Abstract
While a glutaraldehyde crosslinking is most often used to fabricate bioprosthetic heart valves (BHV) using heterograft tissues, it predisposes BHV to calcification and dramatically stiffens the heterograft tissues. Our group previously reported the synthesis and characterization of a novel epoxy-crosslinker, triglycidylamine (TGA). TGA pretreatment of BHV tissues compared to glutaraldehyde results in both calcification resistance in subdermal implants and improved leaflet compliance. In these prior studies, optimal calcification inhibition was noted with the combined use of TGA with mercapto-aminobisphosphonate (MABP). In the present study, we investigated the hypothesis that bovine pericardium cross-linked with TGA-MABP retains these beneficial biomechanical properties in-vivo using a novel mitral valve anterior leaflet (MVAL) ovine valvuloplasty model. Bovine pericardial specimens were crosslinked with either glutaraldehyde or TGA-MABP, from which 1 cm2 sections were implanted in the ovine MVAL after removal of the original tissue of the same size. An array of four sonomicrometry transducers were implanted on the corners and used to compute the complete in-surface strain tensor cardiac cycle over the cardiac cycle at 0 and 4 weeks. Following explant samples were fixed in formalin for histology studies. At 4 weeks both treatment groups experienced no dimensional changes in the unloaded state, indicating no shrinkage. When fully loaded during peak systolic ejection, TGA-MABP valvuloplasty patches were significantly more compliant, which did not change at 4 weeks. In contrast, the glutaraldehyde areal strain increased significantly by 4 weeks. Estimated implant stresses for both treatment groups, based on previously measured biomechanical properties (Biomaterials, vol. 4, 2007), were 40 kPa and 250 kPa in the circumferential and radial directions, respectively, which are comparable to predicted BHV peak stress levels. We conclude that TGA-MABP crosslinked bovine pericardium, when subjected to in-vivo BHV stress levels in a blood contacting environment, maintains stable functionality.
Introduction
Current bioprosthetic heart valves (BHV) are usually defined by their tissue source: the porcine aortic heart valve or bovine pericardium. Tissues are pretreated with a protein crosslinking agent, typically glutaraldehyde (GLUT), to preserve the tissue structure and minimize the immunological reactions to biologically derived tissue. BHV functional durability remains limited to ~15 years, with failure resulting from leaflet structural deterioration mediated by fatigue and/or tissue mineralization. The mechanisms underlying mineralization and techniques to mitigate mineralization have been the subject of extensive research, summarized in several excellent reviews [1, 2]. In general, while mineralization is associated with serious structural deterioration, significant structural damage independent of calcification has also been reported [3]. Studies on explanted BHV have exhibited failure due to both calcification and fatigue either isolated or combined, suggesting a strong mechanistic interrelationship between calcification and fatigue damage [4–6]. Thus, mechanical damage is also a significant factor in the genesis of heterograft biomaterial mineralization. Moreover, the use of BHV biomaterials in percutaneous heart valve designs requires that they are resistant to mechanical damage resulting from the high compressive forces present during the delivery process.
Although used for decades, glutaraldehyde (GLUT) pretreatment has been demonstrated to predispose bioprosthetic implants to calcification because of several important mechanistic factors. Our group reported the synthesis and characterization of a novel crosslinking agent, triglycidyl amine (TGA), a highly polar, water soluble polyepoxy-crosslinking agent [7]. TGA crosslinking enhances biocompatibility, improves mechanical properties per in vitro studies, and inhibits bioprosthetic heart valve degeneration in rat subdermal implant experiments, due to major modifications of the structural proteins of the extracellular matrix (ECM). Further, our in vitro studies of the mechanical properties of TGA-pretreated bovine pericardium have demonstrated significantly greater compliance compared to GLUT-pretreated pericardium [7]. Specifically, TGA-MABP-pretreated pericardium was ~1.6 times as compliant compared to GLUT fixed pericardial tissues under 1 MPa equi-biaxial stress. More recently, Rapoport et al. [8] demonstrated that when biologically derived materials were functionalized with mercapto-aminobisphosphonate combined with TGA cross-linking, rat subdermal explants exhibited reduced alkaline phosphatase activity and no significant calcification in long-term studies.
One important aspect of GLUT cross-linking is that is results in very stiff heterograft tissues, which is likely a significant contributor to BHV fatigue and failure [9]. Hypothetically, the increased compliance of TGA treated tissues may help to improve fatigue resistance since the cross-linked collagen fibers are less tightly bound. Yet, functioning native and BHV leaflets experience highly complex, time-varying external loading patterns resulting from local hemodynamic forces, including both high shear stresses during ejections and large transvalvular pressures during diastole [10]. Leaflet tissues respond to these forces by undergoing large anisotropic in-plane stretching and flexure. Thus, mechanistic studies of biomaterials performance using intact valves can be confounded by the highly complex, regionally variant, time-varying mechanical stresses that occur in the leaflet. Practically, measurement of BHV function in-vivo is possible. Moreover, time course changes in the material are mostly lost since biomaterial assessments can only be performed at the time of valve explant.
To address these limitations, we have investigated the differences between TGA-MABP crosslinked bovine pericardium and glutaraldehyde pretreated bovine pericardium in vivo using a novel technique to evaluate heterograft biomaterials in a blood contacting environment subjected to levels of strain comparable to those in functional bioprostheses. Further, utilizing previous biomechanical data on TGA and GLUT treated bovine pericardium [7] and a structural constitutive model [11], we estimated the in-vivo stresses from the measured in-vivo strain field. Using this approach, we investigated the hypothesis that TGA-MABP crosslinked bovine pericardium would demonstrate stable, and potentially more desirable mechanical properties than observed with GLUT crosslinking, when subjected to physiological stress levels in vivo.
Methods
Crosslinking agents
TGA [7] and MABP [12] were synthesized in our laboratory as previously reported. TGA and MABP purity were assessed by proton NMR, and MABP purification was also documented by phosphorus-NMR. All other reagents used in these studies were obtained from Sigma-Aldrich (St. Louis, MO), and were ACS Reagent Grade quality.
Implant preparation
Fresh bovine pericardium was shipped overnight on ice from St Jude Medical (Minneapolis, MN). Upon arrival, all tissues were rinsed extensively in sterile normal saline solution, and fixed with either glutaraldehyde (GLUT) or with TGA-MABP as follows. GLUT fixed tissues were treated with 0.6% Glutaraldehyde (EM grade, Polysciences, Inc, Warrington, PA) in 50mM HEPES/0.9% NaCl buffer, pH 7.4, at room temperature for seven days with one change, then stored in 0.2% buffered GLUT at room temperature until use. Tissues fixed with TGA-MABP were treated with 100mM TGA plus 20mM MABP in borate mannitol buffer (25 mM sodium tetraborate decahydrate, pH 7.4) at room temperature with daily changes of the TGA-MABP solution for 7 days. Prior to implantation, all materials were rinsed 3×1 hour in an excess of sterile normal saline.
Surgical protocol, patch and sono-crystal implantation
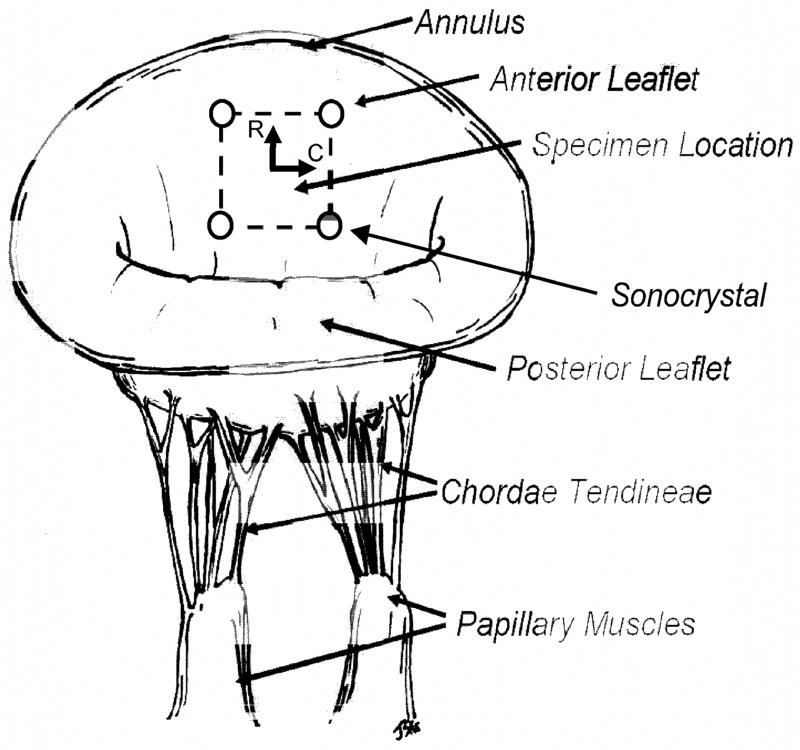
In compliance with guidelines for humane care (NIH Publication No. 85-23, revised 1985) nine male Dorsett sheep (35–45 kg) were induced with sodium thiopental (10–15 mg/kg iv), intubated, anesthetized and ventilated with isofluorane (1.5–2%) and oxygen. The surface ECG and arterial blood pressure were continuously monitored. Through a sterile left lateral thoracotomy on cardiopulmonary bypass, a 10 mm × 10 mm segment was incised from the center of the mitral valve anterior leaflet (MVAL). A similar sized tissue patch of either GLUT or TGA treated bovine pericardium was implanted in this space using a discontinuous running (interrupted in the middle of each side of the patch to prevent a purse string effect) 4-0 prolene suture (Fig. 1). Next, an array of four 1 mm hemispherical PZT-5A piezoelectric transducers (Sonometrics Corp., London, Ontario) were sutured with 6-0 prolene to the four corners of the patch (Fig. 1). Once the crystals were implanted, the leaflets were placed in a coapted position by injecting saline into the LV, the leaflet transducer wires were adjusted to allow a degree of slack that permitted free motion of the leaflets without letting the wires coil in the atrium. The atriotomy was then closed around the wires maintaining this relationship. After the animal was weaned from cardiopulmonary bypass and was hemodynamically stable, an epicardial echocardiogram was performed to assess valve competence. The chest was closed with the sonomicrometer skin bottoms fixed to the skin and the animals were recovered from anesthesia.
Figure 1.
A schematic of the sheep mitral valve apparatus showing the placement of the 4 crystal sonomicrometry transducer array and the location of the implanted valvuloplasty patch on the anterior leaflet.
After recovery, sonomicrometry readings of the crystals were acquired afterwards (see next section for details). This was followed by a 4 week period during which the animal was allowed to be ambulatory within a cage. After this repeat session performed to acquire a new set of readings. After the 4 week data was acquired, the animal was sacrificed, the heart excised and rinsed in normal saline, then the MV anterior leaflet removed for histological evaluations using Movat’s staining procedures [7].
Sonomicrometry data collection
Upon closure, high fidelity pressure transducers (SPC-350, Millar Instruments Inc., Houston, TX) for simultaneous measurements of left ventricular (LVP) and aortic root pressures (ARP) were passed percutaneously into the left ventricle and ascending aorta via a femoral artery. Surface EKG, LVP and ARP were monitored continuously (Hewlett-Packard 78534C monitor). Transducer wires were connected to a Sonometrics Series 5001 Digital Sonomicrometer (Sonometrics Corp., London, Ontario). As described previously [13] sonomicrometry array localization (SAL) was used to determine the three-dimensional coordinates of each transducer every 5 ms throughout the cardiac cycle using sonomicrometry distance data. Three dimensional sonomicrometry positional data were taken following neosynephrine infusion titrated to achieve systolic blood pressures of 150 mmHg. Ventilation was suspended during sonomicrometry measurements. Data was taken for ~15 contiguous cardiac cycles for every data set. Since cycle-to-cycle variations were extremely small, the last cycle was routinely chosen as representative.
Strain computations
Detailed methods for computing dynamic leaflet surface strains have been previously described [14]. Briefly, we adapted our previous approach to determine the 2D in-surface Eulerian (i.e. referenced to the deformed state configuration) strain tensor e at each time point using a finite element-based surface interpolation method. e was referred to a convective, in-surface tangential coordinate system [15],; forthe current study the coordinate axes were aligned to the local circumferential and radial directions of the leaflet (Fig. 1). While the method used for strain calculations allowed bilinear interpolation of e over the patch area, as in our previous studies [14, 16] we utilize the value of e in the center of the patch as representative. The crystal configuration at the minimum left ventricular pressure (LVP) was used as the reference state for all strain calculations, which represented the point of least deformation strain placed on the mitral valve anterior leaflet. The end point for data analysis was chosen to be the minimum LVP following one heart cycle. Thus, the deformation for each valve is based from one full cardiac cycle, beginning and ending with the point of minimal LVP. One benefit of the use of sonocrystals for strain determination is that the 3D positional data from all frames is always available, since there is no need for straight optical pathways as required in the in-vitro studies [14].
In preliminary analyses, we observed that the shear strains were very low and that the principal angles (corresponding to the direction of largest stretch with respect to the circumferential axes) were within ~10° of the radial direction. Taken together, the parameters indicate a low amount of tissue shearing and that the major stretch direction was closely aligned to the radial axis. Thus, in the present study the principal values of e were expressed as percent strain in the radial (the major principal value) and circumferential (the minor principal value) directions. The areal strain, representing the net local change in leaflet area, was also computed.
Stress computations
The present experimental approach also offered an opportunity to estimate the implant dynamic stress histories from the measured strain tensor. This was done under the assumption that the stress and strain fields within the implanted tissue were approximately homogeneous. This assumption allows the use of the measured strains to be directly input into a constitutive model for the implanted heterograft tissues to estimate the resulting stresses.
For the constitutive model we utilized a general structural approach for planar collagenous tissues, as presented in detail in [11]. Briefly, in this approach a representative volume element (RVE) is identified that is large enough to represent the processes associated with the heterograft biomaterial microstructure of the material in some average sense, yet small compared to the characteristic length scale of the microstructure, i.e. the tissue thickness. The RVE is treated as a three-dimensional continuum and it is assumed that the heterograft materials can be modeled as a hyperelastic solid, so that
| (1) |
where S and E are the 2nd Piola-Kirchhoff stress and Green-Lagrange strain tensors, respectively, and W is the tissue strain energy density per unit volume. Using this, the total tissue strain energy W per unit volume can be expressed as
| (2) |
where w is the fiber strain energy function, Ef is the fiber strain computed from the tissue level Green’s strain, and R(θ) is the statistical distribution function representing the angular distribution of the collagen fibers with orientation θ. Using eqn. 1, eqn. 2 becomes in component form (ignoring the negligible shear strains)
| (3) |
Note that the notation for Sf includes a “11” subscript to denote the fact that only stress components along the fiber are considered to contribute to the global tissue stress.
To simulate the effective collagen fiber stress-strain law the simplest formulation (i.e. one with the fewest number of parameters) was desired, which incorporated the effects of collagen volume fraction, uncrimping, exogenous chemical cross-links, and the intrinsic properties of collagen. For this approach, the exponential form
| (4) |
was used, where ci are positive constants. For the fiber angular distribution function R(θ) we utilized a modified beta distribution as described in [11] with mean μ and variance σ, defined over θ ∈ [− π/2, π/2].
To determine the model parameters μ,σ,c1, and c2 for the GLUT and TGA-MABP heterograft tissue types, biaxial mechanical data from both groups from Connolly et al. [7] were utilized. Here the biaxial mechanical data (5 stress-based test protocols for specimens was used, as described in [17]) from each a total 8 specimens per for the GLUT and TGA groups, respectively. Eqns. 3 were fit to the data and solved numerically using Romberg integration [18], with set to zero when Ef≤ 0 since it was assumed that collagen fibers cannot support load when compressed. Further, since the biaxial test specimens were pre-selected so that their collagen fibers were preferentially aligned to the x1 axis, μ was set to 0.
To estimate the in-vivo implant stresses, the components of the Green’s strain tensor E over the cardiac cycle were determined from the same deformation field information used to compute e (see [14] for details). The resulting strain component-time information was then used as input into eqns. 3 to estimate the resultant stress components. Note that while precise collagen fiber measurements were not performed on the implanted pericardial specimens, visually the implant sections were aligned with their apparent collagen fiber preferred direction aligned to the local circumferential axis of the leaflet. Thus we were able to set μ =0, leaving only three parameters (σ,c1, and c2) required for the constitutive model. Since the focus of these simulations were to estimate the peak implant stresses, the mean values of the peak Green’s strain extensional (E11 and E22) components for both implant groups (GLUT and TGA-MABP) were used compute peak stress values, using the material parameters for each tissue specimen from [7].
Explant evaluation
Following the 4 week sonomicrometry studies the animals were euthanized under general anesthesia and the heart was retrieved. The mitral valve apparatus was photographed to document both healing of the implants and the stable localization of the sonomicrometry crystals. The mitral valves were fixed in neutral buffered formalin, and representative samples were subjected to paraffin imbedding and sectioning, followed by staining with Movat’s stain to assess the extracellular matrix of each type of implant.
Statistics
All dimensional, strain, and stress changes were reported as mean ± standard error (SEM). For statistical comparisons, a one-way ANOVA was used, with all differences were deemed statistically significant for p<0.05.
Results
General observations and implant stability and morphology
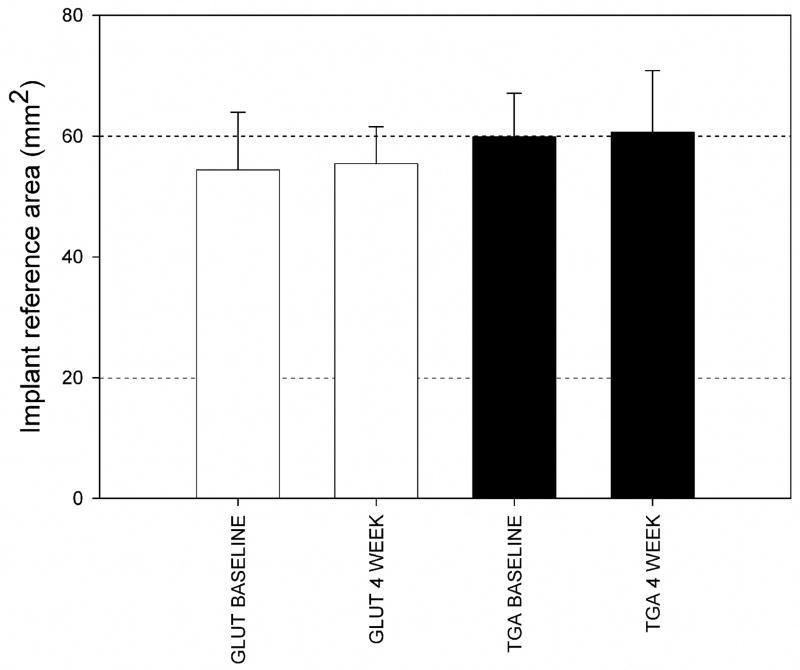
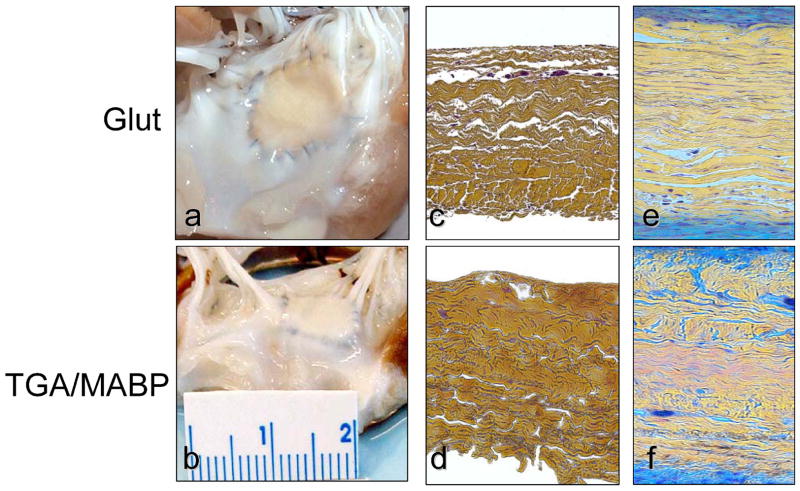
Transdiaphragmatic echocardiography revealed normal leaflet motion in all animals, with actual experimentally realized LV peak pressures within ±1 mmHg of the desired peak pressure. For the present study, a total 5 animals for the GLUT and 8 for the TGA-MABP implant groups were successfully implanted and tested after 4 weeks. The resulting implant dimensions demonstrated ~60 mm2 total area, which did not change significantly after 4 weeks both groups (Fig. 2). Explant histology studies revealed that both the GLUT and TGA-MABP implants demonstrated contiguous integration with the surrounding MV tissues, with no evidence of degeneration or mineralization (Fig. 3). Movat’s staining showed qualitatively greater valve tissue overgrowth of the TGA-MABP implants with increased glycosaminoglycan enriched tissue compared to the glutaraldehyde patches. Taken together, these results indicate that the tissue implants were structurally stable and well integrated into the surrounding host tissue.
Figure 2.
Mean measured implant areas in the reference state (i.e. MVAL unloaded) for both the GLUT and TGA-MABP groups at the 0 and 4 week time points, with mean values ranging from 58 to 60 mm2. There were no statistically significant differences between groups, indicating that all implants were dimensionally stable over the 4 week time period.
Figure 3.
Explant morphology comparing the following: (a) A gross specimen photographs of the glutaraldehyde valvuloplasty site; (b) The TGA valvuloplasty site with more exuberant overgrowth of connective tissue compared to glutaraldehyde fixation (see 3a); Cross-sections (c–f) of: (c) Unimplanted glutaraldehyde fixed bovine pericardium; (d) Unimplanted TGA-MABP pretreated bovine pericardium; (e) A 4 week explant specimen demonstrating overgrowth of the glutaraldehyde fixed specimen with host tissue with intense (blue) glycosaminoglycan staining; (f) A 4 week TGA-MABP explant demonstrating qualitatively greater overgrowth than observed with glutaraldehyde fixation (see 3f). Movats stain for figures c–f, original magnification 50x.
Strain and tissue implant compliance
Strain-time profiles were very regular in shape and smooth, a result due to the high fidelity of the sonocrystal technique (Fig. 4). As reported for the native MVAL [16], the strain- and areal strain-time responses indicated very rapid loading (in ~50 ms) followed by a plateau wherein negligible changes in strain occurred. Implants also experienced large, anisotropic strains, with the radial direction experiencing strain on the order of 20% (Fig. 4-c). Interestingly, in the circumferential direction we noted much smaller, contractile (i.e. negative) strain values on the of order of −5%(Fig. 4-c).
Figure 4.
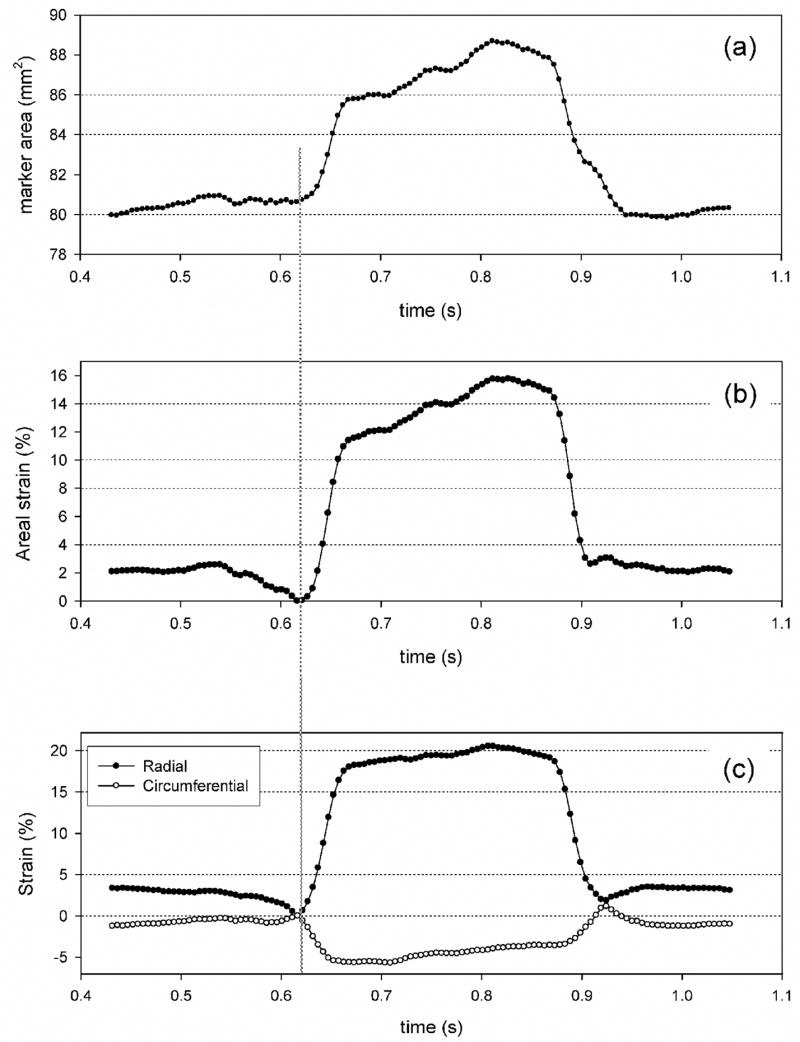
Typical cardiac cycle data for a TGA-MABP valvuloplasty site instrumented with sonomicrometry crystals representing: (a) implant area, (b) areal strain, and (c) individual strain component responses. Smooth responses in deformation were consistently observed, with changes in implant area of ~16% in this example under full systolic loading. Note that in (c) while the total area increased, principal strains were large and positive in the radial direction yet small and contractile in the circumferential direction. Glutaraldehyde pretreated valvuloplasty implants demonstrated comparable results (data not shown).
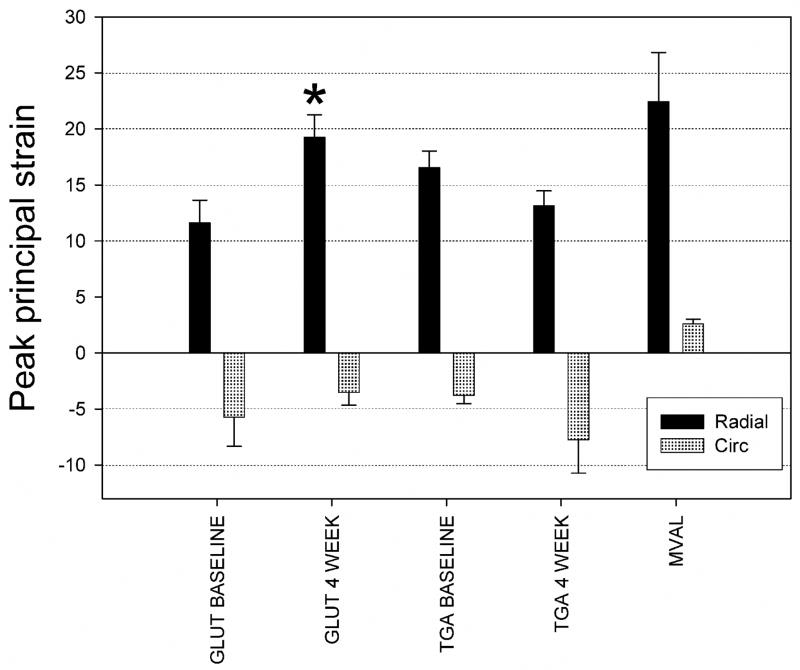
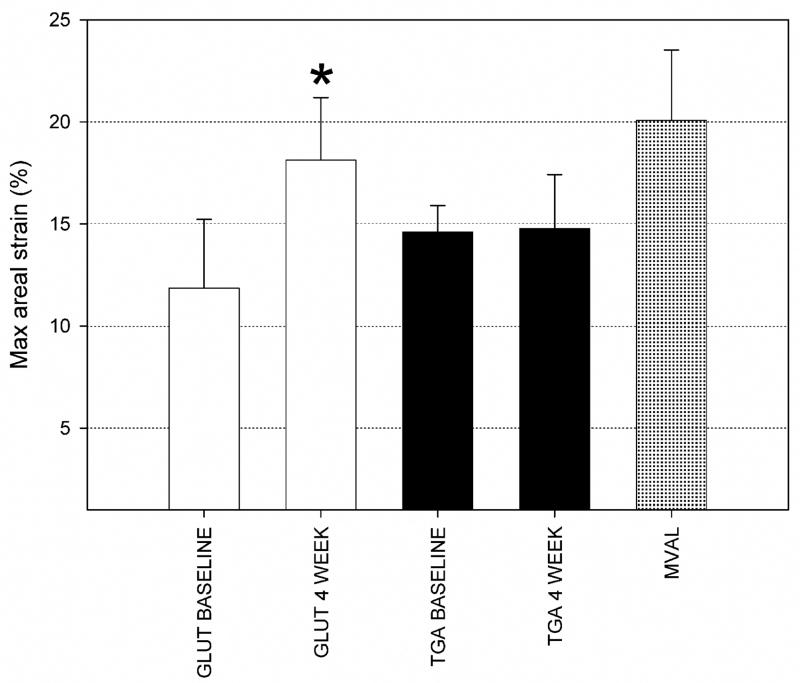
For both implant groups at both the 0 and 4 week timepoints, these trends were maintained (Fig. 5). Mean peak radial strains ranged from 12% to 18%, with mean peak circumferential strains ranging from −7% to −4%. Only the GLUT radial strain component demonstrated a significant change (Fig. 5). These strain levels were also comparable in magnitude to the native mitral valve tissue [16] (Fig. 5). Differences in overall implant compliance were more easily observed when examining the overall areal strain (Fig. 6). Here, we observed an overall areal strain of 12%–15% at the time of implant, with the GLUT group demonstrating an increase in compliance to ~18% at 4 weeks, whereas the TGA group demonstrated no changes. Moreover, the areal compliance of the implant tissues were less but still comparable to the ~20% areal strain reported for the MVAL (Fig. 6).
Figure 5.
Mean results for the peak strains for both TGA-MABP and glutaraldehyde implant groups and timepoints, along with the same data for the MVAL (taken from [16]). There were no statistically significant differences between peak circumferential strains. Only the peak radial strain for the GLUT group showed a statistically significant change at 4 weeks, * indicating p ≤ 0.05.
Figure 6.
Mean peak areal strain results for both implant groups and timepoints, along with the same data for the MVAL (taken from [16]). As for the peak radial strain (Fig. 5), only the GLUT group demonstrated a statistically significant change at 4 weeks, that may reflect the differences in tissue overgrowth (see Figure 3). Moreover, the total implant area changes at 0 weeks were ~12% for the GLUT group and ~15% for the TGA-MABP group, consistent with the increased stiffness of these materials compared to the native MVAL value of ~20%, with * indicating p ≤ 0.05.
Material model parameters and computed in vivo stress behavior
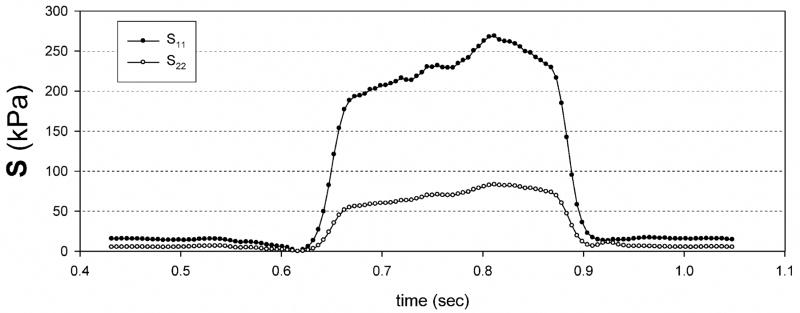
Eqns. 3 fit the biomechanical data from [7] well, with a composite r2 of ~0.98 for both axes (Table 1). The standard deviation σ for the collagen fiber splay function as ~26° for both tissue groups, which is comparable to the ~30° measured using light scattering methods [11]. The resulting stress component-time expectedly responses followed the strain behavior, and also demonstrated substantially larger stress values in the radial direction (Fig. 7). Mean values for the peak stress components were ~40 kPa and ~280 kPa for the circumferential and radial stress components, respectively (Table 1), for both implant groups.
Table 1.
Material parameters and computed peak 2nd Piola-Kirchhoff stresses for both GLUT and TGA-MABP implant tissues. Although the implant tissues produced predictably different material parameters due to the difference between their mechanical behaviors, the resulting computed peak stresses were comparable between both groups, and not statistically different (p=0.988 and 0.979 for S11 and S22, respectively).
| Implant tissue | σ (degrees) | c1(kPa) | c2 | r2 | Peak S11 (kPa) | Peak S22 (kPa) |
|---|---|---|---|---|---|---|
| GLUT | 27.429 ± 17.564 | 20.019 ± 10.409 | 42.926 ± 4.030 | 0.982 ± 0.005 | 40.901 ± 5.020 | 284.981 ± 36.420 |
| TGA | 25.412 ± 4.837 | 0.504 ± 0.378 | 39.747 ± 3.596 | 0.998 ± 0.001 | 42.278 ± 6.309 | 276.519 ± 32.807 |
Figure 7.
Typical computed stress component recordings during the cardiac cycle for a TGA-MABP mitral valvuloplasty site, demonstrating smooth responses under full systolic loading. Glutaraldehyde patch data is not shown, but was qualitatively comparable. Note that while contractile strains occurred in the circumferential direction, positive stresses are produced possibly due to the mechanics of the underlying collagen fiber network of the implant.
Discussion
Effects of TGA-MABP pre-treatment
In the present study the in-vivo performance of a standard GLUT and a novel TGA-MABP crosslinking pre-treatment methods were investigated. While both approaches demonstrated good tissue integration and minimal changes in implant dimensions over the 4 week implant period (Figs. 2, 3), TGA-MABP demonstrated an overall more stable response (Figs. 5,6). It should be noted that the changes the GLUT treated specimens underwent were comparatively small; not surprising given the 4 week time frame. Overall, both heterograft biomaterials performed comparably over the 4 week timeframe. This is an important finding, as it demonstrates that TGA-MABP heterograft biomaterials are able to withstand valvular dynamic stress levels in a blood contacting environment and remain mechanically stable. It should be noted that the greater overgrowth in the TGA-MABP implants could explain some of the differences in measured in-vivo biomechanical properties. While the mechanical properties of the overgrowth are unknown, it is unlikely that it is of sufficient stiffness to appreciably affect the implant response given the very high stresses they are subjected to. While longer-term implants will be required to further assess functional changes, our results provide evidence that TGA-MABP heterograft biomaterials can be used in stress-bearing bioprosthetic implants.
Implant-MVAL biomechanical interactions
When implanting a heterograft biomaterial with different biomechanical properties into the native MVAL, changes in the in-vivo deformation of the implant from the native tissues response during normal valve function should occur. In the present study, we noted that while large radial strains occurred as in the normal leaflet [16], contractile (or negative) strains occurred in the circumferential direction (Figs. 4 and 5). Native heart valvular tissues are highly anisotropic and, due to the highly circumferentially aligned collagen fiber network which is known to induce contractile strains even under positive stress states [19–21]. While native and chemically treated pericardium is also mechanically anisotropic [22, 23], the degree of anisotropy is considerably less than that of valvular tissues. Moreover, the overall stress-strain response of chemically treaded pericardium is more gradual compared to the sharp “L” shaped response of MVAL [24, 25]. Overall, the observed strain-time patterns measured in the present study are consistent with the differences noted in the biomechanical behavior between pericardial heterograft biomaterials [9] and native valvular tissues [20].
Estimated in-vivo stresses
In the present study we estimated the in-vivo stresses from the biaxial mechanical behavior of GLUT and TGA-MABP treated tissues [7]. The computed values (Table 1) are comparable to the ~250 kPa stresses reported in bioprosthetic heart valve finite element simulations [26]. Moreover, the implant stress-rates are likely also comparable to bioprosthetic heart valve tissue (Fig. 7), although comprehensive measurements are lacking in the literature. It is also interesting to note that while contractile strains occurred in the circumferential direction, substantial positive stresses occurred (Table 1). This is a direct result of the coupling due to the implant collagen fiber network [27], which can produce positive stresses [19] in the directions of contractile strains.
Methods for heterograft material assessment
Another unique aspect of this study was the utilization of an in-vivo MVAL valvuloplasty method. This approach allowed the implanted heterograft biomaterials to be subjected to dynamic stresses in a blood contacting environment while allowing direct monitoring of subsequent changes. We were thus able to directly determine if the implanted heterograft biomaterials experience creep or shrinkage (possibly due to mineralization or other host/material interactions), as evidenced by changes in implant dimensions in the reference state. We were also able to determine if implanted heterograft biomaterials experience changes in net mechanical properties (such as stiffness or anisotropy), as evidenced by changes in the realized peak strains under near-identical hemodynamic conditions. This approach was possible since changes it implant in-vivo could be tracked with high dimensional accuracy through the use of the same crystals used throughout the study.
Given the long term clinical expectations for replacement heart valves (15 patient years, or about 600 million cardiac cycles), BHV durability evaluations need to be performed for many millions of cycles. This is accomplished in industrial settings using accelerated wear testing (AWT), wherein fully intact and functioning valves are subjected to hydrodynamically induced opening/closure cycles at rates from 13–25 Hz. Pressure across the closed valve is maintained at a minimum of 90 mmHg for aortic valves, and at a minimum of 120 mmHg for mitral valves. Generally, tissue valves are tested for 200 million cycles (5 equivalent years), with the effects of tissue fatigue described after visual inspection at the end of the test [28]. Important damage information can be obtained from AWT specimens, including changes in collagen fiber architecture and mechanical properties [9, 29–32].
However, while full opening and closure are required, the type of loading using in AWT is clearly not physiologic. Moreover, as previously stated AWT can only test a functioning device; effects of design and biomaterials fatigue responses are intimately intertwined and cannot be separated. The in-vivo methods presented here offer an alternative to assessing the intrinsic performance of novel biomaterials for heart valve tissues in a physiological environment. Implanted tissue specimens are subjected to a blood contacting environment and dynamic stress/strains comparable to those experienced by bioprosthetic heart valve tissues [26]. Since sonocrystals are used, real-time implant dimensional changes can be made that also allow for monitoring of implant dimensions over the course of the test period. For example, in the current study we noted that this approach was sufficiently sensitive to detect previously reported differences between TGA-MABP and GLUT tissue properties (Fig. 6).
In-vivo methods, such as those reported here, would not replace AWT methods, which would constitute a “first-pass” approach to heterograft biomaterial assessment. Rather, they would provide a subsequent, refined test to determine how candidate biomaterials perform in valve-like physiological environments without the confounding effects of a particular valve design. Moreover, levels of integration with the surrounding host tissues (e.g. Fig. 2) would also offer a means to determine the nature and mechanisms of biomaterial/host tissue interactions under in-vivo heart valve tissue stress levels. This would be an advance over other in-vivo methods, such as rat subdermal tests [33], where the implant is in a stress-free state.
Limitations
Suturing sonocrystals onto the MVAL surface introduces the possibility of local tethering effects, as well as tissue damage, both of which can affect strain measurement accuracy. In our previous native MVAL tissue study [16], we determined the extent that suturing of sonocrystals to the anterior mitral valve leaflet had on leaflet mechanical integrity. It was found that placement of the sonocrystals on the MV anterior leaflet surface did not detectably alter MV anterior leaflet tissue properties and hence the local deformation field. In the present study only 4 sonocrystals were used placed at the corners of the implant (Fig. 1), compared to the 9 sonocrystals used in native MVAL study [16]. Since the inter-sonocrystal distances were ~10 mm in both studies, it should be reasonable to assume that placement of the sonocrystals had no measurable effect on strain measurement accuracy. Finally, we note that the measured strain fields were computed at the center of the crystal array, which represent the mean strain components. While the strain fields were not completely homogeneous, overall they exhibited reasonable uniformity as in [16]. Thus, the stress and strain values reported here represent the response of the bulk of the biomaterial implant responses.
Conclusions
Our group previously reported the synthesis and characterization of a novel epoxy-crosslinker, triglycidylamine (TGA). TGA pretreatment of BHV tissues compared to glutaraldehyde results in both calcification resistance in subdermal implants and improved leaflet compliance. In these prior studies, optimal calcification inhibition was noted with the combined use of TGA with mercapto-aminobisphosphonate (MABP). In the present study, we investigated the hypothesis that bovine pericardium cross-linked with TGA-MABP retains these beneficial biomechanical properties in-vivo using a novel mitral valve anterior leaflet (MVAL) ovine valvuloplasty model. TGA-MABP crosslinking of bovine pericardium resulted in a compliant heart valve biomaterial for in vivo use that demonstrated more stable mechanical function than noted with GLUT pretreatment, as shown using a sonomicrometry instrumented mitral valvuloplasty model system. Estimated implant stresses for both TGA-MABP and GLUT groups were 40 kPa and 250 kPa in the circumferential and radial directions, respectively, which are comparable to predicted BHV peak stress levels. We conclude that TGA-MABP crosslinked bovine pericardium, when subjected to in-vivo BHV stress levels in a blood contacting environment, maintains stable functionality.
Acknowledgments
This work was funded by NIH grants HL-073021 and P50 HL74731. Research support was also provided by St. Jude Medical, Inc., St. Paul, MN, and The William J. Rashkind Endowment of The Children’s Hospital of Philadelphia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79(3):1072–80. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Schoen FJ. Editorial: Are immune mechanisms important in tissue heart valve failure? A debate. J Heart Valve Dis. 2001;10(4):458–9. [PubMed] [Google Scholar]
- 3.Sacks MS, Schoen FJ. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J Biomed Mater Res. 2002;62(3):359–71. doi: 10.1002/jbm.10293. [DOI] [PubMed] [Google Scholar]
- 4.Purinya B, Kasyanov V, Volkolakov J, Latsis R, Tetere G. Biomechanical and Structural Properies of the Explanted Bioprosthetic Valve Leaftets. Journal of Biomechanics. 1994;27:1–11. doi: 10.1016/0021-9290(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 5.Mako WJ, Shah A, Vesely I. Mineralization of glutaraldehyde-fixed porcine aortic valve cusps in the subcutaneous rat model: analysis of variations in implant site and cuspal quadrants. J Biomed Mater Res. 1999;45(3):209–13. doi: 10.1002/(sici)1097-4636(19990605)45:3<209::aid-jbm8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Moczar M, Houel R, Ginat M, Clerin V, Wheeldon D, Loisance D. Structural changes in porcine bioprosthetic valves of a left ventricular assist system in human patients. J Heart Valve Dis. 2000;9(1):88–95. discussion 95–6. [PubMed] [Google Scholar]
- 7.Connolly JM, Alferiev I, Clark-Gruel JN, Eidelman N, Sacks M, Palmatory E, Kronsteiner A, Defelice S, Xu J, Ohri R, Narula N, Vyavahare N, Levy RJ. Triglycidylamine Crosslinking of Porcine Aortic Valve Cusps or Bovine Pericardium Results in Improved Biocompatibility, Biomechanics, and Calcification Resistance: Chemical and Biological Mechanisms. Am J Pathol. 2005;166(1):1–13. doi: 10.1016/S0002-9440(10)62227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rapoport HS, Connolly JM, Fulmer J, Dai N, Murti BH, Gorman RC, Gorman JH, Alferiev I, Levy RJ. Mechanisms of the in vivo inhibition of calcification of bioprosthetic porcine aortic valve cusps and aortic wall with triglycidylamine/mercapto bisphosphonate. Biomaterials. 2007;28(4):690–9. doi: 10.1016/j.biomaterials.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacks MS, Mirnajafi A, Sun W, Schmidt P. Bioprosthetic heart valve heterograft biomaterials: structure, mechanical behavior and computational simulation. Expert Rev Med Devices. 2006;3(6):817–34. doi: 10.1586/17434440.3.6.817. [DOI] [PubMed] [Google Scholar]
- 10.Sacks MS, Yoganathan AP. Heart valve function: A biomechanical perspective. Philosophical Transactions of the Royal Society B. doi: 10.1098/rstb.2007.2122. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacks MS. Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J Biomech Eng. 2003;125(2):280–7. doi: 10.1115/1.1544508. [DOI] [PubMed] [Google Scholar]
- 12.Alferiev IS, Connolly JM, Levy RJ. A novel mercapto-bisphosphonate as an efficient anticalcification agent for bioprosthetic tissues. Journal of Organometallic Chemistry. 2005;690:2543–2547. [Google Scholar]
- 13.Gorman JH, 3rd, Gupta KB, Streicher JT, Gorman RC, Jackson BM, Ratcliffe MB, Bogen DK, Edmunds LH., Jr Dynamic three-dimensional imaging of the mitral valve and left ventricle by rapid sonomicrometry array localization. J Thorac Cardiovasc Surg. 1996;112(3):712–26. doi: 10.1016/S0022-5223(96)70056-9. [DOI] [PubMed] [Google Scholar]
- 14.Sacks MS, He Z, Baijens L, Wanant S, Shah P, Sugimoto H, Yoganathan AP. Surface strains in the anterior leaflet of the functioning mitral valve. Annals of Biomedical Engineering. 2002;30(10):1281–90. doi: 10.1114/1.1529194. [DOI] [PubMed] [Google Scholar]
- 15.Sacks MS, Chuong CJ, Templeton GH, Peshock R. In Vivo 3-D Reconstruction and Geometric Characterization of the Right Ventricular Free Wall. Annals of Biomedical Engineering. 1993;21:263–275. doi: 10.1007/BF02368182. [DOI] [PubMed] [Google Scholar]
- 16.Sacks MS, Enomoto Y, Graybill JR, Merryman WD, Zeeshan A, Yoganathan AP, Levy RJ, Gorman RC, Gorman JH., 3rd In-vivo dynamic deformation of the mitral valve anterior leaflet. Ann Thorac Surg. 2006;82(4):1369–77. doi: 10.1016/j.athoracsur.2006.03.117. [DOI] [PubMed] [Google Scholar]
- 17.Sun W, Sacks MS, Sellaro TL, Slaughter WS, Scott MJ. Biaxial mechanical response of bioprosthetic heart valve biomaterials to high in-plane shear. Journal Biomechanical Engineering. 2003;125:372–380. doi: 10.1115/1.1572518. [DOI] [PubMed] [Google Scholar]
- 18.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical Receipes in C. Cambridge: Cambridge University Press; 1988. p. 735. [Google Scholar]
- 19.Billiar KL, Sacks MS. Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: Part II--A structural constitutive model. Journal of Biomechanical Engineering. 2000b;122(4):327–35. doi: 10.1115/1.1287158. [DOI] [PubMed] [Google Scholar]
- 20.Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp--Part I: Experimental results. Journal of Biomechanical Engineering. 2000a;122(1):23–30. doi: 10.1115/1.429624. [DOI] [PubMed] [Google Scholar]
- 21.Adamczyk MM, Vesely I. Characteristics of compressive strains in porcine aortic valves cusps. J Heart Valve Dis. 2002;11(1):75–83. [PubMed] [Google Scholar]
- 22.Sacks MS, Chuong CJ. Orthotropic mechanical properties of chemically treated bovine pericardium. Ann Biomed Eng. 1998;26(5):892–902. doi: 10.1114/1.135. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Langdon SE. Biaxial Mechanical Changes in Bovine Pericardium Cross-linked in EDC under controlled biaxial strain. 21st Annual meeting of the Society of Biomaterials; 1995. p. 86. [Google Scholar]
- 24.Grashow JS, Yoganathan AP, Sacks MS. Biaxial stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Ann Biomed Eng. 2006b;34(2):315–25. doi: 10.1007/s10439-005-9027-y. [DOI] [PubMed] [Google Scholar]
- 25.May-Newman K, Yin FC. Biaxial mechanical behavior of excised porcine mitral valve leaflets. Am J Physiol. 1995;269(4 Pt 2):H1319–27. doi: 10.1152/ajpheart.1995.269.4.H1319. [DOI] [PubMed] [Google Scholar]
- 26.Sun W, Abad A, Sacks MS. Simulated bioprosthetic heart valve deformation under quasi-static loading. Journal of Biomechanial Engineering. 2005;127(6):905–914. doi: 10.1115/1.2049337. [DOI] [PubMed] [Google Scholar]
- 27.Sacks MS. Biaxial mechanical evaluation of planar biological materials. Journal of Elasticity. 2000;61:199–246. [Google Scholar]
- 28.Prosthetic Devices Branch, D.o.C. Respiratory and Neurological Devices, Replacement Heart Valve Guidance. Center for Devices and Radiological Health (FDA); Rockfied, Maryland: 1993. [Google Scholar]
- 29.Gloeckner DC, Billiar KL, Sacks MS. Effects of mechanical fatigue on the bending properties of the porcine bioprosthetic heart valve. Asaio J. 1999;45(1):59–63. doi: 10.1097/00002480-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Wells SM, Sellaro T, Sacks MS. Cyclic loading response of bioprosthetic heart valves: effects of fixation stress state on the collagen fiber architecture. Biomaterials. 2005;26(15):2611–9. doi: 10.1016/j.biomaterials.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Wells SM, Sacks MS. Effects of fixation pressure on the biaxial mechanical behavior of porcine bioprosthetic heart valves with long-term cyclic loading. Biomaterials. 2002;23(11):2389–99. doi: 10.1016/s0142-9612(01)00375-1. [DOI] [PubMed] [Google Scholar]
- 32.Vesely I. The influence of design on bioprosthetic valve durability. J Long Term Eff Med Implants. 2001;11(3–4):137–49. [PubMed] [Google Scholar]
- 33.Schoen F, Levy R. Pathology of Substitute Heart Valves. Journal of Cardiac Surgery. 1994;9:222–227. doi: 10.1111/j.1540-8191.1994.tb00932.x. [DOI] [PubMed] [Google Scholar]