Abstract
Background
Usual interstitial pneumonia (UIP) is a slowly progressive, usually fatal form of idiopathic interstitial pneumonia for which there is no effective treatment. Transbronchial biopsy (TBB) has been utilized only to exclude other diseases such as sarcoidosis, lymphangitic carcinoma, and infection, for example, but TBB is generally considered to have little role in confirming UIP.
Objective
To determine whether diagnostic changes of UIP can be appreciated on TBB specimens.
Design
Retrospective analysis of TBB specimens from patients with proven UIP.
Setting
Two study sites in the United States.
Participants
Twenty-one patients with UIP confirmed by surgical lung biopsy and/or lung explant, and 1 patient with UIP confirmed by clinical and radiographic findings along with follow-up information.
Measurements and results
Adequate tissue for diagnosis was available in 18 cases; in 7 cases (32% overall), there were varying combinations of interstitial fibrosis in a patchwork pattern along with fibroblast foci and/or honeycomb change. These features were considered diagnostic of UIP. Interstitial fibrosis along with fibroblast foci or honeycomb change were seen in two other cases, but the fibrosis lacked a patchwork pattern, and these features were considered consistent with UIP. Nonspecific interstitial fibrosis alone was found in nine cases.
Conclusions
In summary, characteristic histologic features of UIP can be identified on TBB specimens more often than previously appreciated. TBB may be more useful in confirming UIP than previously recognized.
Keywords: diagnosis, interstitial lung disease, pulmonary fibrosis, transbronchial biopsy, usual interstitial pneumonia
Usual interstitial pneumonia (UIP), the most common idiopathic interstitial pneumonia, is a slowly progressive, usually fatal disease that shows little, if any, response to therapy.1–3 It is important to separate UIP from other histologic subsets of idiopathic interstitial pneumonia and from other interstitial lung diseases that have a more favorable response to therapy and better prognosis.1 Traditionally, surgical lung biopsy (SLB), either open thoracotomy or, more recently, video-assisted thoracoscopy, has been advocated for diagnosis in patients with atypical high-resolution CT (HRCT) findings, although it is not without significant morbidity and mortality in these patients.4,5 For example, in one study,4 the 30-day mortality following SLB was 17%. Transbronchial biopsy (TBB) has been utilized only to exclude other diseases such as sarcoidosis, lymphangitic carcinoma, and infection, for example, but TBB is generally considered to have little role in confirming UIP.1 We undertook this study to examine whether diagnostic features of UIP could be found in TBB samples, and whether this procedure might yield sufficient information to support a diagnosis of UIP.
Materials and methods
Written informed consent was obtained from each patient or guardian. The protocol was approved by the local Institutional Review Board or Ethics Committee at each institution, and was in accordance with the recommendations found in the Helsinki Declaration of 1975.
Twenty-two patients with UIP who underwent TBB were identified. In 21 patients, the diagnosis was confirmed by SLB and/or explant specimens (19 by SLB, 2 by explant specimens). The histologic diagnosis of UIP was based on accepted criteria, including patchy interstitial fibrosis alternating with normal lung, fibroblast foci, and honeycomb areas.3,6 In one case with typical clinical and radiologic findings (subpleural honeycombing on HRCT),7,8 additional tissue specimens were considered unnecessary.
Sixteen patients were identified in the files of the Lung Transplant Service of the University of Pennsylvania School of Medicine between May 1993 and September 2000. Open-lung biopsy was undertaken in 14 patients, while 2 patients underwent explant at the time of lung transplantation. All patients were evaluated at the University of Pennsylvania for lung transplantation, but the diagnostic SLBs were performed at outlying hospitals.
Six patients were identified in the files of the Pathology Department at Upstate Medical University, Syracuse, between January 1990 and May 2003. Five patients underwent subsequent SLB; in one patient, TBB findings along with clinical and radiographic findings were considered diagnostic.
From one to six (mean, 2.5) hematoxylin-eosin–stained slides were available in each case. In three cases, there was also one Trichrome-stained slide. The number of pieces of alveolated lung parenchyma and bronchial wall were recorded, and the following pathologic features were evaluated: interstitial fibrosis, a patchwork pattern to the fibrosis characterized by juxtaposition of interstitial fibrosis and normal lung without transition zones, fibroblast foci, and honeycomb change. The findings were considered diagnostic of UIP if there was patchwork interstitial fibrosis along with fibroblast foci or honeycomb change, or both; findings were consistent with UIP if there was interstitial fibrosis lacking a patchwork pattern with either fibroblast foci or honeycomb change; findings were nonspecific if there was only interstitial fibrosis; and findings were insufficient if only bronchial wall was present.
Results
Clinical Features
There were 9 men and 13 women (age range, 31 to 83 years; mean, 51 years). All patients had dyspnea at presentation, and it had been present from 1 to 60 months (mean, 16 months) in 19 patients in whom this information was known. One patient (case 9) was discovered incidentally during a testicular cancer workup to have interstitial lung infiltrates on chest radiographs, and then retrospectively noted that shortness of breath had been present for 6 months. Two patients (cases 11 and 20) had a family history of pulmonary fibrosis. Radiographic studies in all patients showed bilateral interstitial infiltrates, and HRCT results in four of five patients were typical of UIP. Pulmonary function studies showed a restrictive pattern. Prednisone therapy was initiated in 19 patients without improvement. SLB was undertaken in 19 patients from 2 weeks to 17 months (mean, 4 months) following TBB, while 2 patients underwent lung transplantation 3 years after TBB with no SLB. Five patients eventually underwent lung transplantation after an interval of 2 to 5 years (mean, 3.2 years).
Additional biopsy specimens were not obtained after TBB in one patient in whom the clinical and radiographic findings were considered typical of UIP. This patient (case 22) had a 1-year history of worsening dyspnea and pulmonary function test results along with HRCT showing extensive interstitial scarring and peripheral honeycomb change.
Pathologic Findings
Two pathologists (E.A.B., A.L.K.) reviewed the TBB specimens jointly. The pathologic findings (TBB) are summarized in Table 1. Eighteen specimens were considered adequate, containing at least one fragment of alveolated lung parenchyma (mean, 2.5; range, 1 to 5), while 4 specimens contained only bronchial wall and were considered insufficient for diagnosis.
Table 1. Pathologic Findings on TBB in UIP Patients.
| Case No. | Site of TBB | No. of Pieces of Alveolated Parenchyma | No. of Pieces of Bronchial Wall | Pathologic Findings | Pathologic Diagnosis |
|---|---|---|---|---|---|
| 2 | RLL | 5 | 1 | Patchwork fibrosis, ff, HCC | UIP |
| 3 | na | 3 | 1 | Patchwork fibrosis, ff | UIP |
| 4 | RLL | 3 | 1 | Patchwork fibrosis, ff | UIP |
| 10 | RLL and RUL | 3 | 3 | Patchwork fibrosis, HCC | UIP |
| 16 | na | 2 | 4 | Patchwork fibrosis, ff | UIP |
| 17 | LUL | 5 | 1 | Patchwork fibrosis, ff | UIP |
| 22 | na | 2 | 2 | Patchwork fibrosis, ff | UIP |
| 13 | RUL | 3 | 0 | Fibrosis, ff | Consistent with UIP |
| 14 | RLL | 2 | 1 | Fibrosis, HCC | Consistent with UIP |
| 1 | RLL | 1 | 0 | Fibrosis | Nonspecific |
| 5 | LLL | 2 | 1 | Patchwork fibrosis | Nonspecific |
| 7 | RLL | 2 | 6 | Fibrosis | Nonspecific |
| 9 | RLL | 2 | 0 | Fibrosis | Nonspecific |
| 11 | na | 2 | 0 | Fibrosis | Nonspecific |
| 12 | RUL | 1 | 2 | Patchwork fibrosis | Nonspecific |
| 15 | na | 2 | 0 | Fibrosis | Nonspecific |
| 18 | LLL | 4 | 2 | Patchwork fibrosis | Nonspecific |
| 19 | Left lung | 3 | 0 | Fibrosis | Nonspecific |
| 6 | RML | 0 | 4 | Only bronchial wall | Insufficient |
| 8 | RLL | 0 | 5 | Only bronchial wall | Insufficient |
| 20 | na | 0 | 5 | Only bronchial wall | Insufficient |
| 21 | RLL | 0 | 3 | Only bronchial wall | Insufficient |
ff = fibroblast foci; HCC = honeycomb change; RLL = right lower lobe; RUL = right upper lobe; RML = right middle lobe; LUL = left upper lobe; LLL = left lower lobe; na = not available.
Specimens in seven cases contained features considered diagnostic of UIP. In one patient (case 2), there was a combination of focal interstitial fibrosis with a patchwork pattern, fibroblast foci, and areas of honeycomb change, a combination of features that would be diagnostic even without clinical information (Fig 1). The patchwork pattern was characterized by the juxtaposition of foci of collagen-type interstitial fibrosis next to relatively normal lung with no intervening transition zones (Fig 1, left, A). The honeycomb change was characterized by enlarged, restructured air spaces surrounded by fibrosis (Fig 1, center, B). The enlarged airspaces were lined by cuboidal epithelium and filled with mucus containing inflammatory cells and macrophages. Fibroblast foci appeared as small aggregates of spindle-shaped cells embedded in a lightly stained, myxoid stroma (Fig 1, right, C). Six other cases showed features considered diagnostic of UIP in the setting of a patient with chronic interstitial lung disease. The typical patchwork pattern of interstitial fibrosis with alternating areas of interstitial fibrosis and normal alveolar septa was present in all (Fig 2, left, A). Fibroblast foci were additionally found in five cases (Fig 2, right, B), while areas of honeycomb change were present in one case (Fig 3). In two patients (cases 16 and 22), the fibroblast foci were especially numerous and easily identified at low magnification (Fig 4), while in other cases the fibroblast foci were present in atelectatic, fibrotic lung parenchyma, making their recognition difficult at low magnification. However, the lightly stained nature of the myxoid matrix containing the spindle-shaped myofibroblasts contrasted with the surrounding collagen fibrosis and helped in their identification.
FIGURE 1.
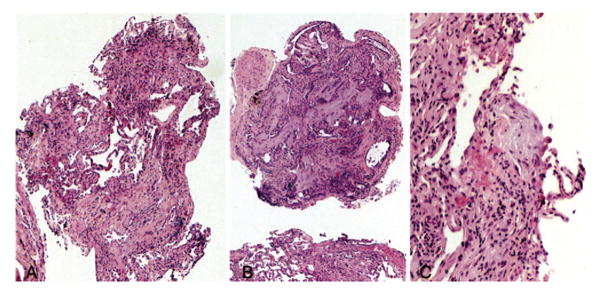
Case 2. Left, A: Low-magnification photomicrograph showing the characteristic patchwork pattern of fibrosis in UIP. Note the relatively normal lung in the center adjacent to marked interstitial fibrosis in surrounding parenchyma (hematoxylin-eosin, original × 10). Center, B: Low-magnification view showing honeycomb change in another fragment characterized by enlarged, irregular air spaces filled with mucin (hematoxylin-eosin, original × 10). Right, C: Higher-magnification view showing a fibroblast focus. Note the interstitial location of spindle-shaped cells embedded in a myxoid background. The surrounding lung parenchyma shows collagen-type fibrosis and mild chronic inflammation (hematoxylin-eosin, original × 20).
FIGURE 2.
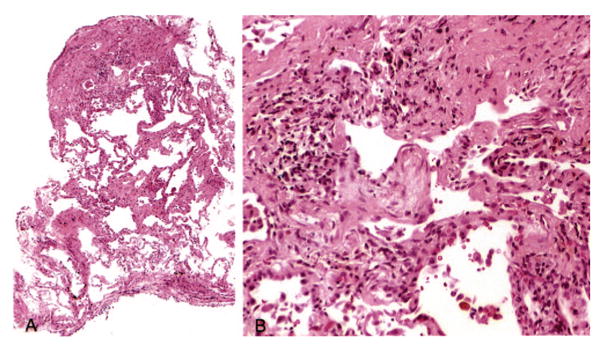
Case 22. Left, A: Low-magnification photomicrograph showing variegated fibrosis (hematoxylin-eosin, original × 10). Note the patchwork pattern of involvement with areas of interstitial fibrosis alternating with normal lung. Right, B. Higher-magnification view of a fibroblast focus present in the central upper portion on left, A, showing interstitial location and characteristic lightly stained myxoid stroma containing spindle-shaped cells (hematoxylin-eosin, original × 20).
FIGURE 3.
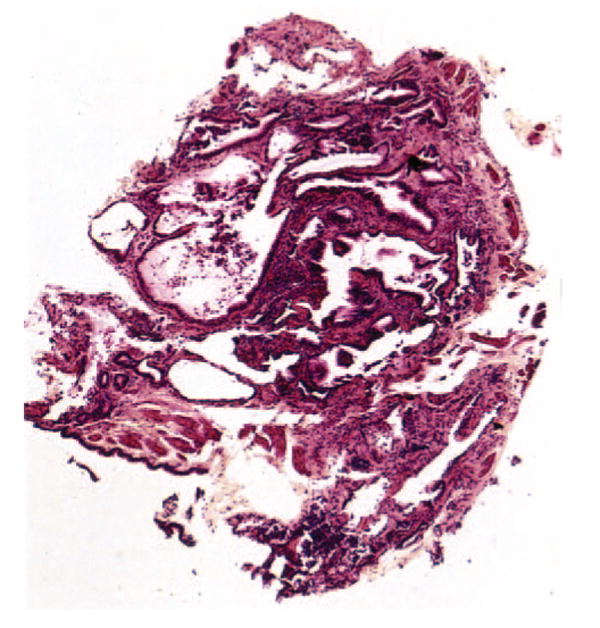
Case 14. Low-magnification photomicrograph of honeycomb change. The airspaces are enlarged, deformed, irregularly shaped, and lined by bronchial type epithelium (hematoxylin-eosin, original × 10).
FIGURE 4.

Case 16. Prominent fibroblast foci in a background of interstitial collagen-type fibrosis. Fibroblast foci are recognized by their myxoid appearance, with spindle-shaped cells arranged parallel to the alveolar lining (hematoxylin-eosin, original × 10).
Two cases were considered consistent with UIP. They showed a combination of collagen-type fibrosis with either fibroblast foci or honeycomb change, but they lacked the characteristic patchwork pattern to the fibrosis.
Nine patients had only interstitial fibrosis and mild chronic inflammation. Although a patchwork pattern to the fibrosis was noted in two cases, fibroblast foci and honeycomb change were not identified. These findings were considered nonspecific.
The number of alveolated lung fragments sampled was slightly greater in the nine diagnostic and consistent cases (mean, 3.1; range, 2 to 5) than in the nine nonspecific cases (mean, 2.1; range 1 to 4), although this difference was not significant. Similarly, the difference was not significant when comparing patients with diagnostic biopsy findings (mean, 3.3; range, 2 to 5) to those with either consistent with (mean, 2.5; range, 2 to 3) or nonspecific findings (mean, 2.1; range, 1 to 4). Diagnostic changes were noted with as few as two lung fragments, although one case with four fragments showed only nonspecific changes.
Discussion
Our findings suggest that TBB may be more useful than previously recognized in confirming UIP. The fact that 7 of our 22 cases showed changes considered diagnostic of UIP and an additional 2 cases were considered consistent with UIP indicates that significant changes can be recognized even in these small samples. It should be remembered, however, that all patients had clinical and radiographic features compatible with UIP, including chronic dyspnea, restrictive pulmonary function, interstitial infiltrates on chest radiographs, and subpleural honeycombing on HRCT. Because of the small size of TBB specimen, the pathologic findings on TBB must be correlated with the clinical and radiographic findings before making a diagnosis in cases of chronic interstitial lung disease. If the clinical and or radiographic features do not fit, consideration should be given to a larger biopsy specimen.
Because UIP is characterized by chronic inflammation and fibrosis and these features are common nonspecific findings in peribronchial parenchyma, it has generally been assumed that TBB cannot be utilized in diagnosing UIP.1 Our study, however, shows that certain characteristic features of UIP, including patchwork pattern of involvement by fibrosis and temporal variability with fibroblast foci, collagen, and honeycomb change, previously thought to be recognizable only on SLB specimens, can sometimes be seen on TBB specimens. The patchwork pattern is characterized by areas of interstitial fibrosis juxtaposed to areas of relatively normal lung. Its presence helps to distinguish the changes from nonspecific peribronchial fibrosis in which there is a gradual transition from abnormal to normal. Likewise, evidence of temporal heterogeneity characterized by a mixture of fibroblast foci and inactive-appearing collagen fibrosis would be an unusual nonspecific finding in peribronchial parenchyma. Fibroblast foci appear as small aggregates of spindle-shaped cells within a myxoid matrix. They are localized in the interstitium beneath the alveolar epithelium, and the long axis of the component spindle-shaped cells runs parallel to the alveolar lining. Although fibroblast foci are not specific for UIP, their presence in the right clinical and pathological setting supports the diagnosis.
Honeycomb change is recognized by enlarged, sometimes irregularly shaped airspaces that often contain inspissated mucin, macrophages, and other inflammatory cells. The airspaces are usually lined by bronchiolar type epithelium but may sometimes be lined by low cuboidal or flattened alveolar epithelium. When lined by bronchial epithelium, the spaces are distinguished from bronchioles by the absence of smooth-muscle bundles in their walls. Sometimes, the epithelial lining is absent and pools of inspissated mucus within fibrotic parenchyma comprise the main finding.
The small size of the specimens obtained by TBB has been considered to be a major impediment to the successful use of TBB for diagnosing interstitial lung disease.1 Not surprisingly, diagnostic biopsies in our study tended to contain more alveolated lung fragments than biopsies showing only nonspecific findings, although the difference was not important. Diagnostic changes of UIP, however, were found in cases with as few as two pieces of alveolated parenchyma and, conversely, one case with four pieces showed only nonspecific changes. Clearly, the bronchoscopist should take as many pieces as safely possible, although the diagnosis is sometimes possible with minimal tissue.
A common problem encountered in interpreting TBB specimens is the frequent presence of artifactual atelectasis that may both obscure diagnostic features and also be interpreted incorrectly as interstitial fibrosis.2 Since alveolar epithelial hyperplasia accompanies most forms of interstitial lung disease, identification of these cells in difficult cases helps confirm interstitial fibrosis rather than atelectasis. The presence of atelectasis can also cause difficulty in distinguishing fibroblast foci from intraluminal fibroblast plugs of bronchiolitis obliterans-organizing pneumonia (BOOP). The fibroblast plugs of BOOP are usually larger than fibroblast foci and often have elongated or branching shapes. The presence of smooth-muscle bundles in the surrounding interstitium indicating the wall of an alveolar duct or respiratory bronchiole is also helpful in indicating BOOP rather than fibroblast foci. In difficult cases, the finding elsewhere of interstitial collagen-type fibrosis and/or honeycomb change favors UIP and thus fibroblast foci, since BOOP is usually unassociated with chronic lung scarring. The radiographic findings can also be extremely helpful in difficult cases.
Recently, HRCT has been shown to be diagnostic of UIP in approximately one half of cases, and biopsy specimens therefore may not always be necessary.7,8 HRCT is most valuable in academic settings, however, and SLB is still widely utilized in community hospitals. Since many patients are elderly and severely ill, it is important for the pathologist to carefully examine TBB specimens to identify features supportive of UIP that can potentially obviate a subsequent more invasive procedure since SLB is not without adverse effects in these patients.
Our study is limited in that we approached the cases with the clear understanding that a diagnosis of UIP was ensured. Whether the histologic criteria proposed can successfully separate patients with UIP from those with other forms of diffuse lung disease when applied in a “blinded” and prospective fashion is beyond the scope of the current study. We demonstrate the potential sensitivity of this diagnostic technique in patients proven to have idiopathic pulmonary fibrosis, but the study design precludes speculation regarding specificity of the proposed histologic criteria in a more heterogeneous group of patients with other forms of diffuse lung disease.
Summary
Characteristic histologic features of UIP can be identified on TBB specimens more often than previously appreciated. Interstitial fibrosis with a patchwork pattern along with fibroblast foci and/or honeycomb change on TBB further support the diagnosis of UIP in the appropriate clinical and radiographic setting.
Acknowledgments
This work was funded by NIH-NHLBI P50 HL67665.
Abbreviations
- BOOP
bronchiolitis obliterans organizing pneumonia
- HRCT
high-resolution CT
- SLB
surgical lung biopsy
- TBB
transbronchial lung biopsy
- UIP
usual interstitial pneumonia
Footnotes
Publisher's Disclaimer: CHEST is the official journal of the American College of Chest Physicians. It has been published monthly since 1935. Copyright 2007 by the American College of Chest Physicians, 3300 Dundee Road, Northbrook IL 60062. All rights reserved. No part of this article or PDF may be reproduced or distributed without the prior written permission of the copyright holder (http://www.chestjournal.org/misc/reprints.shtml). ISSN: 0012-3692.
References
- 1.American Thoracic Society/European Respiratory Society. International multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 2.Katzenstein A-LA. Katzenstein and Askin's surgical pathology of non-neoplastic lung disease. 3rd. Philadelphia, PA: WB Saunders Company; 1997. [Google Scholar]
- 3.Katzenstein A-LA, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 4.Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J. 2001;17:175–179. doi: 10.1183/09031936.01.17201750. [DOI] [PubMed] [Google Scholar]
- 5.Tiitto L, Heiskanen U, Bloigu R, et al. Thoracoscopic lung biopsy is a safe procedure in diagnosing usual interstitial pneumonia. Chest. 2005;128:2375–2380. doi: 10.1378/chest.128.4.2375. [DOI] [PubMed] [Google Scholar]
- 6.Katzenstein A-LA, Zisman DA, Litzky LA, et al. Usual interstitial pneumonia: histologic study of biopsy and explant specimens. Am J Surg Pathol. 2002;26:1567–1577. doi: 10.1097/00000478-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Hunninghake GW, Lynch DA, Galvin JR, et al. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest. 2003;124:1215–1223. doi: 10.1378/chest.124.4.1215. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Mageto YN, Lockhart D, et al. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest. 1999;116:1168–1174. doi: 10.1378/chest.116.5.1168. [DOI] [PubMed] [Google Scholar]


