Abstract
By releasing multiple pro-inflammatory mediators upon activation, mast cells are critical effector cells in the pathogenesis of allergic inflammation. The traditional viewpoint of antigen-dependent mast cell activation is that of a Th2-driven process whereby antigen-specific IgE molecules are produced by B cells followed by binding of the IgE to high affinity IgE receptors (FcεRI) expressed on mast cells. Subsequent antigen-dependent aggregation of the FcεRI initiates an intracellular signalling cascade that culminates in mediator release. Mast cell responses, including cell growth, survival, chemotaxis, and cell adhesion, however, can also be regulated by other receptors expressed on mast cells. Furthermore, FcεRI-mediated mast cell mediator release can be significantly modified by ligation of specific classes of these receptors. One such class of receptors is the G protein-coupled receptors (GPCR). In this review, we describe how sub-populations of GPCRs can either enhance or inhibit FcεRI-mediated mast cell activation depending on the particular G protein utilized for relaying signalling. Furthermore, we discuss the potential mechanisms whereby the signalling responses utilized by the FcεRI for mast cell activation are influenced by those initiated by GPCRs to produce these diverse responses.
Keywords: Mast cells, FcεRI, G protein-coupled receptors, IgE, antigen, signalling
Mast cell activation
Mast cells play a role in both the innate and adaptive immune responses which contribute to the body’s defence mechanisms against invading pathogens and parasites [1–3]. However, when mast cells are inappropriately activated following antigen exposure, the resulting release of multiple pro-inflammatory mediators leads to the initiation of the clinical manifestations associated with allergic inflammation [4]. This response occurs as a consequence of the antigen binding to IgE molecules which occupy high affinity receptors for IgE (FcεRI) on the mast cell surface and resulting cross-linking of these receptors [5, 6]. FcεRI aggregation induces a cascade of intracellular signalling events which lead to the rapid release of histamine and proteolytic enzymes, as a consequence of degranulation, and the release of leukotrienes (LTs) and prostaglandins (PGs), as a result of phospholipase A2 (PLA2)-mediated phospholipid hydrolysis [4, 7]. Following enhanced gene expression, a variety of cytokines and chemokines are also released from the activated mast cells, however this is a delayed response observed several hours after degranulation and the release of phospholipid-derived mediators [4].
The FcεRI-mediated process described above is likely to be an over-simplification of what is actually occurring during an ongoing reaction in vivo. In this respect, the immediate microenvironment surrounding the cells in their resident tissues likely contains a multiplicity of factors which, under specific conditions, may modify antigen-dependent mast cell activation [6]. Support for this concept has come from studies conducted in vivo which have demonstrated that other receptors, apart from FcεRI, can quite significantly influence mast cell activation [6]. The most widely investigated of these interactions is the process by which the receptor for stem cell factor (SCF), Kit, modifies mast cell responses elicited by aggregated FcεRI [8–11]. For example, studies, conducted in both human and mouse mast cells, have revealed that concurrent administration of SCF with antigen produces a marked enhancement of both degranulation and cytokine production compared to that observed with antigen alone [9, 11]. Although this interaction between SCF and antigen has yet to be demonstrated in a physiological setting, the critical role that SCF plays in mast cell growth, differentiation and survival [12, 13] would suggest that such an integrated activation process may indeed occur in vivo. It has also recently been demonstrated that concurrent activation of toll like receptors (TLR) 2 and TLR4 with FcεRI aggregation also results in a marked enhancement of cytokine production [14]. However, this response is observed in the absence of a potentiation of degranulation and appears to involve a distinctly different integration process than that utilized by Kit.
In addition to the above examples, there is an increasing volume of literature describing how members of the G protein-coupled receptor (GPCR) superfamily can also impact mediator release. The responses elicited by these GPCR agonists, however, are quite divergent ranging from induction of chemotaxis and adhesion to potentiation of FcεRI-mediated mast cell activation [6, 15]. Indeed multiple or even divergent responses in mast cells evoked by a single ligand have been reported [16–20]. To discuss how these responses may be regulated it is first necessary to briefly describe the properties of GPCRs.
GPCRs
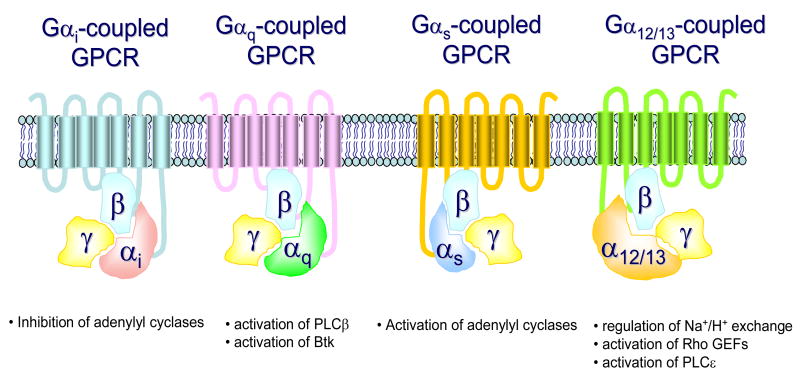
GPCRS are a diverse group of receptors characterized by their 7 transmembrane spanning regions and their association with heterotrimeric GTP-binding proteins (G protein s) [21]. G proteins exist as a complex of Gα and Gβγ subunits. Upon GPCR ligation, GDP is displaced from the Gα subunit, thus permitting GTP to bind to the Gα subunit resulting in dissociation of this subunit from the Gβγ subunits [22, 23]. This allows the free subunits to mediate downstream signalling. The G protein α subunit family is divided into four major groups based on sequence homologies and functional similarities (Gαs, Gαi, Gαq, and Gα12) [23, 24]. Gαs stimulates adenylyl cyclase, Gαi inhibits adenylyl cyclase, Gα12/13 stimulates the guanine nucleotide exchange activity of Rho, and Gαq activates phospholipase (PL)Cβ and Bruton’s tyrosine kinase (Btk) (Fig. 1) [25–27]. The ability of Gi to relay signals is inhibited by pre-treatment with pertussis toxin, therefore this agent has been utilized to demonstrate the involvement of this particular G protein in cellular responses [28]. The dissociated Gβγ-dimer also regulates a variety of intracellular effectors, including PLCβ, phosphoinositide 3-kinase (PI3K), ion channels, and adenylyl cyclase [29]. Thus, the abilities of the individual G proteins to elicit specific responses are not only dependent on the cell types in which they are expressed but also the specific G proteins to which they are linked.
Fig 1.
Major classes of GPCRs and the signals relayed by the respective Gα subunits.
GPCRs and mast cell function
In the following sections we describe how mast cell function is modified by GPCRs, and discuss the potential mechanisms by which these processes may be regulated. We have not endeavoured to comprehensively list all the GPCRs expressed on mast cells or their respective effects, but, rather, have focussed on several classes of GPCR which have been documented to modify FcεRI-mediated mast cell activation or by themselves to induce mast cell mediator release. These receptors include the receptor for the complement component C3a, receptors for the bioactive lipids, sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA), receptors for adenosine and adenine nucleotides, receptors for chemokines such as MIP-1α and the β2 adrenergic receptor. The majority of the studies described below have been conducted in either mouse or rat mast cells. When examined in human mast cells, the results have often been contrasting and the potential reasons for this are also discussed in the following sections. It should also be pointed out, that a number of other GPCRs, for example those for the chemokines [15], fractalkine [30], eotaxin [31], monocyte chemoattractant protein (MCP) [15], and the bioactive amine, 5-hydroxytryptamine [32], can induce chemotaxis and/or cell adhesion in the absence of an apparent ability to modify FcεRI-mediated responses or to induce mast cell activation in the absence of antigen. This category of GPCR is not further discussed in this review.
C3a
The anaphylatoxic peptide C3a is generated at the site of immune complex-dependent inflammation [33]. In addition to its ability to act as a mast cell chemoattractant [34], C3a also can modulate mast cell degranulation and the release of chemokines such as MCP-1 and Rantes [35, 36]. The actions of C3a on degranulation, however, differ depending on the particular mast cell subtype. In the mucosal type of mast cells such as RBH-2H3 cells [37] or mouse bone marrow-derived mast cells (BMMCs) [38], C3a inhibits FcεRI-mediated degranulation, apparently independently of the C3a receptor. In these systems, C3a appears to inhibit degranulation through suppression of the phosphorylation of the FcεRI β subunit by an undefined mechanism. In contrast, C3a markedly induces degranulation and/or chemokine production in the HMC-1 and LAD-2 human mast cell lines [36], in primary cultures of human CD34+-derived mast cells [39], and in rat peritoneal mast cells [40] by a C3a receptor-mediated mechanism. These responses are associated with an increase in intracellular calcium concentrations ([Ca2+]i). The effect of C3a on migration, calcium mobilization, and PLC activation in mast cells is blocked by pertussis toxin, indicating that the functional mast cell C3a receptor is coupled to Gi [34, 40, 41]. In addition to its ability to induce degranulation in the absence of antigen, C3a can act in an additive manner with antigen/IgG1 via the FcγRI [39] and antigen/IgE via the FcεRI (Kuehn and Gilfillan, unpublished data) to enhance degranulation in human CD34+-derived mast cells. Unlike with Kit and other GPCRs, however, there appears to be no synergy in these interactions (Kuehn and Gilfillan, unpublished data). The data, however, do suggest that the regulatory signalling pathways leading to degranulation elicited by the C3a receptor is different from that regulated by the Fc receptors [6].
Sphingosine-1-phosphate
In multiple cell types, the bioactive lysophospholipid, S1P, regulates quite diverse biological responses including chemotactic motility, calcium homeostasis, cell survival, and cell differentiation [42, 43]. S1P is formed by the sphingosine kinase (SK) 1- and 2-dependent phosphorylation of sphingosine and several cell types of hematopoietic lineage, including mast cells, display enhanced S1P levels following activation of these enzymes as a consequence of immune stimulation [44, 45]. Five GPCRs with alternative functions have been described for S1P and these have been designated S1P1–5 [46]. In addition to acting through these cell surface receptors to modify cellular function, S1P may also act intracellularly to mobilize intracellular calcium and such a mechanism may contribute, in part, to the calcium signal observed in activated mast cell [47, 48]. Mast cells express both the S1P1 and S1P2 receptors [43, 45]. The S1P1 receptor, which is linked to Gi, regulates mast cell migration in a pertussis toxin-sensitive manner [49] similar to that observed in T cells [50], whereas the SIP2 receptor regulates degranulation in a pertussis toxin insensitive manner [44, 49]. Thus, although the S1P2 receptor is linked to Gi, Gq and G12/13, the ability of S1P to promote degranulation through the S1P2 receptor is likely mediated via Gq and/or G12/13, rather Gi. The concentrations of S1P required for degranulation are higher than those required for chemotaxis [16, 45] and these higher concentrations of S1P also inhibits chemotaxis [45, 49]. This has led to the suggestion that in allergic inflammatory reactions, mast cells would migrate to target tissues at low S1P concentration gradients, however once resident in the target tissues the higher S1P concentrations would prevent further migration, and mast cell degranulation would occur following ligation of the S1P2 receptor [16].
Recent evidence has suggested that whereas intrinsic (mast cell) sources of S1P, released via the ATP binding cassette (ABC)C1 transporter [51], may act on the S1P1 receptor to control chemotaxis, both intrinsic and extrinsic sources of S1P may act on the S1P2 receptor to modify degranulation [52]. Experiments using S1P2 knock out mice have also suggested that the S1P generated in mast cells following FcεRI aggregation, acts in an autocrine/paracrine manner to contribute to the degranulation observed following antigen challenge [45]. Our studies (Kuehn and Gilfillan, unpublished observations) do suggest that S1P can act in a synergistic manner with antigen to enhance degranulation in mouse BMMCs, however, whether this is due to an intracellular or extracellular response has not yet been determined. Despite the significant impairment of S1P-mediated degranulation in S1P2−/− mouse BMMCs, the concentrations required to elicit degranulation (μM) are markedly higher than the Kd (20–27 nM) for the SIP2 receptor. This, thus, raises the question as to whether the ability of S1P to promote degranulation of mast cells is entirely mediated by a cell surface receptor.
Lysophosphatidic acid
LPA is another lipid-derived molecule that can influence mast cell activation through a GPCR-mediated mechanism. LPA has been reported to both enhance the development of human mast cells from progenitor cells [53] and to promote chemokine generation in the mature cells [54]. Furthermore, LPA has also been reported to induce degranulation in mouse and rat mast cells [55, 56]. All four GPCR receptors for LPA (LPA1– LPA4) are expressed in human mast cells [53] and the effects of LPA on mast cell proliferation appears to be mediated by either LPA1 or LPA3 receptors via Gi [53, 55], whereas the effects of LPA on chemokine generation appear to be mediated via the LPA2 receptor [54]. It is unclear, however, which LPA receptor may mediated the degranulation response observed in the mouse mast cells.
Prostaglandin E2
The arachidonic acid metabolite PGE2 is an important modulator in inflammatory responses which is produced in mast cells, dendritic cells, epithelial cells, fibroblast, and macrophage [57–59]. PGE2 binds four receptor subtypes (EP1, EP2, EP3, and EP4) [60]. The EP2 and EP4 receptors are coupled with Gs which enhances adenylyl cyclase activity thus increases intracellular cyclic adenosine monophosphate (cAMP) levels, whereas the EP1 receptor is coupled with Gq and thus signals through intracellular calcium. The EP3 receptor is coupled with multiple G proteins (Gi, Gq, and Gs) [60]. PGE2 modulates FcεRI-mediated mast cell activation in quite contrasting manners depending on the predominance of the receptor subtype ligated in the particular mast cell system. In this respect, PGE2 has been demonstrated to enhance FcεRI-mediated degranulation and the production of cytokines, including IL-6 and GM-CSF, in mouse BMMCs [61, 62]. These responses appear to be mediated via the EP3 receptor [62]. Similarly, it has been reported that PGE2 also enhances antigen-dependent degranulation in cultures of peripheral blood-derived human mast cells through the EP1 and/or EP3 receptors [18]. In contrast, other studies conducted in human mast cells, have provided evidence that PGE2 inhibits FcεRI-mediated histamine, PGD2, LTC4, and TNF-α production via the EP2 receptor which relays this inhibition through elevated cAMP levels [19, 20]. Thus, the relative balance between the expression of EP1/EP3 receptors and EP2 receptors appears to largely dictate the response in a particular model of mast cell function. Little information, however, exists concerning the relative expression of the EP receptors on human mast cells in a physiological setting and how the expression of these receptors may be regulated. Further studies are therefore required to establish how PGE2 modifies mast cell activation in disease states in vivo.
Adenosine
Adenosine has been proposed to play an important role in asthma and chronic obstructive pulmonary disease , and the clinical treatment of asthmatic responses by methylxanithines may, in part, be linked to the ability of these compounds to act as adenosine receptor antagonists [63–65]. As with PGE2, adenosine can produce quite contrasting responses on mast cell activation. For example, adenosine is reported to potentiate FcεRI-mediated degranulation and LT release in mouse BMMCs [66], RBL 2H3 [67, 68], and human lung mast cells [17] in a pertussis toxin sensitive manner; indicating that these responses are mediated via Gi. In addition, in the HMC-1 human mast cell line, adenosine also stimulates the production of IL-4 [69] and IL-8 [70]. In contrast, adenosine has also been reported to inhibit FcεRI-mediated degranulation of human mast cells [17, 71]. Furthermore, following pertussis toxin pre-treatment of RBL 2H3 cells, adenosine inhibits LT production in these cells [67].
These diverse responses produced by adenosine again are directly attributable to the multiple receptors for this ligand expressed on mast cells. Adenosine binds to P1 purinoceptors (purinergic receptors) and four P1 purinoceptors subtypes (A1, A2A, A2B, and A3) have been described. The A2A, A2B, and A3 receptors have been reported to be expressed on mast cells [65, 72]. The ability of adenosine to inhibit FcεRI-mediated degranulation is mimicked by the A2A-specific agonist, CGS21680 [73], suggesting that it is this receptor that is responsible for down regulating mast cell responses, whereas the A2B receptor upregulates mast cell activation [70, 74]. Both the A2A and A2B receptors are linked to Gs, thus when ligated, will result in increased levels of cAMP following adenylyl cyclase activation. Increased levels of cAMP in mast cells would result in inhibition of FcεRI-mediated mast cell mediator release explaining the ability of tha A2A receptor to down-regulate mast cell activation. The A2B but not A2A receptor is also linked to Gq, which regulates PLCβ activation [63, 70]. Thus, the A2B receptor appears to enhance mediator release through PLC activation and elevation of [Ca2+]i generation. In the mouse and rat, it is the Gi-linked A3 receptor that appears to be the major receptor involved in the ability of adenosine to enhance FcεRI-mediated degranulation [75]. However, in the human, the A3 receptor does not appear to be involved in the regulation of degranulation [76], thus, the ability of adenosine to upregulate degranulation in the human appers to be primarily via the A2B adenosine receptor.
Other purinoceptor agonisits
The bioactive purine and pyrimidine nucleotides, ADP, ATP, and UTP can influence cellular activation by binding to specific cell surface P2 purinoceptors [77]. P2 purinoceptors are subdivided into two major functional groups: P2X receptors, which form ligand-gated ion channels and pores, and P2Y receptors, which are coupled to G proteins [78]. Human mast cells have been documented to express P2Y1, P2Y2, P2Y11, P2Y12, and P2Y13 purinoceptors in addition to several P2X subtypes [79]. As with PGE2 and adenosine, adenine nucleotides produce contrasting responses in mast cells. In rat and mouse mast cells, ATP has been described to induce Ca2+-dependent degranulation via P2X and P2Y receptors [80, 81]. Furthermore, ATP has also been shown to potentiate FcεRI-mediated histamine release in rat mast cells [82]. Similarly, ATP and UTP have been reported to enhance FcεRI-mediated degranulation in human mast cells and this response appears to be mediated through P2Y but not P2X receptors [83]. However, although P2 purinoceptor agonists such as ADP produce an increase in intracellular calcium levels and the activation of the MAP kinases ERK and p38 through P2Y1 and/or P2Y12, these agents appear to only minimally affect deganulation and eicosanoid production in the absence of antigen [79]. In contrast to their effects on FcεRI-mediated degranulation, ADP, ATP, and ATP analogues block cytokine production induced by both a TLR2 agonist and LTD4 via the Gs-induced elevation of cAMP [79]. These observations suggest that, as with other GPCRs, multiple P2 purinoceptors with opposing function are expressed in mast cells.
Chemokines
Mast cells produce both chemokines and cytokines following cellular activation [84, 85]. Thus, as with S1P, PGE2 and adenosine, these represent compounds that are not only produced by mast cells, but which can also act upon mast cells to modify mast cell function. Unlike cytokines which utilize associating signalling subunits to relay their responses, receptors for chemokines are G protein-coupled. Chemokine receptors are divided into four different families (CXCR, CR, CX3CR, and CCR) based on structural homology and the position of cystein residues [86]. CC-chemokine receptors, particularly the CCR3 receptors, have been proposed to play an important role in allergic diseases [87, 88]. Mast cells express CCR3 and this receptor binds a variety of chemokines including eotaxin, RANTES, MCP [84, 87, 89]. Unlike eotaxin and MCP, which induce mast cell chemotaxis but not degranulation [15, 31], RANTES has been demonstrated to induce mast cell degranulation [90] and PGD2 generation [91], in addition to its chemotactic properties [92]. Similarly MIP-1α, which binds CCR1 and is produced in mast cells following FcεRI aggregation, also induces chemotaxis and enhances FcεRI-mediated calcium generation and degranulation in RBL-CCR1 cells (RBL 2H3 cells expressing human CCR1) [93] and mouse BMMCs [15, 66]. Such synergy may have physiological relevance as antigen-induced mast cell responses in vivo were reported to be substantially reduced in MIP-1α–knock out mice [94], suggesting that MIP-1α is an important cofactor for FcεRI-mediated responses in vivo.
β2-adrenergic agonists
The last category of GPCRs that modify antigen mediated mast cell activation, the β2 adrenoceptor do so by inhibiting mast cell activation without having other apparent actions. β2-adrenoceptor agonists are used as bronchodilators in the therapeutic management of asthma [95, 96]. Both short-acting β2-agonists, such as salbutamol and terbutaline, and longer-acting agonists such as salmeterol and formoterol, have also been demonstrated to inhibit the FcεRI-mediated degranulation and the release of PGD2, and LTs from human mast cells [96–98]. Moreover, β2 agonists have been shown to inhibit IgE-dependent cytokine production such as GM-CSF and TNF-α in these cells [98, 99]. As with other GPCRs that downregulate FcεRI-mediated degranulation, the inhibitory properties of β2 agonists are mediated by the Gs-dependent activation of adenylate cyclase, and subsequent increase in intracellular cAMP [100].
Integration of signalling
The complexity of the signalling cascade associated with the FcεRI has been extensively described in several recent reviews [6, 85, 101, 102] and it is not the aim of this current review to revisit this theme. However, to understand how GPCRs may influence mast cell responses, it is necessary to provide a brief overview of these signalling processes.
FcεRI signalling
Although the basic principles of the receptor-distal signalling pathways utilized by the aggregated FcεRI for mediator release have been largely elucidated, the events that allow aggregated FcεRI to initiate these processes are still not fully understood. However, these initial events appear to involve, and require, FcεRI translocation to glycolipid-enriched membrane domains within the plasma membrane termed lipid rafts [103, 104]. Hence, the signalling FcεRIβ and γ chains are tyrosine phosphorylated by the action of the Src Kinase Lyn, which is constitutively activated within these domains [105–107]. The phosphorylation sites within the β and γ chains are contained within immunoreceptor tyrosine-based activation motifs (ITAMS) which provide docking sites for the src homology 2 (SH2) domains of associating signalling molecules [101]. In particular, the phosphorylated ITAMs of the γ chains recruit the tyrosine kinase, Syk [108] which, following its phosphorylation and activation, phosphorylates downstream substrates including the transmembrane adaptor molecules LAT and NTAL (also known as LAB and LAT2) [109, 110].
The multiple tyrosines phosphorylated on LAT and NTAL allow the further recruitment of signalling molecules and by this means a multi-molecular receptor-signalling complex or signalosome is constructed [110, 111]. The complex centred around LAT allows for the recruitment and, thus activation of PLCγ1 and PLCγ2 [6, 109] both by direct binding and indirect binding via the cytosolic adaptor molecules Gads and SLP76 [112]. PLC catalyzes the hydrolysis of phosphatidylinosoitol (4,5)-bis phosphate (PIP2) with resulting liberation of inositol (1,4,5)-trisphosphate (IP3) and diacylglycerol (DAG) [113] which respectively liberate Ca2+ [114] from the endoplasmic reticulum and induce the activation of protein kinase C (PKC) [115, 116]. Using knock out approaches [109], calcium buffering experiments [116] and pharmacological inhibitors [117], it has been demonstrated that these signalling processes are essential for the ability of antigen to elicit a degranulation response.
A parallel complementary pathway which is initiated following activation of the tyrosine kinase Fyn is also required for optimal degranulation [118]. This pathway, which follows phosphorylation of the cytosolic adaptor molecule Gab2, leads to the activation of class 1A PI3K [118]. In the mast cell, the p110δ PI3K subunit isoform appears to be the predominant type utilized by the FcεRI for deganulation and cytokine production [119]. PI3K catalyses the phosphorylation of plasma-membrane associated PIP2 to form phosphatidylinositol (3,4,5)-triphosphate (PIP3). This molecular configuration binds pleckstrin homology (PH) domains contained in a subset of critical signalling molecules allowing these molecules to be recruited into the signalosome. These signalling molecules include PLCγ1 and PLCγ2, the Btk, and the serine/threonine kinases PDK1 and AKT [120, 121]. The use of knock out [122] and transgenic mice [119], and pharmacological inhibitors [117, 119] have demonstrated a critical role for PI3K in mast cell activation, though mouse mast cell appear to show a greater dependency on PI3K mediated responses as do human mast cells. Regardless, it has been proposed that PI3K regulates an amplification pathway also involving NTAL and Btk, for ongoing signalling events initiated by the LAT-PLCγ1 pathway [6].
Many of the same signalling events described above are also critical for eicosanoid production and cytokine gene expression [101, 123], however, the terminal events diverge from those required for degranulation. The eicosanoids are generated as a consequence of MAP kinase-dependent activation of PLA2 which liberates the precursor, arachidonic acid, from phospholipids [124]. The MAP kinase pathway also regulates cytokine gene expression by inducing the phosphorylation of transcription factors such as the AP1 components Fos and Jun [125]. Cytokine production is also regulated by other PI3K and calcium dependent process and the downstream activation of other transcription factors such as NF-κB and NFAT [119, 125, 126].
GPCR-mediated signalling
The ability of GPCRs to regulate mast cell responses such as degranulation and cytokine production requires that the initial signals generated by these receptors ultimately impact on the terminal signalling processes described above for FcεRI. Unlike the FcεRI receptor, GPCRs do not appear to require tyrosine kinases for the initiation of degranulation or cytokine generation but, as described earlier, GPCRs regulate their function through Gα subunits and βγ subunits [6, 23, 27]. Thus, how GPCRs influence mast cell function depends directly upon the class of G protein engaged and how the individual subunits regulate effector proteins. Although the initiating events utilized by the FcεRI and GPCRs may differ, there are common elements to these pathways at more receptor-distal stages. In this respect, as with the FcεRI, the signalling cascades which allow GPCRs to induce degranulation or potentiate FcεRI-mediated degranulation ultimately lead to the activation of the critical signalling enzymes PLC and PI3K [27, 66]. However, unlike the FcεRI which utilizes PLCγ1 and PLCγ2 [6, 109], and primarily the p110β subunit isoform of PI3K [119] for signalling, GPCRs activate PLCγ [27, 113] and the p110γ subunit isoform of PI3K [127]. Regardless, both PLCβ and PLCγ isoforms, when activated, will lead to the generation of IP3 and DAG leading to calcium mobilization and PKC activation respectively. In addition, the p110β and γ subunit isoforms of PI3K will both induce the formation of membrane-associated PIP3 allowing the membrane recruitment of PH domain-containing signalling molecules [121].
Transmission of signalling via Gi (Gαi and/or βγ) alone is sufficient for the GPCRs to initiate activation of both PLCβ and PI3Kγ [27] and indeed the majority of the GPCRs that promote degranulation of mast cells, whether in the presence or absence of antigen, are linked to Gi as evidenced by their sensitivity to pertussis toxin. The exception being the S1P2 receptor which, although linked to Gi, likely mediates its effects via G12/13 or Gq leading to the respective activation of either PLCβ, or of the GTP-binding proteins Rho [44, 46]. The ability of specific GPCRs, e.g. the EP2, A2A, and β2 adrenergic receptor to down-regulate FcεRI-mediated mast cell activation is dependent on that fact that these receptors induce adenylyl cyclase-dependent cAMP production via Gs. cAMP has been demonstrated to negatively regulate mast cell function [100] although the precise mechanism controlling this response still remains unclear. However, conditions that elevate cAMP have been demonstrated to stimulated the phosphorylation of the transcription regulator CREB and to induce the expression of the CREB dependent inducible cAMP early repressor [79]. It is therefore possible that, at least the inhibitory effect of cAMP on cytokine production is linked to these events.
It is still unclear why the signals generated by adenosine and PGE2 are insufficient by themselves to induce degranulation yet those induced by C3a and S1P are capable of inducing degranulation in the absence of antigen. One possible explanation is that adenosine and PGE2 can simultaneously activate both inhibitory pathways, via Gs, and stimulatory pathways, via Gi, thus the ability to induce degranulation is kept in check by the inhibitory signals, whereas, both C3a and S1P appear to be only positively regulating mast cell function through Gi, and/or Gq. A further explanation may be that the signals produced by adenosine and PGE2 are of sufficient strength and duration to allow degranulation to proceed. For example, the calcium signal produced by these agents is transient, and not of sufficient strength to effectively deplete the intracellular stores to trigger store-operated calcium entry necessary for degranulation [62, 72]. C3a, in contrast, produces a maintained calcium signal [36] which appears to be of sufficient magnitude and duration to induce a degranulation response in the absence of antigen. S1P is unique in the sense that it does not require Gi to elicit its effects on degranulation [44, 45]. As it is also linked to G12/13, and Gq, it is possible that its ability to induce degranulation of mast cells is linked to PLCβ activation as mediated by Gq.
The activation of PLCs and PI3K by both Gi-coupled GPCRs and FcεRI provides a mechanism by which the signals indiuced by these receptors may be integrated for the synergistic enhancement of mast cell mediator release. Based on data obtained from BMMCs derived from the bone marrow of PI3Kγ knock out mice, it has been proposed that the ability of several GPCRs, including those for adenosine, MIP-1α, and Rantes, to synergistically enhance antigen-induced intracellular calcium modulation and degranulation, is regulated by Gi dependent PI3Kγ activation [66]. However, Figure 2b of this study [66] suggest that although reduced, adenosine can still synergistically enhance FcεRI-mediated degranulation in the absence of PI3Kγ. Furthermore, our unpublished observations (Kuehn and Gilfillan) suggest that the ability of PGE2 to synergistically enhance FcεRI-mediated degranulation in BMMCs is independent of PI3K but rather involves Gi-dependent trans-synergy in the activation of PLCγ and PLCβ leading to a marked enhancement of store operated calcium entry. Whether this applies for the other Gi-linked receptors which modify mast cell function remains to be established
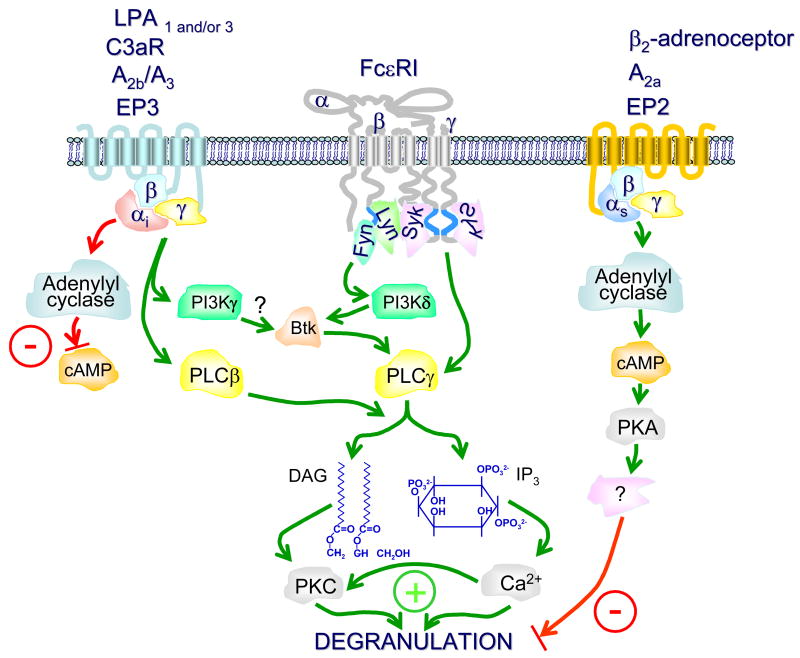
Fig 2.
The respective balance between Gi- and Gs-dependent signalling pathways and their roles in the modification of FcαRI-mediated mast cell degranulation. For clarity, many of the intermediary steps in the FcαRI-dependent signalling pathway have been omitted. For further details concerning this pathway and the pathways regulating cytokine production and eicosanoid release, the readers are referred to the text and references [6, 101]. FcαRI aggregation leads to the phospholipase Cγ (PLCγ)-mediated production of diaclglycerol (DAG) and inositol trisphosphate (IP3) which respectively activate protein kinase C (PKC) and mobilize calcium from intracellular stores, essential signals for degranulation. Phosphoinositide 3-kinase (PI3K), primarily the p110δ subunit, contributes to a maintained calcium signal partly through the Bruton’s tyrosine kinase (Btk)-dependent enhancement of PLCγ activation. Gi-coupled receptors impact these events positively by downregulating the production of cyclic adenosine monophosphate (cAMP) which is a negative signal for mast cell activation and by promoting the activation of PI3Kγ and PLCβ. In contrast Gs-coupled receptors impact FcεRI-mediated mast cell activation by enhancing the production of cAMP which, via activation of protein kinase A (PKA), inhibits mast cell activation by an, as yet, unknown mechanism. S1P2 and P2Y which are coupled to Gq-to induce calcium flux and result in degranulation (not shown in this figure). The green lines and positive symbols designate activating signalling pathways and the red lines and -symbols represent inhibiting signalling pathways.
Conclusions
In this review we have discussed how GPCRs can either induce mast cell activation or modify antigen-dependent mast cell activation. The majority of the studies described have been carried out in cultured mouse mast cell and it is apparent from the limited studies conducted in human mast cells that there may significant differences in the nature of the responses generated by GPCRs between mouse and human. These differences may be related to interspecies differences in the expression of subclasses of receptors for specific ligands and the nature of the G proteins which are coupled to these receptors. In this respect the balance between Gi- and Gs-coupled receptors (Fig. 2) appears to be critical, with the former G protein up-regulating mast cell responses likely via PLCβ and p110γ PI3K regulated pathways, and the latter G protein down-regulating mast cell responses following adenylyl cyclase-dependent enhanced cAMP levels. Further studies are thus required to determine how the relative expression of the GPCRs modifying mast cell responses are regulated and to determine to what extent G proteins contribute to mast cell reaction in normal and disease settings in the human.
Table 1.
Ligands, and their GPCR expressed on mast cells that influence mast-cell activation. Although other GPCR ligands such as eotaxin and fractalkine etc. can induce mast cell chemotaxis, we have only listed the ligands that effect mast cell mediator release and/or cytokine or chemokine production.
| Ligand | Receptor : G-protein | species | Mast cell response |
|---|---|---|---|
| C3a | C3aR : Gi | Human | |
| S1P | S1P1 : Gi
S1P2: Gi,Gq,G12 |
Mouse | |
| LPA | LPA1 & 2:Gi,Gq,G12
LPA3 : Gi,Gq LPA4 : Gs |
Human |
|
| Mouse |
|
||
| PGE2 | EP1 : Gq
EP2 : Gs EP3 : Gi,Gq,Gs EP4 : Gs |
Human | |
| Mouse | |||
| Adenosine | A2A : Gs
A2B : Gs, Gq A3 : Gi, Gq |
Human | |
| Mouse | |||
|
ATP, ADP
UTP, UDP |
P2Y1 : Gq
P2Y2 : Gi,Gq P2Y11 : Gs, Gq P2Y12 & 13 : Gi |
Human | |
| Mouse | |||
| β2-adrenergic agonists | β2-adrenoceptor | Human | |
| MIP-1α | CCR1 | Mouse | |
| Rantes | CCR1,3,4 | Mouse |
Acknowledgments
Research in the authors’ laboratory is supported by the NIAID Intramural Program within the National Institutes of Health, USA. Due to the restricted space available, not all pertinent literature could be referenced in this article. This does not imply that studies not quoted are of lesser merit.
Abbreviations
- BMMCs
bone marrow-derived mast cells
- cAMP
cyclic adenosine monophosphate
- EP
E prostanoid receptor
- FcεRI
High affinity IgE-receptor
- GPCR
G protein-coupled receptor
- IgE
immunoglobulin E
- LPA
lysophosphatidic acid
- LT
leukotriene
- MIP-1α
macrophage inflammatory protein-1α
- MCP
monocyte chemoattractant protein
- PI3K
phosphoinositide 3-kinase
- PGE2
prostaglandin E2
- PLC
phospholipase C
- Rantes
regulated upon activation, normal T cell expressed and secreted
- S1P
sphingosine-1-phosphate
- TLR
Toll like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–99. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 3.Tkaczyk C, Jensen BM, Iwaki S, Gilfillan AM. Adaptive and innate immune reactions regulating mast cell activation: from receptor-mediated signaling to responses. Immunol Allergy Clin North Am. 2006;26:427–50. doi: 10.1016/j.iac.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 5.Metzger H. The receptor with high affinity for IgE. Immunol Rev. 1992;125:37–48. doi: 10.1111/j.1600-065x.1992.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 6.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–30. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 7.Fujishima H, Sanchez Mejia RO, Bingham CO, 3rd, Lam BK, Sapirstein A, Bonventre JV, et al. Cytosolic phospholipase A2 is essential for both the immediate and the delayed phases of eicosanoid generation in mouse bone marrow-derived mast cells. Proc Natl Acad Sci U S A. 1999;96:4803–7. doi: 10.1073/pnas.96.9.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tkaczyk C, Horejsi V, Iwaki S, Draber P, Samelson LE, Satterthwaite AB, et al. NTAL phosphorylation is a pivotal link between the signaling cascades leading to human mast cell degranulation following Kit activation and FcεRI aggregation. Blood. 2004;104:207–14. doi: 10.1182/blood-2003-08-2769. [DOI] [PubMed] [Google Scholar]
- 9.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104:2410–7. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff SC, Dahinden CA. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med. 1992;175:237–44. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwaki S, Tkaczyk C, Satterthwaite AB, Halcomb K, Beaven MA, Metcalfe DD, et al. Btk plays a crucial role in the amplification of Fc epsilonRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–70. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- 12.Galli SJ, Tsai M, Wershil BK. The c-kit receptor, stem cell factor, and mast cells. What each is teaching us about the others. Am J Pathol. 1993;142:965–74. [PMC free article] [PubMed] [Google Scholar]
- 13.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcεR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–8. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taub D, Dastych J, Inamura N, Upton J, Kelvin D, Metcalfe D, et al. Bone marrow-derived murine mast cells migrate, but do not degranulate, in response to chemokines. J Immunol. 1995;154:2393–402. [PubMed] [Google Scholar]
- 16.Olivera A, Rivera J. Sphingolipids and the balancing of immune cell function: lessons from the mast cell. J Immunol. 2005;174:1153–8. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 17.Hughes PJ, Holgate ST, Church MK. Adenosine inhibits and potentiates IgE-dependent histamine release from human lung mast cells by an A2-purinoceptor mediated mechanism. Biochem Pharmacol. 1984;33:3847–52. doi: 10.1016/0006-2952(84)90050-9. [DOI] [PubMed] [Google Scholar]
- 18.Wang XS, Lau HY. Prostaglandin E potentiates the immunologically stimulated histamine release from human peripheral blood-derived mast cells through EP1/EP3 receptors. Allergy. 2006;61:503–6. doi: 10.1111/j.1398-9995.2006.01043.x. [DOI] [PubMed] [Google Scholar]
- 19.Kay LJ, Yeo WW, Peachell PT. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br J Pharmacol. 2006;147:707–13. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107:3243–50. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. Embo J. 1999;18:1723–9. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. Faseb J. 1997;11:346–54. [PubMed] [Google Scholar]
- 23.Hamm HE, Gilchrist A. Heterotrimeric G proteins. Curr Opin Cell Biol. 1996;8:189–96. doi: 10.1016/s0955-0674(96)80065-2. [DOI] [PubMed] [Google Scholar]
- 24.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 25.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–8. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 26.Bence K, Ma W, Kozasa T, Huang XY. Direct stimulation of Bruton's tyrosine kinase by G(q)-protein alpha-subunit. Nature. 1997;389:296–9. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 27.Kehrl JH. Heterotrimeric G protein signaling: roles in immune function and fine-tuning by RGS proteins. Immunity. 1998;8:1–10. doi: 10.1016/s1074-7613(00)80453-7. [DOI] [PubMed] [Google Scholar]
- 28.Albert PR, Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–18. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 29.Clapham DE, Neer EJ. G protein beta gamma subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 30.Papadopoulos EJ, Fitzhugh DJ, Tkaczyk C, Gilfillan AM, Sassetti C, Metcalfe DD, et al. Mast cells migrate, but do not degranulate, in response to fractalkine, a membrane-bound chemokine expressed constitutively in diverse cells of the skin. Eur J Immunol. 2000;30:2355–61. doi: 10.1002/1521-4141(2000)30:8<2355::AID-IMMU2355>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Romagnani P, De Paulis A, Beltrame C, Annunziato F, Dente V, Maggi E, et al. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol. 1999;155:1195–204. doi: 10.1016/S0002-9440(10)65222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, et al. 5-hydroxytryptamine induces mast cell adhesion and migration. J Immunol. 2006;177:6422–32. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 33.Ali H, Panettieri RA., Jr Anaphylatoxin C3a receptors in asthma. Respir Res. 2005;6:19. doi: 10.1186/1465-9921-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, et al. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863–70. [PubMed] [Google Scholar]
- 35.Thangam EB, Venkatesha RT, Zaidi AK, Jordan-Sciutto KL, Goncharov DA, Krymskaya VP, et al. Airway smooth muscle cells enhance C3a-induced mast cell degranulation following cell-cell contact. Faseb J. 2005;19:798–800. doi: 10.1096/fj.04-2797fje. [DOI] [PubMed] [Google Scholar]
- 36.Venkatesha RT, Berla Thangam E, Zaidi AK, Ali H. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol. 2005;42:581–7. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Erdei A, Andreev S, Pecht I. Complement peptide C3a inhibits IgE-mediated triggering of rat mucosal mast cells. Int Immunol. 1995;7:1433–9. doi: 10.1093/intimm/7.9.1433. [DOI] [PubMed] [Google Scholar]
- 38.Andrasfalvy M, Peterfy H, Toth G, Matko J, Abramson J, Kerekes K, et al. The beta subunit of the type I Fcepsilon receptor is a target for peptides inhibiting IgE-mediated secretory response of mast cells. J Immunol. 2005;175:2801–6. doi: 10.4049/jimmunol.175.5.2801. [DOI] [PubMed] [Google Scholar]
- 39.Woolhiser MR, Brockow K, Metcalfe DD. Activation of human mast cells by aggregated IgG through FcgammaRI: additive effects of C3a. Clin Immunol. 2004;110:172–80. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Mousli M, Hugli TE, Landry Y, Bronner C. A mechanism of action for anaphylatoxin C3a stimulation of mast cells. J Immunol. 1992;148:2456–61. [PubMed] [Google Scholar]
- 41.Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, et al. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996;157:1693–8. [PubMed] [Google Scholar]
- 42.Goetzl EJ, Wang W, McGiffert C, Huang MC, Graler MH. Sphingosine 1-phosphate and its G protein-coupled receptors constitute a multifunctional immunoregulatory system. J Cell Biochem. 2004;92:1104–14. doi: 10.1002/jcb.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–70. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 44.Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–25. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 45.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, et al. Transactivation of sphingosine-1-phosphate receptors by FcepsilonRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–70. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siehler S, Manning DR. Pathways of transduction engaged by sphingosine 1-phosphate through G protein-coupled receptors. Biochim Biophys Acta. 2002;1582:94–9. doi: 10.1016/s1388-1981(02)00142-7. [DOI] [PubMed] [Google Scholar]
- 47.Lee HS, Park CS, Lee YM, Suk HY, Clemons TC, Choi OH. Antigen-induced Ca2+ mobilization in RBL-2H3 cells: role of I(1,4,5)P3 and S1P and necessity of I(1,4,5)P3 production. Cell Calcium. 2005;38:581–92. doi: 10.1016/j.ceca.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380:634–6. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 49.Jolly PS, Bektas M, Watterson KR, Sankala H, Payne SG, Milstien S, et al. Expression of SphK1 impairs degranulation and motility of RBL-2H3 mast cells by desensitizing S1P receptors. Blood. 2005;105:4736–42. doi: 10.1182/blood-2004-12-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuyuki H, Maeda Y, Yano K, Sugahara K, Chiba K, Kohno T, et al. Involvement of sphingosine 1-phosphate (S1P) receptor type 1 and type 4 in migratory response of mouse T cells toward S1P. Cell Mol Immunol. 2006;3:429–37. [PubMed] [Google Scholar]
- 51.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103:16394–9. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olivera A, Mizugishi K, Tikhonova A, Ciaccia L, Odom S, Proia RL, et al. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–97. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Bagga S, Price KS, Lin DA, Friend DS, Austen KF, Boyce JA. Lysophosphatidic acid accelerates the development of human mast cells. Blood. 2004;104:4080–7. doi: 10.1182/blood-2004-03-1166. [DOI] [PubMed] [Google Scholar]
- 54.Lin DA, Boyce JA. IL-4 regulates MEK expression required for lysophosphatidic acid-mediated chemokine generation by human mast cells. J Immunol. 2005;175:5430–8. doi: 10.4049/jimmunol.175.8.5430. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto T, Ohata H, Honda K. Lysophosphatidic acid (LPA) induces plasma exudation and histamine release in mice via LPA receptors. J Pharmacol Sci. 2006;100:82–7. doi: 10.1254/jphs.fpj05030x. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto T, Ohata H, Momose K, Honda K. Lysophosphatidic acid induces histamine release from mast cells and skin fragments. Pharmacology. 2005;75:13–20. doi: 10.1159/000085784. [DOI] [PubMed] [Google Scholar]
- 57.Marshall JS, Gomi K, Blennerhassett MG, Bienenstock J. Nerve growth factor modifies the expression of inflammatory cytokines by mast cells via a prostanoid-dependent mechanism. J Immunol. 1999;162:4271–6. [PubMed] [Google Scholar]
- 58.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 59.Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004;25:40–6. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 61.Gomi K, Zhu FG, Marshall JS. Prostaglandin E2 selectively enhances the IgE-mediated production of IL-6 and granulocyte-macrophage colony-stimulating factor by mast cells through an EP1/EP3-dependent mechanism. J Immunol. 2000;165:6545–52. doi: 10.4049/jimmunol.165.11.6545. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen M, Solle M, Audoly LP, Tilley SL, Stock JL, McNeish JD, et al. Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J Immunol. 2002;169:4586–93. doi: 10.4049/jimmunol.169.8.4586. [DOI] [PubMed] [Google Scholar]
- 63.Polosa R, Holgate ST. Adenosine receptors as promising therapeutic targets for drug development in chronic airway inflammation. Curr Drug Targets. 2006;7:699–706. doi: 10.2174/138945006777435236. [DOI] [PubMed] [Google Scholar]
- 64.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148:91–7. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 65.Forsythe P, Ennis M. Adenosine, mast cells and asthma. Inflamm Res. 1999;48:301–7. doi: 10.1007/s000110050464. [DOI] [PubMed] [Google Scholar]
- 66.Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, et al. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–51. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 67.Gilfillan AM, Wiggan GA, Welton AF. Pertussis toxin pretreatment reveals differential effects of adenosine analogs on IgE-dependent histamine and peptidoleukotriene release from RBL-2H3 cells. Biochim Biophys Acta. 1990;1052:467–74. doi: 10.1016/0167-4889(90)90157-9. [DOI] [PubMed] [Google Scholar]
- 68.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–90. [PubMed] [Google Scholar]
- 69.Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I. Cross-talk between G(s)- and G(q)-coupled pathways in regulation of interleukin-4 by A(2B) adenosine receptors in human mast cells. Mol Pharmacol. 2006;70:727–35. doi: 10.1124/mol.106.022780. [DOI] [PubMed] [Google Scholar]
- 70.Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest. 1995;96:1979–86. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duffy SM, Cruse G, Brightling CE, Bradding P. Adenosine closes the K+ channel KCa3.1 in human lung mast cells and inhibits their migration via the adenosine A2A receptor. Eur J Immunol. 2007;37:1653–62. doi: 10.1002/eji.200637024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, et al. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003;171:338–45. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki H, Takei M, Nakahata T, Fukamachi H. Inhibitory effect of adenosine on degranulation of human cultured mast cells upon cross-linking of Fc epsilon RI. Biochem Biophys Res Commun. 1998;242:697–702. doi: 10.1006/bbrc.1997.8040. [DOI] [PubMed] [Google Scholar]
- 74.Linden J, Thai T, Figler H, Jin X, Robeva AS. Characterization of human A(2B) adenosine receptors: radioligand binding, western blotting, and coupling to G(q) in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol. 1999;56:705–13. [PubMed] [Google Scholar]
- 75.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000;275:4429–34. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 76.Yamano K, Inoue M, Masaki S, Saki M, Ichimura M, Satoh M. Human adenosine A(3) receptor leads to intracellular Ca(2+) mobilization but is insufficient to activate the signaling pathway via phosphoinositide 3-kinase gamma in mice. Biochem Pharmacol. 2005;70:1487–96. doi: 10.1016/j.bcp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 77.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–75. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 78.Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 79.Feng C, Mery AG, Beller EM, Favot C, Boyce JA. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173:7539–47. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- 80.Jaffar ZH, Pearce FL. Histamine secretion from mast cells stimulated with ATP. Agents Actions. 1990;30:64–6. doi: 10.1007/BF01968999. [DOI] [PubMed] [Google Scholar]
- 81.Saito H, Sakaguchi N, Ebisawa M, Matsumoto K, Akasawa A, Iikura Y. The stimuli releasing histamine from murine bone marrow-derived mast cells. 2. Mechanisms involved in histamine release induced by extracellular ATP and its metabolites. Arerugi. 1991;40:680–8. [PubMed] [Google Scholar]
- 82.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–4. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 83.Schulman ES, Glaum MC, Post T, Wang Y, Raible DG, Mohanty J, et al. ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am J Respir Cell Mol Biol. 1999;20:530–7. doi: 10.1165/ajrcmb.20.3.3387. [DOI] [PubMed] [Google Scholar]
- 84.Nakajima T, Inagaki N, Tanaka H, Tanaka A, Yoshikawa M, Tamari M, et al. Marked increase in CC chemokine gene expression in both human and mouse mast cell transcriptomes following Fcepsilon receptor I cross-linking: an interspecies comparison. Blood. 2002;100:3861–8. doi: 10.1182/blood-2002-02-0602. [DOI] [PubMed] [Google Scholar]
- 85.Kambayashi T, Koretzky GA. Proximal signaling events in Fc epsilon RI-mediated mast cell activation. J Allergy Clin Immunol. 2007;119:544–52. doi: 10.1016/j.jaci.2007.01.017. quiz 53–4. [DOI] [PubMed] [Google Scholar]
- 86.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 87.Murray LA, Syed F, Li L, Griswold DE, Das AM. Role of chemokines in severe asthma. Curr Drug Targets. 2006;7:579–88. doi: 10.2174/138945006776818674. [DOI] [PubMed] [Google Scholar]
- 88.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38:881–5. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 89.Pease JE. Asthma, allergy and chemokines. Curr Drug Targets. 2006;7:3–12. doi: 10.2174/138945006775270204. [DOI] [PubMed] [Google Scholar]
- 90.Zhao ZZ, Sugerman PB, Zhou XJ, Walsh LJ, Savage NW. Mast cell degranulation and the role of T cell RANTES in oral lichen planus. Oral Dis. 2001;7:246–51. [PubMed] [Google Scholar]
- 91.Castellani ML, Petrarca C, Frydas S, Conti CM, Salini V, Conti P, et al. Rat basophilic leukemia cells (RBL-2H3) generate prostaglandin D2 (PGD2) after regulated upon activation, normal T-cell expressed and secreted (RANTES) activation. Int J Biol Markers. 2006;21:211–7. doi: 10.1177/172460080602100403. [DOI] [PubMed] [Google Scholar]
- 92.Juremalm M, Olsson N, Nilsson G. Selective CCL5/RANTES-induced mast cell migration through interactions with chemokine receptors CCR1 and CCR4. Biochem Biophys Res Commun. 2002;297:480–5. doi: 10.1016/s0006-291x(02)02244-1. [DOI] [PubMed] [Google Scholar]
- 93.Toda M, Dawson M, Nakamura T, Munro PM, Richardson RM, Bailly M, et al. Impact of engagement of FcepsilonRI and CC chemokine receptor 1 on mast cell activation and motility. J Biol Chem. 2004;279:48443–8. doi: 10.1074/jbc.M408725200. [DOI] [PubMed] [Google Scholar]
- 94.Miyazaki D, Nakamura T, Toda M, Cheung-Chau KW, Richardson RM, Ono SJ. Macrophage inflammatory protein-1alpha as a costimulatory signal for mast cell-mediated immediate hypersensitivity reactions. J Clin Invest. 2005;115:434–42. doi: 10.1172/JCI18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Waldeck B. Beta-adrenoceptor agonists and asthma--100 years of development. Eur J Pharmacol. 2002;445:1–12. doi: 10.1016/s0014-2999(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 96.Peachell P. Regulation of mast cells by beta-agonists. Clin Rev Allergy Immunol. 2006;31:131–42. doi: 10.1385/CRIAI:31:2:131. [DOI] [PubMed] [Google Scholar]
- 97.Wang XS, Lau HY. Beta-adrenoceptor-mediated inhibition of mediator release from human peripheral blood-derived mast cells. Clin Exp Pharmacol Physiol. 2006;33:746–50. doi: 10.1111/j.1440-1681.2006.04435.x. [DOI] [PubMed] [Google Scholar]
- 98.Akabane H, Murata M, Kubota M, Takashima E, Tanaka H, Inagaki N, et al. Effects of salmeterol xinafoate and fluticasone propionate on immunological activation of human cultured mast cells. Allergol Int. 2006;55:387–93. doi: 10.2332/allergolint.55.387. [DOI] [PubMed] [Google Scholar]
- 99.Bissonnette EY, Befus AD. Anti-inflammatory effect of beta 2-agonists: inhibition of TNF-alpha release from human mast cells. J Allergy Clin Immunol. 1997;100:825–31. doi: 10.1016/s0091-6749(97)70280-x. [DOI] [PubMed] [Google Scholar]
- 100.Weston MC, Peachell PT. Regulation of human mast cell and basophil function by cAMP. Gen Pharmacol. 1998;31:715–9. doi: 10.1016/s0306-3623(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 101.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–25. doi: 10.1016/j.jaci.2006.04.015. quiz 26. [DOI] [PubMed] [Google Scholar]
- 102.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–78. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 103.Kovarova M, Tolar P, Arudchandran R, Draberova L, Rivera J, Draber P. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcepsilon receptor I aggregation. Mol Cell Biol. 2001;21:8318–28. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Field KA, Holowka D, Baird B. Structural aspects of the association of FcepsilonRI with detergent-resistant membranes. J Biol Chem. 1999;274:1753–8. doi: 10.1074/jbc.274.3.1753. [DOI] [PubMed] [Google Scholar]
- 105.Yamashita T, Mao SY, Metzger H. Aggregation of the high-affinity IgE receptor and enhanced activity of p53/56lyn protein-tyrosine kinase. Proc Natl Acad Sci U S A. 1994;91:11251–5. doi: 10.1073/pnas.91.23.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vonakis BM, Chen H, Haleem-Smith H, Metzger H. The unique domain as the site on Lyn kinase for its constitutive association with the high affinity receptor for IgE. J Biol Chem. 1997;272:24072–80. doi: 10.1074/jbc.272.38.24072. [DOI] [PubMed] [Google Scholar]
- 107.Eiseman E, Bolen JB. Engagement of the high-affinity IgE receptor activates src protein-related tyrosine kinases. Nature. 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 108.Chen T, Repetto B, Chizzonite R, Pullar C, Burghardt C, Dharm E, et al. Interaction of phosphorylated FcepsilonRIgamma immunoglobulin receptor tyrosine activation motif-based peptides with dual and single SH2 domains of p72syk. Assessment of binding parameters and real time binding kinetics. J Biol Chem. 1996;271:25308–15. doi: 10.1074/jbc.271.41.25308. [DOI] [PubMed] [Google Scholar]
- 109.Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, et al. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity. 2000;12:525–35. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 110.Iwaki S, Jensen BM, Gilfillan AM. Ntal/Lab/Lat2. Int J Biochem Cell Biol. 2007;39:868–73. doi: 10.1016/j.biocel.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rivera J. NTAL/LAB and LAT: a balancing act in mast-cell activation and function. Trends Immunol. 2005;26:119–22. doi: 10.1016/j.it.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 112.Silverman MA, Shoag J, Wu J, Koretzky GA. Disruption of SLP-76 interaction with Gads inhibits dynamic clustering of SLP-76 and FcepsilonRI signaling in mast cells. Mol Cell Biol. 2006;26:1826–38. doi: 10.1128/MCB.26.5.1826-1838.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beaven MA, Metzger H. Signal transduction by Fc receptors: the Fc epsilon RI case. Immunol Today. 1993;14:222–6. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- 115.Bell RM, Burns DJ. Lipid activation of protein kinase C. J Biol Chem. 1991;266:4661–4. [PubMed] [Google Scholar]
- 116.Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, et al. Ca(2+)-dependent and Ca(2+)-independent isozymes of protein kinase C mediate exocytosis in antigen-stimulated rat basophilic RBL-2H3 cells. Reconstitution of secretory responses with Ca2+ and purified isozymes in washed permeabilized cells. J Biol Chem. 1993;268:1749–56. [PubMed] [Google Scholar]
- 117.Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. The phospholipase Cγ1-dependent pathway of FcεRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278:48474–84. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- 118.Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, et al. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–8. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 119.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, et al. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–11. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 120.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–45. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 121.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 122.Lu-Kuo JM, Fruman DA, Joyal DM, Cantley LC, Katz HR. Impaired kit- but not FcepsilonRI-initiated mast cell activation in the absence of phosphoinositide 3-kinase p85alpha gene products. J Biol Chem. 2000;275:6022–9. doi: 10.1074/jbc.275.8.6022. [DOI] [PubMed] [Google Scholar]
- 123.Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, et al. Impaired FcepsilonRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J Immunol. 2005;175:7602–10. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- 124.Bingham CO, 3rd, Austen KF. Phospholipase A2 enzymes in eicosanoid generation. Proc Assoc Am Physicians. 1999;111:516–24. doi: 10.1046/j.1525-1381.1999.99321.x. [DOI] [PubMed] [Google Scholar]
- 125.Lorentz A, Klopp I, Gebhardt T, Manns MP, Bischoff SC. Role of activator protein 1, nuclear factor-kappaB, and nuclear factor of activated T cells in IgE receptor-mediated cytokine expression in mature human mast cells. J Allergy Clin Immunol. 2003;111:1062–8. doi: 10.1067/mai.2003.1342. [DOI] [PubMed] [Google Scholar]
- 126.Kitaura J, Asai K, Maeda-Yamamoto M, Kawakami Y, Kikkawa U, Kawakami T. Akt-dependent cytokine production in mast cells. J Exp Med. 2000;192:729–40. doi: 10.1084/jem.192.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 2002;14:7–18. doi: 10.1006/smim.2001.0337. [DOI] [PubMed] [Google Scholar]
- 128.Nakamura H, Saito H, Ikura Y. The stimuli releasing histamine from murine bone marrow-derived mast cells. 1. The presence of P2-purinoceptors. Arerugi. 1989;38:1359–63. [PubMed] [Google Scholar]
- 129.Budagian V, Bulanova E, Brovko L, Orinska Z, Fayad R, Paus R, et al. Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-kappa B. J Biol Chem. 2003;278:1549–60. doi: 10.1074/jbc.M206383200. [DOI] [PubMed] [Google Scholar]