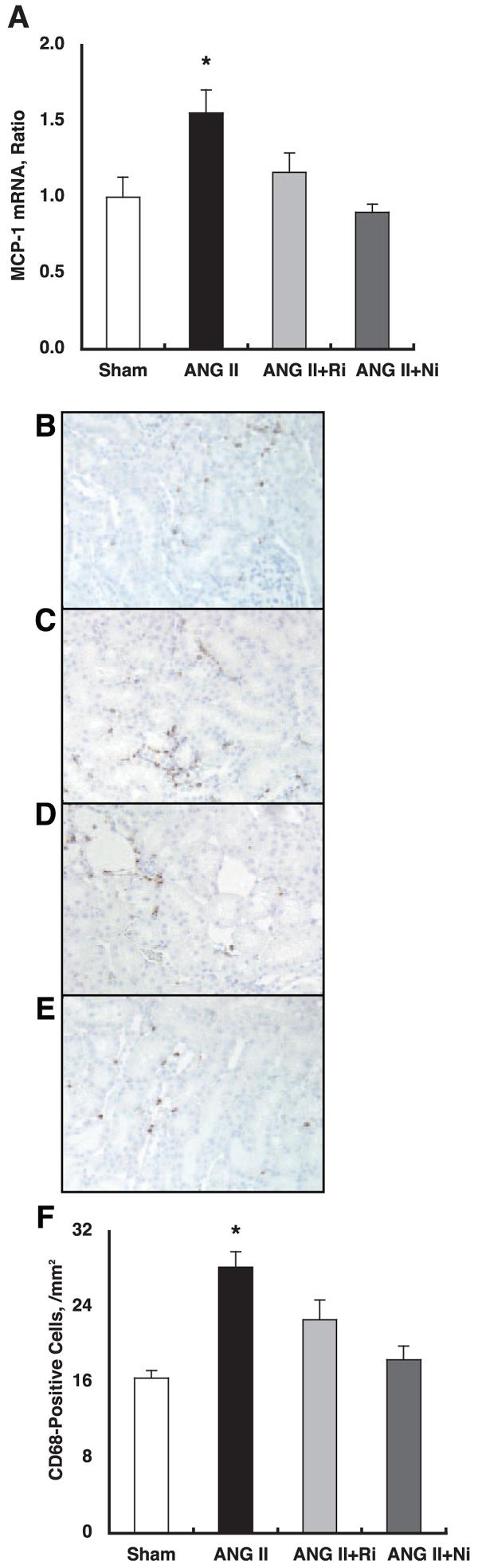
A: chronic ANG II infusion significantly increased intrarenal MCP1 mRNA levels (1.55 ± 0.15 for ANG II vs. 1.00 ± 0.13 arbitrary units for Sham). Both treatments completely blocked ANG II-induced enhancement of intrarenal MCP1 mRNA levels (1.16 ± 0.13 for ANG II+fasudil and 0.90 ± 0.05 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group. B–E: interstitial macrophage/monocyte infiltration was evaluated by CD68-positive cell number, which is a surface marker for macrophages and monocytes, using zinc-saturated, formalin-fixed, paraffin-embedded kidney samples from Sham (B), ANG II (C), ANG II+fasudil (D), and ANG II+parthenolide (E) groups. CD68-positive cells are stained brown. F: CD68-positive cell numbers were significantly increased by chronic ANG II infusion (28 ± 2 cells/mm2) compared with Sham (16 ± 1). Both treatments completely blocked ANG II-induced enhancement of interstitial macrophage/monocyte infiltration (23 ± 2 for ANG II+fasudil and 18 ± 1 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group.
Interstitial macrophage/monocyte infiltration
The interstitial macrophage/monocyte infiltration was evaluated by CD68-positive cell number, which is a surface marker for macrophages and monocytes, using zinc-saturated, formalin-fixed, paraffin-embedded kidney samples from the Sham (Fig. 2B), ANG II (Fig. 2C), ANG II+fasudil (Fig. 2D), and ANG II+parthenolide (Fig. 2E) groups. CD68-positive cells are stained brown. Figure 2F demonstrated that CD68-positive cell numbers were significantly increased by chronic ANG II infusion (28 ± 2 cells/mm2) compared with Sham (16 ± 1 cells/mm2). Both treatments completely blocked ANG II-induced enhancement of interstitial macrophage/monocyte infiltration (23 ± 2 for ANG II+fasudil and 18 ± 1 for ANG II+parthenolide, respectively).
TGF-β1 mRNA
As demonstrated in Fig. 3A, chronic ANG II infusion significantly increased intrarenal TGF-β1 mRNA levels (1.52 1 ± 0.16 for ANG II vs. 1.00 ± 0.08 arbitrary units for Sham). Both treatments completely blocked ANG II-induced enhancement of intrarenal TGF-β1 mRNA levels (0.95 ± 0.12 for ANG II+fasudil and 0.74 ± 0.07 arbitrary units for ANG II+parthenolide, respectively).
Fig. 3.
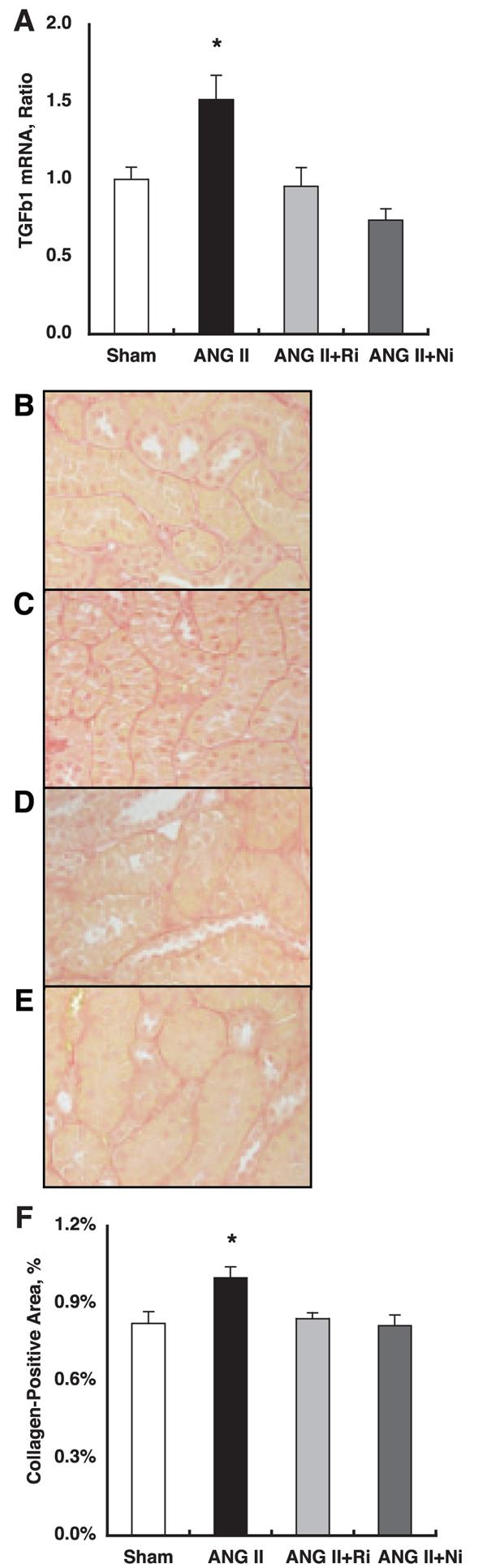
A: chronic ANG II infusion significantly increased intrarenal transforming growth factor (TGF)-β1 mRNA levels (1.52 ± 0.16 for ANG II vs. 1.00 ± 0.08 arbitrary units for Sham). Both treatments completely blocked ANG II-induced enhancement of intrarenal TGF-β1 mRNA levels (0.95 ± 0.12 for ANG II+fasudil and 0.74 ± 0.07 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group. B–E: interstitial collagen-positive area was stained by Picro-sirius red using zinc-saturated, formalinfixed, paraffin-embedded kidney samples from Sham (B), ANG II (C), ANG II+fasudil (D), and ANG II+parthenolide (E) groups. Collagen-positive area is stained pink. F: an established computer-aided semiautomatic quantification system demonstrates that the interstitial collagen-positive area was significantly increased by chronic ANG II infusion (1.00 ± 0.04%) compared with Sham (0.82 ± 0.05). Both treatments completely blocked ANG II-induced enhancement of interstitial collagen-positive area (0.84 ± 0.02 for ANG II+fasudil and 0.81 ± 0.04 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group.
Interstitial collagen-positive area
The interstitial collagen-positive area was stained by Picro-sirius red using zinc-saturated, formalin-fixed, paraffin-embedded kidney samples from Sham (Fig. 3B), ANG II (Fig. 3C), ANG II+fasudil (Fig. 3D), and ANG II±parthenolide (Fig. 3E) groups. The collagen-positive area is stained pink. With the use of an established computer-aided semiautomatic quantification system, Fig. 3F demonstrated that the interstitial collagen-positive area was significantly increased by chronic ANG II infusion (1.00 ± 0.04%) compared with Sham (0.82 ± 0.05). Both treatments completely blocked ANG II-induced enhancement of the interstitial collagen-positive area (0.84 ± 0.02 for ANG II+fasudil and 0.81 ± 0.04 for ANG II+parthenolide, respectively).
Urinary protein excretion
As demonstrated in Fig. 4A, chronic ANG II infusion significantly increased urinary protein excretion (43 ± 6 for ANG II vs. 11 ± 2 mg/day for Sham). Both treatments completely blocked ANG II-induced enhancement of urinary protein excretion (28 ± 6 for ANG II+fasudil and 23 ± 3 mg/day for ANG II+parthenolide, respectively).
Fig. 4.
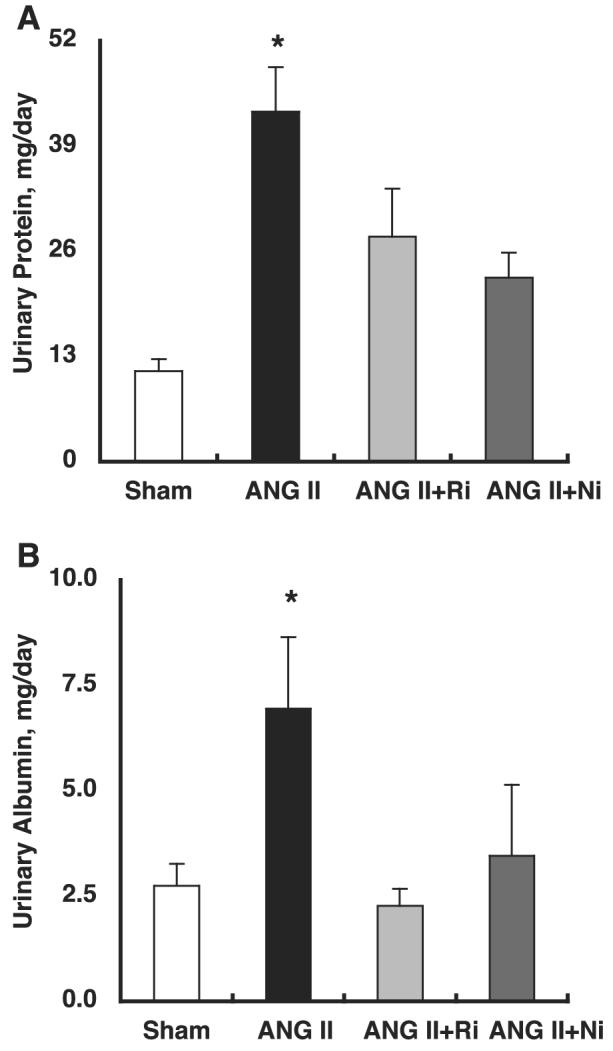
A: chronic ANG II infusion significantly increased urinary protein excretion (43 ± 6 for ANG II vs. 11 ± 2 mg/day for Sham). Both treatments completely blocked ANG II-induced enhancement of urinary protein excretion (28 ± 6 for ANG II+fasudil and 23 ± 3 for ANG II+parthenolide, respectively). B: chronic ANG II infusion significantly increased urinary albumin excretion (6.9 ± 1.7 for ANG II vs. 2.7 ± 0.5 mg/day for Sham). Both treatments completely blocked ANG II-induced enhancement of urinary albumin excretion (2.3 ± 0.4 for ANG II+fasudil and 3.5 ± 1.7 for ANG II+parthenolide, respectively). *P < 0.05 compared with the Sham group.
Urinary albumin excretion
As demonstrated in Fig. 4B, chronic ANG II infusion significantly increased urinary albumin excretion (6.9 ± 1.7 for ANG II vs. 2.7 ± 0.5 mg/day for Sham). Both treatments completely blocked ANG II-induced enhancement of urinary albumin excretion (2.3 ± 0.4 for ANG II+fasudil and 3.5 ± 1.7 mg/day for ANG II+parthenolide, respectively).
Western blot analysis
As demonstrated in Fig. 5A, chronic ANG II infusion significantly increased phosphorylation of IκBα (1.49 ± 0.09 for ANG II vs. 1.00 ± 0.07 arbitrary units for Sham). Both treatments completely blocked ANG II-induced phosphorylation of IκBα (1.03 ± 0.02 for ANG II+ fasudil and 1.05 ± 0.07 arbitrary units for ANG II+ parthenolide, respectively). As demonstrated in Fig. 5B (85 kDa), chronic ANG II infusion did not change phosphorylation of IκBKα (0.99 ± 0.07 for ANG II vs. 1.00 ± 0.09 arbitrary units for Sham). Either treatment did not alter phosphorylation of IκBKα (1.00 ± 0.05 for ANG II+fasudil and 1.08 ± 0.07 arbitrary units for ANG II+parthenolide, respectively). As demonstrated in Fig. 5B (87-kDa), chronic ANG II infusion did not change phosphorylation of IκBKβ (0.94 ± 0.05 for ANG II vs. 1.00 ± 0.07 arbitrary units for Sham). Either treatment did not alter phosphorylation of IκBKβ (0.93 ± 0.09 for ANG II+fasudil and 1.00 ± 0.07 arbitrary units for ANG II+parthenolide, respectively). As demonstrated in Fig. 5C, chronic ANG II infusion did not change phosphorylation of NF-κB-inducing kinase (0.90 ± 0.05 for ANG II vs. 1.00 ± 0.07 arbitrary units for Sham). Either treatment did not alter phosphorylation of NF-κB-inducing kinase (0.89 ± 0.07 for ANG II+fasudil and 0.94 ± 0.07 for ANG II+parthenolide, respectively). To verify equal loading, membranes were reprobed with β-actin antibody. As demonstrated in Fig. 5D, similar densities were observed among the four groups.
Fig. 5.
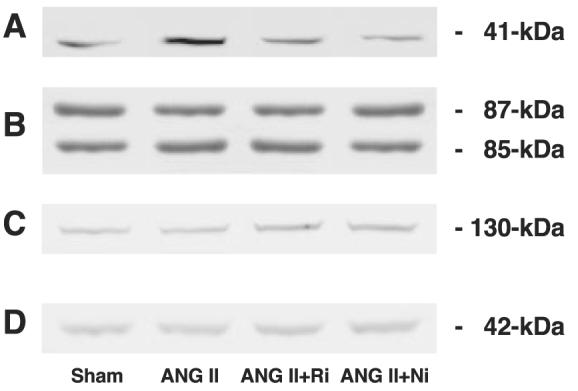
Representative Western blot analysis. To elucidate at which level ANG II-induced Rho activation stimulates the NF-κB pathway, related kinase activities were evaluated by Western blot analysis. Chronic ANG II infusion significantly increased phosphorylation of IκBα (A; 1.49 ± 0.09 for ANG II vs. 1.00 ± 0.07 arbitrary units for Sham). Both treatments completely blocked ANG II-induced phosphorylation of IκBα (1.03 ± 0.02 for ANG II+fasudil and 1.05 ± 0.07 for ANG II+parthenolide, respectively). Chronic ANG II infusion did not change phosphorylation of IκBKα (B; 85 kDa, 0.99 ± 0.07 for ANG II vs. 1.00 ± 0.09 arbitrary units for Sham). Either treatment did not alter phosphorylation of IκBKα (1.00 ± 0.05 for ANG II+fasudil and 1.08 ± 0.07 for ANG II+parthenolide, respectively). Chronic ANG II infusion did not change phosphorylation of IκBKβ (B; 87 kDa, 0.94 ± 0.05 for ANG II vs. 1.00 ± 0.07 arbitrary units for Sham). Either treatment did not alter phosphor-ylation of IκBKβ (0.93 ± 0.09 for ANG II+fasudil and 1.00 ± 0.07 for ANG II+parthenolide, respectively). Chronic ANG II infusion did not change phosphorylation of NF-κB-inducing kinase (C; 0.90 ± 0.05 for ANG II vs. 1.00 ± 0.07 arbitrary units for Sham). Either treatment did not alter phosphor-ylation of NF-κB-inducing kinase (0.89 ± 0.07 for ANG II+fasudil and 0.94 ± 0.07 for ANG II+parthenolide, respectively). To verify equal loading, membranes were reprobed with β-actin antibody. Similar densities were observed among the 4 groups (D). These data clearly indicate that ANG II-induced Rho activation stimulates NF-κB pathway at phosphorylation of IκBα levels and that this mechanism is independent of IκBKα, IκBKβ, and NF-κB-inducing kinase.
DISCUSSION
A small GTPase, Rho, and its downstream effector molecule, Rho kinase, play an important role in various cellular functions (49). Rho/Rho kinase inhibits myosin phosphatase by phosphorylating its myosin-binding subunit, favoring accumulation of phosphorylated myosin light chain and enhanced contraction of vascular smooth muscle cell (46). It has been established that Rho/Rho kinase participates in cell adhesion/ migration and proliferation (1, 64) and is involved in various models of cardiovascular disorders, independently of systemic BP (66). Thus the inhibition of Rho kinase is reported to suppress the neointimal formation of balloon-injured rat carotid arteries (63) and attenuate the formation of the coronary injury induced by chronic treatment with MCP1 and/or low-density lipoprotein (40). Recently, a couple of studies have examined the role of Rho kinase in renal cells in culture (12, 20). Endlich et al. (12) found that a novel Rho kinase inhibitor, Y-27632, inhibited the reorganization of cytoskeleton induced by mechanical stress in cultured renal podocytes. Furthermore, the inhibition of Rho kinase activity is reported to prevent the TGF-β1-induced increase in connective tissue growth factor accumulation in cultured human renal fibroblast cells (20). Although these in vitro observations strongly suggest a substantial role of Rho kinase in mediating the progression of renal injury, direct in vivo evidence for the contribution of Rho kinase to the development of renal disease is insufficient. We have recently reported that NF-κB-dependent upregulation of MCP1 and TGF-β1 plays an important role in the development of renal injury in ANG II-dependent hypertension (54). Interestingly, Perona et al. (55) demonstrated that Rho induces the transcriptional activity of NF-κB by stimulating the phosphor-ylation of the IκBα in Ser-32 and Ser-36 residues. However, it has not been established whether a Rho kinase inhibitor and/or NF-κB inhibitor exerts renoprotective effects on ANG II-dependent hypertension. Therefore, the present study was performed to determine the effectiveness of Rho kinase inhibitor fasudil and NF-κB inhibitor parthenolide in renal injury in ANG II-infused hypertensive rats. Our study provides a firm foundation that fasudil as well as parthenolide markedly attenuated the progression of renal injury in ANG II-infused hyper-tensive rats.
Emerging evidence has demonstrated that macrophage/ monocyte infiltration is one of the key mechanisms in the progression of renal fibrosis (34, 78). Consistent with a previous study (54), chronic treatment with ANG II resulted in enhancement of CD68-positive macrophage/monocyte infiltration with an increase in expression of MCP1 mRNA as a potent chemotactic factor of macrophage/monocytes (34, 78). In the present study, we demonstrated that the inhibition of Rho kinase with fasudil strongly suppressed the expression of MCP1 mRNA and macrophage/monocyte infiltration. These findings are in agreement with those of previous studies indicating the protective effects of Rho kinase inhibitors on macrophage/monocyte infiltration and interstitial fibrosis in different renal disease models (26, 69). Furthermore, in vitro studies using chemotactic factors have demonstrated that neutrophil chemotaxis is significantly inhibited by fasudil (61). Collectively, these data suggest that the renoprotective effects of Rho kinase inhibitor on ANG II-induced renal injury are mediated, at least in part, by inhibition of macrophage/monocyte infiltration.
Renal fibrosis is characterized mainly by an excessive synthesis and accumulation of extracellular matrix components, including collagen (48). Kagami et al. (25) demonstrated that ANG II stimulates extracellular matrix protein synthesis through induction of TGF-β1 expression in rat glomerular cells and this induction is via an ANG II type 1 receptor-dependent mechanism. The cytokine TGF-β1 has been shown to play roles in the process of fibrogenesis and collagen synthesis via multiple pathways such as G1 phase arrest, cell-size enlargement, protein synthesis induction, an inhibitory effect on proteinase activity, and extracellular matrix enhancement (6, 80). Consistent with a previous study (54), the present study demonstrated that in chronic ANG II-infused rats, renal fibrosis is associated with increases in collagen-positive area accompanied by increases in TGF-β1 mRNA expression. These data suggest a possible contribution of the TGF-β1 pathway to ANG II-induced renal fibrosis. Of interest, recent studies also have indicated that Rho kinase plays a role in mediating renal fibrosis through a TGF-β1-dependent mechanism (20, 45, 53, 60). Nishikimi et al. (53) showed that increased TGF-β1 and collagen expression in injured kidneys of Dahl salt-sensitive hypertensive rats was associated with augmented gene expression of several Rho kinase families. Furthermore, treatment with a subdepressor dose of fasudil attenuated augmentation of TGF-β1 and collagen expression and improved renal injury in these animals (53). Similarly, another specific Rho kinase inhibitor, Y27632, suppressed interstitial fibrosis and augmentation of TGF-β1 and collagen expression in mouse kidneys with unilateral ureteral obstruction (45). Recent in vitro studies also demonstrated that Rho kinase is an essential factor for mechanical stretch-induced TGF-β1 synthesis in hepatic stel-late cells (60). Furthermore, the inhibition of Rho kinase activity prevents TGF-β1-induced increases in collagen accumulation in cultured human renal fibroblasts (20). In our study, Rho kinase inhibition with fasudil significantly attenuated ANG II-induced renal fibrosis and augmentation of TGF-β1 mRNA expression as well as collagen in the kidney. These data suggest that Rho kinase plays an important role in mediating ANG II-induced activation of the TGF-β1-dependent pathway, leading to collagen accumulation and the progression of renal fibrosis.
In the present study, treatment with a Rho kinase inhibitor did not alter BP but markedly attenuated the progression of renal injury in ANG II-infused rats, suggesting a potential contribution of ANG II-induced Rho kinase activation to renal injury independently of BP changes. A BP-independent renoprotective effect of Rho kinase inhibitors is also recently demonstrated in a different model of renal injury (69). Sun el al. (69) reported that treatment with fasudil did not alter BP but significantly ameliorated proteinuria and renal injury in rats that received aldosterone infusion. It is well known that ANG II is one of the most potent vasoconstrictor in the body, and it is well established that ANG II-mediated vascular tone constitutes an important determinant of glomerular hemodynamics (50). However, ANG II has multiple effects and the ANG II-induced vasoconstriction and high BP are only a small part of the roles of ANG II. For example, ANG II causes aldosterone secretion (52), cell infiltration and migration (11), thrombosis (74), and superoxide production (16, 17, 77). ANG II also modulate transporters (4, 5) and channels (9, 76) in proximal tubules as well as distal tubules. All of these factors are involved in ANG II-induced renal injury independently of the hypertension-induced renal injury. The variety of roles of ANG II may account for the BP-independent renoprotective effect of Rho kinase inhibitors in this study.
We selected 3 mg·kg−1·day−1 as the dose of fasudil treatment in the present study, because we previously reported that this dose of fasudil treatment had no effect on systolic BP and a higher dose (10 mg·kg−1·day−1) decreased systolic BP in rats (26). It is reported that relatively higher doses of fasudil (14–48 mg·kg−1·day−1) produce a blocking action against calcium entry (2, 23) as well as an inhibitory effect on protein kinase C (3, 23). We did not examine the intracellular calcium levels or protein kinase C levels in this study. However, according to the BP profiles, it seems unlikely that a relatively low dose of Fasudil exerts such nonspecific actions in this study.
Parthenolide is a sesquiterpene lactone and an active constituent derived from the Mexican Indian medicinal herb feverfew (Tanacetum parthenium). We selected 1 mg·kg−1·day−1 as the dose of parthenolide treatment in the present study, because it was previously reported that this dose of parthenolide treatment exerted anti-inflammatory effects without altering the normal behavior of mice and rats (24, 38). A recent report demonstrates that parthenolide directly alkylates the p65 subunit of NF-κB, thereby inhibiting DNA binding (14). Moreover, it is shown that parthenolide directly binds to and inhibits IκBKβ, the kinase subunit known to play a critical role in cytokine-mediated signaling (37). The inhibitory effects of parthenolide seem to be specific for NF-κB because they did not influence the activity of other transcription factors (19).
The effectiveness of a Rho kinase inhibitor in renal and cardiovascular injury in experimental animal models is also reported in previous studies (26, 27, 42, 45, 53, 59, 62, 72). Mukai et al. (42) provided evidence that upregulation of Rho kinase plays a key role in the pathogenesis of hypertensive vascular disease in spontaneously hypertensive rats. Kataoka et al. (27) presented an important role of Rho kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats through the MCP1 and TGF-β1 pathways. Nagatoya et al. (45) demonstrated that a Rho kinase inhibitor prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction through suppressing the migration of macrophages. Satoh et al. (62) also exhibited that Rho kinase inhibition produces a reduction of macrophage infiltration and attenuates interstitial fibrosis in rat kidneys with unilateral ureteral obstruction. Kanda et al. (26) showed evidence that fasudil improved glomerular and tubulointerstitial injury in subtotally nephrectomized, spontaneously hypertensive rats through an upregulation of p27kip1, a cyclin-dependent kinase inhibitor, and the subsequent inhibition of cell proliferation. Nishikimi et al. (53) revealed that fasudil attenuates glomerulosclerosis in Dahl salt-sensitive rats fed a high-salt diet with the reduction of mRNA expression levels of TGF-β1, collagen I, and collagen III in the renal cortex. Teraishi et al. (72) reported that the Rho kinase pathway plays a key role in the pathogenesis of ischemia-reperfusion-induced acute renal failure in rats through the suppression of the enhanced myeloperoxidase activity. Ruperez et al. (59) illustrated that the Rho kinase pathway is involved in renal damage in rats caused by ANG II through the regulation of proinflammatory and profibrotic mediators. Our results extend these findings and provide novel information by showing that the Rho-NF-κB axis plays an important role through NF-κB-dependent upregulation of the MCP1 and TGF-β1 pathways in the development of renal injury in ANG II-dependent hypertensive rats independently of changes in BP.
Several authors have also shown the effectiveness of NF-κB inhibitors in models of renal damage (13, 38, 39, 43, 73, 75). Muller et al. (43) provided the evidence that NF-κB inhibition ameliorates inflammatory renal injury through prevention of the NF-κB-dependent transactivation of the intercellular adhesion molecule and inducible nitric oxide synthase in double-transgenic rats harboring both human renin and angiotensinogen genes. Lopez-Franco et al. (38) demonstrated that an NF-κB inhibitor prevented proteinuria in an anti-Thy 1.1 nephritis rat model through the diminished renal expression of MCP1 and inducible nitric oxide synthase. Volpini et al. (75) presented an important role of NF-κB in the pathogenesis of tubulointerstitial nephritis induced by gentamicin in rats through the attenuation of macrophage infiltration. Tugcu et al. (73) also exhibited that NF-κB inhibitor ameliorates the nephrotoxicity induced by gentamicin in rats via the suppression of inducible nitric oxide synthase levels. Mitaka et al. (39) showed evidence that a blockade of NF-κB activation prevents hypodynamic shock and gastric hypoperfusion induced by endotoxin in anesthetized dogs, maybe through the suppression of inducible nitric oxide synthase levels. Fujihara et al. (13) revealed that the activation of the NF-κB system plays an important role in the pathogenesis of renal injury in the ⅚ renal ablation model of rats through the prevention of interstitial macrophage infiltration. Our results are supported by these findings by showing that an NF-κB inhibitor attenuates the MCP1 mRNA levels as well as interstitial macrophage infiltration and thus ameliorates renal injury in ANG II-dependent hypertensive rats.
It is reported that NF-κB activation is related to ANG II type 1 receptor-mediated pathways and is believed to be dependent on activation of the Rho proteins that regulate intracellular signaling (68). It is also demonstrated that treatment with a Rho kinase inhibitor diminished ANG II-induced NF-κB DNA binding activity in the kidneys of rats (59). Our data are consistent with these findings. Moreover, our data clearly indicated that parthenolide did not alter ANG II-induced Rho kinase activation although fasudil abolished ANG II-induced Rho kinase activation. Our findings are supported by previous in vitro studies (7, 10, 21, 55, 65, 81). Perona et al. (55) demonstrated that Rho-induced NF-κB activation stimulates translocation of RelA (p65) to the nucleus in NIH-3T3 murine fibroblast cells. They also reported that Rho-dependent activation of NF-κB is mediated by phosphorylation of IκBα and is reversed by overexpression of IκBα (55). Hodge et al. (21) provided evidence that Rho activation is required for increased NF-κB activity in PC-3 human prostate cancer cells. Zhao et al. (81) exhibited that neurotensin, a neuropeptide, stimulates IL-8 expression in NCM460 human colonic epithelial cells through Rho-mediated NF-κB pathways. Chen et al. (10) showed that a Rho exchange factor mediates NF-κB activation in human peripheral blood monocytes. Boyer et al. (7) presented that Rho GTPase instructs NF-κB activation by conveying IκBα to the ruffling membranes in HEp-2 epithelial carcinoma cells. Shimizu et al. (65) reveled that NF-κB activation is involved through the Rho/Rho kinase pathway in lipopolysaccharide-induced IL-8 production in cultured human cervical stromal cells. These data clearly indicate that there is a mechanistic linkage between Rho and NF-κB. Taken together, the present data suggest that the Rho-NF-κB axis plays a crucial role in the development of ANG II-induced renal injury independently of BP regulation.
To elucidate at which level ANG II-induced Rho activation stimulates the NF-κB pathway, related kinase activities were evaluated by Western blot analysis. As demonstrated in Fig. 5, ANG II-induced Rho activation stimulates the NF-κB pathway at phosphorylation of IκBα levels and this mechanism is independent from IκBKα, IκBKβ, and NF-κB-inducing kinase. These data are supported by the previous findings in vitro. Perona et al. (55) and Montaner et al. (41) demonstrated that Rho-induced NF-κB activation stimulates phosphorylation of IκBα. Moreover, Cammarano et al. (8) presented that whereas Rac, another Rho family GTPase, stimulates the activity of the IκBKβ, Rho activates NF-κB without activating either IκBKα or IκBKβ. These data clearly indicated that ANG II-induced Rho activation stimulates the NF-κB pathway at phosphorylation of IκBα levels and this mechanism is independent from IκBKα, IκBKβ, and NF-κB-inducing kinase.
In summary, this study was performed to determine the effectiveness of a Rho kinase inhibitor and NF-κB inhibitor in renal injury in ANG II-infused hypertensive rats. After 12 days of ANG II infusion, systolic BP (Fig. 1A), Rho kinase activity (Fig. 1B), NF-κB activity (Fig. 1,C and D), renal ANG II contents (Fig. 1E), MCP1 mRNA (Fig. 2A), interstitial macrophage/monocyte infiltration (Fig. 2, B-F), TGF-β1 mRNA (Fig. 3A), interstitial collagen-positive area (Fig. 3, B-F), urinary protein excretion (Fig. 4A), urinary albumin excretion (Fig. 4B), and phosphorylation of IκBα (Fig. 5A) were significantly enhanced compared with the Sham group. While fasudil or parthenolide did not alter systolic BP, both treatments completely blocked ANG II-induced enhancement of NF-κB activity, renal ANG II contents, MCP1 mRNA, interstitial macrophage/monocyte infiltration, TGF-β1 mRNA, interstitial collagen-positive area, urinary protein excretion, urinary albumin excretion, and phosphorylation of IκBα. Importantly, parthenolide did not alter ANG II-induced Rho kinase activation although fasudil abolished ANG II-induced Rho kinase activation. Moreover, ANG II infusion did not alter phosphorylation of IκBKα, IκBKβ, or NF-κB-inducing kinase. These data indicate that the Rho-NF-κB axis plays a crucial role in the development of ANG II-induced renal injury independently of BP regulation. Although the mechanistic linkage between Rho/Rho kinase and NF-κB is reported in previous studies in vitro and in vivo, this study is providing for the first time in vivo evidence that NF-κB is located downstream of the Rho/Rho kinase pathway by virtue of the fact that ANG II-induced Rho activation stimulates the NF-κB pathway at phosphorylation of IκBα levels and that this mechanism is independent of IκBKα, IκBKβ, and NF-κB-inducing kinase. A Rho kinase inhibitor and NF-κB inhibitor exert renoprotective effects, at least partially, through BP-independent mechanisms in ANG II-infused rats.
ACKNOWLEDGMENTS
The authors acknowledge excellent technical assistances from My-Linh Rauv, Duy V. Tran, Dale M. Seth, and Mark A. Cabrera (Tulane University).
GRANTS
This study was supported by National Institutes of Health Grants R01-DK-072408, P20-RR-017659, and R01-HL-026371, the Health Excellence Fund of the Louisiana Board of Regents, and Sankyo Co., Ltd. (Tokyo, Japan).
Footnotes
Portions of this study were presented in an abstract form at the 60th Annual Fall Conference and Scientific Sessions of the Council for High Blood Pressure Research in association with the Council on the Kidney in Cardiovascular Disease in San Antonio, TX (Hypertension 48: e75, 2006).
REFERENCES
- 1.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 2.Asano T, Ikegaki I, Satoh S, Suzuki Y, Shibuya M, Takayasu M, Hidaka H. Mechanism of action of a novel antivasospasm drug, HA1077. J Pharmacol Exp Ther. 1987;241:1033–1040. [PubMed] [Google Scholar]
- 3.Asano T, Suzuki T, Tsuchiya M, Satoh S, Ikegaki I, Shibuya M, Suzuki Y, Hidaka H. Vasodilator actions of HA1077 in vitro and in vivo putatively mediated by the inhibition of protein kinase. Br J Pharmacol. 1989;98:1091–1100. doi: 10.1111/j.1476-5381.1989.tb12652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berk BC, Vallega G, Muslin AJ, Gordon HM, Canessa M, Alexander RW. Spontaneously hypertensive rat vascular smooth muscle cells in culture exhibit increased growth and Na+/H+ exchange. J Clin Invest. 1989;83:822–829. doi: 10.1172/JCI113964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA. Cytokines in kidney disease: the role of transforming growth factor-beta. Am J Kidney Dis. 1993;22:105–113. doi: 10.1016/s0272-6386(12)70175-0. [DOI] [PubMed] [Google Scholar]
- 7.Boyer L, Travaglione S, Falzano L, Gauthier NC, Popoff MR, Lemichez E, Fiorentini C, Fabbri A. Rac GTPase instructs nuclear factor-kappaB activation by conveying the SCF complex and IkBalpha to the ruffling membranes. Mol Biol Cell. 2004;15:1124–1133. doi: 10.1091/mbc.E03-05-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cammarano MS, Minden A. Dbl and the Rho GTPases activate NF kappa B by I kappa B kinase (IKK)-dependent and IKK-independent pathways. J Biol Chem. 2001;276:25876–25882. doi: 10.1074/jbc.M011345200. [DOI] [PubMed] [Google Scholar]
- 9.Carmines PK, Navar LG. Disparate effects of Ca channel blockade on afferent and efferent arteriolar responses to ANG II. Am J Physiol Renal Fluid Electrolyte Physiol. 1989;256:F1015–F1020. doi: 10.1152/ajprenal.1989.256.6.F1015. [DOI] [PubMed] [Google Scholar]
- 10.Chen LY, Zuraw BL, Ye RD, Pan ZK. A Rho exchange factor mediates fMet-Leu-Phe-induced NF-kappaB activation in human peripheral blood monocytes. J Biol Chem. 2004;279:7208–7212. doi: 10.1074/jbc.M309542200. [DOI] [PubMed] [Google Scholar]
- 11.Dubey RK, Jackson EK, Luscher TF. Nitric oxide inhibits angiotensin II-induced migration of rat aortic smooth muscle cell. Role of cyclic-nucleotides and angiotensin1 receptors. J Clin Invest. 1995;96:141–149. doi: 10.1172/JCI118014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol. 2001;12:413–422. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- 13.Fujihara CK, Antunes GR, Mattar AL, Malheiros DM, Vieira JM, Jr, Zatz R. Chronic inhibition of nuclear factor-κB attenuates renal injury in the 5/6 renal ablation model. Am J Physiol Renal Physiol. 2007;292:F92–F99. doi: 10.1152/ajprenal.00184.2006. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- 15.Graciano ML, Cavaglieri RdC, Delle H, Dominguez WV, Casarini DE, Malheiros DM, Noronha IL. Intrarenal renin-angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol. 2004;15:1805–1815. doi: 10.1097/01.asn.0000131528.00773.a9. [DOI] [PubMed] [Google Scholar]
- 16.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 17.Hannken T, Schroeder R, Stahl RA, Wolf G. Angiotensin II-mediated expression of p27Kip1 and induction of cellular hypertrophy in renal tubular cells depend on the generation of oxygen radicals. Kidney Int. 1998;54:1923–1933. doi: 10.1046/j.1523-1755.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 18.Harrison-Bernard LM, El-Dahr SS, O'Leary DF, Navar LG. Regulation of angiotensin II type 1 receptor mRNA and protein in angiotensin II-induced hypertension. Hypertension. 1999;33:340–346. doi: 10.1161/01.hyp.33.1.340. [DOI] [PubMed] [Google Scholar]
- 19.Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- 20.Heusinger-Ribeiro J, Eberlein M, Wahab NA, Goppelt-Struebe M. Expression of connective tissue growth factor in human renal fibroblasts: regulatory roles of RhoA and cAMP. J Am Soc Nephrol. 2001;12:1853–1861. doi: 10.1681/ASN.V1291853. [DOI] [PubMed] [Google Scholar]
- 21.Hodge JC, Bub J, Kaul S, Kajdacsy-Balla A, Lindholm PF. Requirement of RhoA activity for increased nuclear factor kappaB activity and PC-3 human prostate cancer cell invasion. Cancer Res. 2003;63:1359–1364. [PubMed] [Google Scholar]
- 22.Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin-angiotensin system. Contrib Nephrol. 2004;143:117–130. doi: 10.1159/000078716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishida T, Takanashi Y, Kiwada H. Safe and efficient drug delivery system with liposomes for intrathecal application of an antivasospastic drug, fasudil. Biol Pharm Bull. 2006;29:397–402. doi: 10.1248/bpb.29.397. [DOI] [PubMed] [Google Scholar]
- 24.Jain NK, Kulkarni SK. Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J Ethnopharmacol. 1999;68:251–259. doi: 10.1016/s0378-8741(99)00115-4. [DOI] [PubMed] [Google Scholar]
- 25.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanda T, Wakino S, Hayashi K, Homma K, Ozawa Y, Saruta T. Effect of fasudil on Rho-kinase and nephropathy in subtotally nephrectomized spontaneously hypertensive rats. Kidney Int. 2003;64:2009–2019. doi: 10.1046/j.1523-1755.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 28.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobori H, Hayashi M, Saruta T. Thyroid hormone stimulates renin gene expression through the thyroid hormone response element. Hypertension. 2001;37:99–104. doi: 10.1161/01.hyp.37.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem Biophys Res Commun. 2004;315:746–750. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, Cohen EP, Navar LG. Young Scholars Award Lecture: intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens. 2006;19:541–550. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Franco O, Suzuki Y, Sanjuan G, Blanco J, Hernandez-Vargas P, Yo Y, Kopp J, Egido J, Gomez-Guerrero C. Nuclear factor-kappa B inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol. 2002;161:1497–1505. doi: 10.1016/s0002-9440(10)64425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitaka C, Hirata Y, Narumi Y, Yokoyama K, Makita K, Katsuyama K, Imai T. Blockade of nuclear factor-kappaB activation prevents hypodynamic shock and gastric hypoperfusion induced by endotoxin in anesthetized dogs. Intensive Care Med. 2005;31:718–723. doi: 10.1007/s00134-005-2617-1. [DOI] [PubMed] [Google Scholar]
- 40.Miyata K, Shimokawa H, Kandabashi T, Higo T, Morishige K, Eto Y, Egashira K, Kaibuchi K, Takeshita A. Rho-kinase is involved in macrophage-mediated formation of coronary vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol. 2000;20:2351–2358. doi: 10.1161/01.atv.20.11.2351. [DOI] [PubMed] [Google Scholar]
- 41.Montaner S, Perona R, Saniger L, Lacal JC. Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- 42.Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J, Kaibuchi K, Takeshita A. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J. 2001;15:1062–1064. doi: 10.1096/fj.00-0735fje. [DOI] [PubMed] [Google Scholar]
- 43.Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension. 2000;35:193–201. doi: 10.1161/01.hyp.35.1.193. [DOI] [PubMed] [Google Scholar]
- 44.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, Shokoji T, Kimura S, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Temporary angiotensin blockade at the prediabetic stage attenuates the development of type 2 diabetic nephropathy. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagatoya K, Moriyama T, Kawada N, Takeji M, Oseto S, Murozono T, Ando A, Imai E, Hori M. Y-27632 prevents tubulointerstitial fibrosis in mouse kidneys with unilateral ureteral obstruction. Kidney Int. 2002;61:1684–1695. doi: 10.1046/j.1523-1755.2002.00328.x. [DOI] [PubMed] [Google Scholar]
- 46.Nagumo H, Sasaki Y, Ono Y, Okamoto H, Seto M, Takuwa Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am J Physiol Cell Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura A, Hayashi K, Ozawa Y, Fujiwara K, Okubo K, Kanda T, Wakino S, Saruta T. Vessel- and vasoconstrictor-dependent role of Rho/Rho-kinase in renal microvascular tone. J Vasc Res. 2003;40:244–251. doi: 10.1159/000071888. [DOI] [PubMed] [Google Scholar]
- 48.Nangaku M, Pippin J, Couser WG. C6 mediates chronic progression of tubulointerstitial damage in rats with remnant kidneys. J Am Soc Nephrol. 2002;13:928–936. doi: 10.1681/ASN.V134928. [DOI] [PubMed] [Google Scholar]
- 49.Narumiya S. The small GTPase Rho: cellular functions and signal transduction. J Biochem. 1996;120:215–228. doi: 10.1093/oxfordjournals.jbchem.a021401. [DOI] [PubMed] [Google Scholar]
- 50.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Navar LG, Kobori H, Prieto-Carrasquero MC. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep. 2003;5:135–143. doi: 10.1007/s11906-003-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive states. J Renin Angiotensin Aldosterone Syst. 2001;2:S176–S184. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikimi T, Akimoto K, Wang X, Mori Y, Tadokoro K, Ishikawa Y, Shimokawa H, Ono H, Matsuoka H. Fasudil, a Rho-kinase inhibitor, attenuates glomerulosclerosis in Dahl salt-sensitive rats. J Hypertens. 2004;22:1787–1796. doi: 10.1097/00004872-200409000-00024. [DOI] [PubMed] [Google Scholar]
- 54.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol. 2007;292:F330–F339. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perona R, Montaner S, Saniger L, Sanchez-Perez I, Bravo R, Lacal JC. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 56.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruiz-Ortega M, Lorenzo O, Ruperez M, Konig S, Wittig B, Egido J. Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms. Circ Res. 2000;86:1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 59.Ruperez M, Sanchez-Lopez E, Blanco-Colio LM, Esteban V, Rodriguez-Vita J, Plaza JJ, Egido J, Ruiz-Ortega M. The Rho-kinase pathway regulates angiotensin II-induced renal damage. Kidney Int Suppl. 2005:S39–45. doi: 10.1111/j.1523-1755.2005.09908.x. [DOI] [PubMed] [Google Scholar]
- 60.Sakata R, Ueno T, Nakamura T, Ueno H, Sata M. Mechanical stretch induces TGF-beta synthesis in hepatic stellate cells. Eur J Clin Invest. 2004;34:129–136. doi: 10.1111/j.1365-2362.2004.01302.x. [DOI] [PubMed] [Google Scholar]
- 61.Satoh S, Kobayashi T, Hitomi A, Ikegaki I, Suzuki Y, Shibuya M, Yoshida J, Asano T. Inhibition of neutrophil migration by a protein kinase inhibitor for the treatment of ischemic brain infarction. Jpn J Pharmacol. 1999;80:41–48. doi: 10.1254/jjp.80.41. [DOI] [PubMed] [Google Scholar]
- 62.Satoh S, Yamaguchi T, Hitomi A, Sato N, Shiraiwa K, Ikegaki I, Asano T, Shimokawa H. Fasudil attenuates interstitial fibrosis in rat kidneys with unilateral ureteral obstruction. Eur J Pharmacol. 2002;455:169–174. doi: 10.1016/s0014-2999(02)02619-5. [DOI] [PubMed] [Google Scholar]
- 63.Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- 64.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ Res. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu S, Tahara M, Ogata S, Hashimoto K, Morishige K, Tasaka K, Murata Y. Involvement of nuclear factor-kB activation through RhoA/Rho-kinase pathway in LPS-induced IL-8 production in human cervical stromal cells. Mol Hum Reprod. 2007;13 doi: 10.1093/molehr/gal113. doi:10.1093/molehr/gal1113. [DOI] [PubMed] [Google Scholar]
- 66.Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2002;39:319–327. doi: 10.1097/00005344-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 67.Shome K, Rizzo MA, Vasudevan C, Andresen B, Romero G. The activation of phospholipase D by endothelin-1, angiotensin II, and platelet-derived growth factor in vascular smooth muscle A10 cells is mediated by small G proteins of the ADP-ribosylation factor family. Endocrinology. 2000;141:2200–2208. doi: 10.1210/endo.141.6.7517. [DOI] [PubMed] [Google Scholar]
- 68.Strawn WB. Pathophysiological and clinical implications of AT(1) and AT(2) angiotensin II receptors in metabolic disorders: hypercholesterolaemia and diabetes. Drugs. 2002;62:31–41. [PubMed] [Google Scholar]
- 69.Sun GP, Kohno M, Guo P, Nagai Y, Miyata K, Fan YY, Kimura S, Kiyomoto H, Ohmori K, Li DT, Abe Y, Nishiyama A. Involvements of Rho-kinase and TGF-beta pathways in aldosterone-induced renal injury. J Am Soc Nephrol. 2006;17:2193–2201. doi: 10.1681/ASN.2005121375. [DOI] [PubMed] [Google Scholar]
- 70.Suzaki Y, Ozawa Y, Kobori H. Intrarenal Oxidative Stress and Augmented Angiotensinogen are Precedent to Renal Injury in Zucker Diabetic Fatty Rats. Int J Biol Sci. 2007;3:40–46. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzaki Y, Prieto-Carrasquero MC, Kobori H. Intratubular renin-angiotensin system in hypertension. Curr Hypertens Rev. 2006;2:151–157. doi: 10.2174/157340206776877325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teraishi K, Kurata H, Nakajima A, Takaoka M, Matsumura Y. Preventive effect of Y-27632, a selective Rho-kinase inhibitor, on ischemia/reperfusion-induced acute renal failure in rats. Eur J Pharmacol. 2004;505:205–211. doi: 10.1016/j.ejphar.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 73.Tugcu V, Ozbek E, Tasci AI, Kemahli E, Somay A, Bas M, Karaca C, Altug T, Cekmen MB, Ozdogan HK. Selective nuclear factor kappa-B inhibitors, pyrolidium dithiocarbamate and sulfasalazine, prevent the nephrotoxicity induced by gentamicin. BJU Int. 2006;98:680–686. doi: 10.1111/j.1464-410X.2006.06321.x. [DOI] [PubMed] [Google Scholar]
- 74.Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volpini RA, Costa RS, da Silva CG, Coimbra TM. Inhibition of nuclear factor-kappaB activation attenuates tubulointerstitial nephritis induced by gentamicin. Nephron Physiol. 2004;98:97–106. doi: 10.1159/000081558. [DOI] [PubMed] [Google Scholar]
- 76.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 77.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat: role of AT1 receptors. Kidney Int. 2003;63:202–208. doi: 10.1046/j.1523-1755.2003.00729.x. [DOI] [PubMed] [Google Scholar]
- 78.Xu ZG, Lanting L, Vaziri ND, Li Z, Sepassi L, Rodriguez-Iturbe B, Natarajan R. Upregulation of angiotensin II type 1 receptor, inflamma-tory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney: reversal by angiotensin II type 1 receptor blockade. Circulation. 2005;111:1962–1969. doi: 10.1161/01.CIR.0000161831.07637.63. [DOI] [PubMed] [Google Scholar]
- 79.Yamakawa T, Tanaka S, Numaguchi K, Yamakawa Y, Motley ED, Ichihara S, Inagami T. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 2000;35:313–318. doi: 10.1161/01.hyp.35.1.313. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao D, Kuhnt-Moore S, Zeng H, Wu JS, Moyer MP, Pothoulakis C. Neurotensin stimulates IL-8 expression in human colonic epithelial cells through Rho GTPase-mediated NF-!B pathways. Am J Physiol Cell Physiol. 2003;284:C1397–C1404. doi: 10.1152/ajpcell.00328.2002. [DOI] [PubMed] [Google Scholar]