The immune system normally defends the body against the constant threat of myriad organisms and substances in the surrounding environment. In some individuals immune reactivity becomes inappropriate or excessive, and autoimmune or chronic inflammatory diseases ensue, which usually persist for most of the patient’s life. While chronic inflammation can affect practically any organ, this process is distinctive in the gastrointestinal tract: Whereas most organs contain only a sprinkling of immune cells, the normal gut contains a rich lymphoid compartment that maintains a low level of “physiological” intestinal inflammation. Enteric flora and food antigens are believed to induce and sustain this physiological intestinal inflammation that is transformed into destructive and persistent “pathological” inflammation when the gut is involved by Crohn disease (CD), ulcerative colitis (UC), or other forms of chronic inflammatory bowel disease (IBD).
Recent advances in the understanding of IBD pathogenesis, especially in regard to regulation of mucosal cytokines, raised hopes of uncovering specific imbalances of pro- and anti-inflammatory mediators that could explain the mechanisms and chronicity of inflammation (1). Numerous cytokine abnormalities that may contribute to IBD pathogenesis are indeed found in the mucosa of CD and UC patients (2). Remarkably, however, chronic inflammation thrives uncontrolled in the gut of IBD patients despite the induction of potent immunosuppressive and anti-inflammatory cytokines, such as IL-1 receptor antagonist, IL-10, and TGF-&bgr; (3–5). This paradoxical situation has led to the assumption that the anti-inflammatory defenses induced are inadequate to offset a seemingly invincible proinflammatory offense. Why should this be so? Why should proinflammatory cytokines like IFN-&ggr; and TNF-&agr; have the upper hand against equally powerful anti-inflammatory mediators? This key issue has been addressed in this issue of the JCI by Monteleone et al., who describe specific defects of TGF-&bgr;1–mediated immunosuppression in the mucosa of IBD patients (6).
TGF-&bgr; signaling in healthy and inflamed tissues
TGF-&bgr; is a cytokine produced by both immune and nonimmune cells, and it exhibits a broad range of functions, primus inter pares being the modulation of immune responses. TGF-&bgr; controls the differentiation, proliferation, and state of activation of all immune cells, and is implicated in immune abnormalities linked to cancer, autoimmunity, opportunistic infections, and fibrotic complications (7). In addition, induction of TGF-&bgr; is the step sine qua non in the regulation of one of the major functions of the intestinal immune system, the induction of oral tolerance. In this process, immune reactivity against orally ingested antigens is selectively suppressed, largely through the effects of TGF-&bgr; and its downstream targets.
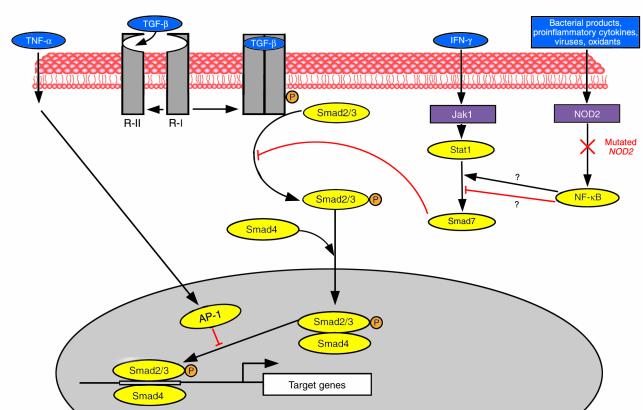
TGF-&bgr;–triggered signals are transduced by Smads, a family of proteins that serve as substrates for TGF-&bgr; receptor type I and type II and contains several members (8). The type I receptor recognizes Smad2 and -3 which, en route to the nucleus, associate with Smad4, forming complexes that participate in DNA binding and recruitment of transcription factors (Figure 1). In addition to these agonistic Smads, antagonist or inhibitory Smads also exist, like Smad7, which blocks activated receptors and interferes with phosphorylation of Smad2 and -3. Interestingly, both IFN-&ggr; and TNF-&agr; inhibit the TGF-&bgr;/Smad signaling pathways (9, 10), suggesting that these proinflammatory cytokines act, at least in part, by blocking the effects of immunosuppressive cytokines like TGF-&bgr;.
Figure 1.
Schematic representation of the TGF-&bgr;/Smad pathway and its interrelationship with mediators of inflammatory signals. Ligation of TGF-&bgr; to the constitutively active receptor type II (R-II) causes recruitment and phosphorylation (P) of receptor type I (R-I) and formation of a receptor complex. The activated receptor I phosphorylates receptor-regulated Smad2 and -3, which then form a complex with the common mediator Smad4. The Smad2/3-Smad4 complex translocates into the nucleus together with DNA-binding cofactors and binds to enhancers specific for TGF-&bgr; target genes. The inhibitory Smad7 antagonizes TGF-&bgr; signaling by interfering with the binding of Smad2 and -3 with the activated receptor complex. IFN-&ggr; inhibits the TGF-&bgr;/Smad signaling pathway by upregulating the expression of Smad7. TNF-&agr; inhibits the pathway by inducing AP-1 components (c-Jun and JunB) that directly interfere with the interaction of the Smad2/3-Smad4 complex with DNA. Activation of NF-&kgr;B by a variety of inflammatory stimuli may also regulate the TGF-&bgr;/Smad pathway, but whether this involves activation or inhibition of Smad7 is still unclear. The NOD2 mutation, recently described in some CD patients, is depicted to suggest how it could hypothetically diminish the anti-inflammatory action of TFG-&bgr; by impairing NF-&kgr;B activity.
Work in a variety of murine models provides irrefutable evidence that eliminating TFG-&bgr; or disrupting its downstream signaling cascade leads to inflammatory disease. Thus, deletion of the TGF&bgr;1 gene causes systemic inflammation and early death (11). Similarly, expression of a dominant-negative TGF-&bgr; type II receptor leads to CD4+ T-cell hyperactivity and autoimmunity (12), and the targeted disruption of Smad3 (13) or the overexpression of Smad7 (14) cause inflammation at mucosal surfaces or in the airways, respectively. Until now, defective TGF-&bgr; signaling in humans has been associated with cancer or rare genetic disorders (hereditary chondrodysplasia, hereditary hemorrhagic telangectasia, and persistent müllerian duct syndrome) (15). In the present work, Monteleone et al. show for the first time that disruption of the TGF-&bgr; signaling cascade is also detrimental to common human conditions like IBD, and that it renders patients unable to mount an effective anti-inflammatory response in the gut.
That TGF-&bgr; is essential to mucosal immunity and oral tolerance and that it plays a major role in intestinal inflammation have been established by a number of observations in normal animals and mice with experimental IBD. Following an antigen-specific oral challenge, TFG-&bgr; production is markedly upregulated to maintain tolerance in murine gut-associated lymphoid tissue (16). Upregulation of intestinal TGF-&bgr; has been documented in a variety of murine colitis models, including those induced by haptens (both the Th1 type, induced by trinitrobenzene sulfonic acid, and the type Th2, induced by oxazolone) (17, 18), transfer of CD45RBhigh CD4+ T cells (19), or deletion of the IL2 gene (20).
Smads in IBD
Upregulation of TFG-&bgr; has also been documented in CD and UC patients (5), but why this response fails to control IBD remained unclear until the present report. Monteleone et al. assessed the expression level of agonistic and inhibitory Smads in patients’ intestines. They found consistent defects of TGF-&bgr; signaling in whole mucosal tissue, purified CD3+ lamina propria T cells, and CD3– lamina propria mononuclear cells (6). Compared with normal controls, phosphorylation of Smad3 was significantly reduced in both CD and UC samples, while the amount of Smad7 protein was significantly increased, with an inverse relationship between Smad7 and phosphorylated Smad3 in individual samples (Figure 2). Crucially, Monteleone et al. found that they could restore the ability of patients’ lamina propria mononuclear cells to inhibit IFN-&ggr; and TNF-&agr; production in response to exogenous TGF-&bgr; by treating with specific Smad7 anti-sense oligonucleotides. Cellular levels of phosphorylated Smad3 increased in parallel with this treatment. The authors concluded that blockade of TGF-&bgr; signaling by excessive Smad7 helps maintain the elevated production of proinflammatory cytokines in IBD and that silencing of Smad7 expression restores the ability of TGF-&bgr; to suppress inflammation.
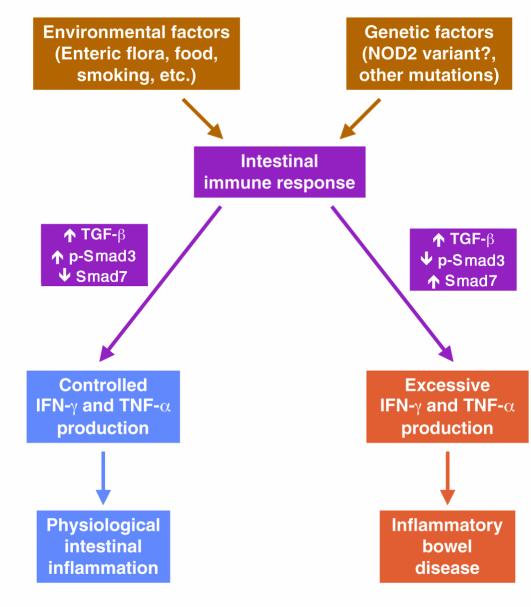
Figure 2.
Effect of defective TGF-&bgr; signaling on the outcome of the intestinal immune response. Environmental antigens activate the intestinal immune system, a response that is modulated by the genetic make-up of the host. In normal individuals the enhanced intestinal production of TGF-&bgr; is accompanied by an increase of phosphorylated Smad3 (p-Smad3) with a concomitant decrease of inhibitory Smad7. This allows the expression of TGF-&bgr; anti-inflammatory activity, which maintains IFN-&ggr; and TNF-&agr; within limits compatible with physiological intestinal inflammation. In susceptible individuals, activation of the immune system also leads to increased production of TGF-&bgr;, but inappropriately high levels of Smad7 inhibit p-Smad3 resulting in defective TGF-&bgr; signaling. As a consequence, excessive amounts of IFN-&ggr; and TNF-&agr; are produced resulting in chronic intestinal inflammation clinically manifested as IBD.
Novel and important findings such as these provoke new questions and stimulate speculation. An obvious question arising from the present report is whether impairment of the TGF-&bgr;/Smad signaling pathway could explain the well-established hyperactivity of the intestinal NF-&kgr;B system in IBD patients (21). There is evidence of a crosstalk between the Smad and NF-&kgr;B signaling cascades at the transcriptional level, although conflicting reports describe both inhibition and enhancement of Smad7 transcription by activated NF-&kgr;B (22, 23), effects that would be expected to enhance or inhibit TGF-&bgr; anti-inflammatory function, respectively.
The molecular basis of Smad7 overexpression in IBD is not known and may well be heterogeneous. Although several different genetic loci have been described in both CD and UC patients, the recent discovery of the 3020insC variant of the NOD2 gene in CD is especially intriguing (24, 25). The wild-type NOD2 gene product activates the NF-&kgr;B system (26), and thus, at least according one report (23), suppresses Smad7, allowing the anti-inflammatory properties of TFG-&bgr; to be fully expressed. It is tempting to suggest that the mutated NOD2 protein impairs the NF-&kgr;B response to bacterial and inflammatory signals and that a consequent derepression of Smad7 blocks the TGF-&bgr;/Smad signaling cascade at an early step (Figure 1). Such a model leads to readily testable predictions, but it is obviously speculative at the moment, especially in light of the uncertainties surrounding NF-&kgr;B’s effects on Smad7 (22, 23).
Other questions inspired by the findings of Monteleone et al. relate to the generality of the Smad imbalance in IBD and associated conditions. Defective TFG-&bgr; signaling appears not to be specific for IBD, since Monteleone and colleagues found decreased Smad3 phosphorylation and increased Smad7 not only in CD and UC, but also in other forms of chronic inflammation, such as indeterminate colitis and pouchitis. Thus, the quest for IBD-specific immune defects (if indeed they exist) must go on. In addition, levels of Smad3 and -7 in uninvolved mucosa of IBD patients were similar to those present in the mucosa of healthy controls, suggesting that TGF-&bgr; signaling defects are secondary to inflammation. Thus, the quest for primary immune abnormalities in IBD (if indeed they exist) must also go on. Moreover, mucosal samples or immune cells from patients with acute infectious or self-limited colitis were not evaluated. Thus, the modulation of TGF-&bgr; signaling needs to be examined during short-lived bouts of gut inflammation and in patients with long-standing IBD who go in and out of clinical exacerbations and remissions. Finally, and perhaps most importantly, it remains uncertain what the physiological TGF-&bgr;–dependent immunosuppression is directed against in the healthy gut mucosa. Based on current concepts of IBD pathogenesis, bacterial antigens are prime suspects, but this must still be proven.
Prospects for novel IBD therapies
The finding of a novel immunoregulatory defect in IBD sheds new light on the cause and mechanisms of IBD and should excite basic and clinical investigators alike. But for the frustrated physician caring for anxious patients suffering from CD or UC, this news triggers an entirely different reaction: Will this information translate into new and better treatments? It is obviously too early to know whether any practical therapeutic applications will derive from knowledge of a TGF-&bgr; signaling defect in IBD, but one point is worth noting. In the last decade, we have witnessed major advances in the treatment of IBD, many of which are based on a more precise understanding of the mechanisms of intestinal inflammation. Although grounded in solid reasoning, these therapies have resulted in disparate outcomes. Blocking specific proinflammatory mediators has met with considerable success, as seen with the administration of TNF-&agr; antibodies to CD patients (27). On the other hand, the results of infusing these patients with large amounts of immunosuppressive IL-10 have been rather disappointing (28). These and other biotherapies in the pipeline are all based on a shared “exogenous approach”, in which the mucosal cytokine milieu surrounding the inflammatory cells is altered to suppress inflammation. Unfortunately, we know almost nothing about the response of patients’ immunocytes to the deluge of cytokines or cytokine-regulatory agents infused in vivo. For instance, if IL-10 responses were defective in CD patients — as TGF-&bgr; responses appear to be in IBD — we should expect nothing but failure from IL-10 therapy regardless of how much of the cytokine is supplied to the patient. Other studies show that cytokines are not the sole determinants of immune responses, and that some signaling molecules work upstream of cytokine signals, as is the case for T-bet in Th1 differentiation (29). In addition, other molecules function as endogenous negative regulators of immune reactivity, as CIS3 does in intestinal inflammation (30). Alternative therapeutic strategies, targeting the intracellular pathways that transduce the effects of cytokines, may therefore be required. Such an “endogenous” approach has yielded remarkable success in human malignancies, as recently reported with the use of a specific tyrosine kinase inhibitor in myeloid leukemia (31). Agents analogous to the Smad7 anti-sense oligonucleotides used by Monteleone et al. (6) could provide both a novel means to affect TGF-&bgr; signaling in vivo and an important test of the “endogenous” approach to treating chronic inflammatory disease.
Footnotes
See the related article beginning on page 601.
References
- 1.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 2.Fiocchi, C. 1996. Cytokines in inflammatory bowel disease. R.G. Landes Company. Austin, Texas, USA. 270 pp.
- 3.Casini-Raggi V, et al. Mucosal imbalance of interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease: a novel mechanism of chronic inflammation. J Immunol. 1995;154:2434–2440. [PubMed] [Google Scholar]
- 4.Autschbach F, et al. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998;153:121–130. doi: 10.1016/S0002-9440(10)65552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factor &agr; and &bgr; in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 6.Monteleone G, et al. Blocking Smad7 restores TGF-&bgr;1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–609. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-&bgr; Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 8.Massague J, Wotton D. Transcriptional control by the TGF-&bgr;/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-&bgr;/SMAD signalling by the interferon-&ggr;/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 10.Verrecchia F, Pessah M, Atfi A, Mauviel A. Tumor necrosis factor-&agr; inhibits transforming growth factor-&bgr;/Smad signaling in human dermal fibroblasts via AP-1 activation. J Biol Chem. 2000;275:30226–30231. doi: 10.1074/jbc.M005310200. [DOI] [PubMed] [Google Scholar]
- 11.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-&bgr;1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelik L, Flavell RA. Abrogation of TGF&bgr; signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-&bgr; EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakao A, et al. Blockade of transforming growth factor &bgr;/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000;192:151–158. doi: 10.1084/jem.192.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massague J. TGF-&bgr; signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 16.Gonnella PA, et al. In situ response in gut-associated lymphoid tissue (GALT) following oral antigen in TCR-transgenic mice. J Immunol. 1998;160:4708–4718. [PubMed] [Google Scholar]
- 17.Neurath MF, et al. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta–mediated oral tolerance. J Exp Med. 1996;183:1–12. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role of transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludviksson BR, Ehrhardt RO, Strober W. TGF-&bgr; production regulates the development of the 2,4,6-trinitrophenol-conjugated keyhole limpet hemocyanin-induced colonic inflammation in IL-2-deficient mice. J Immunol. 1997;159:3622–3628. [PubMed] [Google Scholar]
- 21.Jobin C, Sartor RS. The I&kgr;/NF-&kgr;B system: a key determinant of mucosal inflammation and protection. Am J Physiol. 2001;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 22.Bitzer M, et al. A mechanism of suppression of TGF-&bgr;/SMAD signaling by NF-&kgr;B/RelA. Genes Dev. 2000;14:187–197. [PMC free article] [PubMed] [Google Scholar]
- 23.Nagarajan RP, et al. Repression of transforming-growth-factor-beta–mediated transcription by nuclear factor &kgr;B. Biochem J. 2000;348:591–596. [PMC free article] [PubMed] [Google Scholar]
- 24.Hugot J-P, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 25.Ogura Y, et al. A frameshift mutation in Nod2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 26.Ogura Y, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-&kgr;B. J Biol Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 27.Targan SR, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 28.Bickston SJ, Cominelli F. Recombinant interleukin 10 for the treatment of active Crohn’s disease: lessons in biologic therapy. Gastroenterology. 2000;119:1781–1783. doi: 10.1053/gast.2000.20822. [DOI] [PubMed] [Google Scholar]
- 29.Mullen AC, et al. Role of T-bet in commitment of Th1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki A, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]