Abstract
Background
Roflumilast is a targeted oral once‐daily administered phosphodiesterase 4 (PDE4) inhibitor with clinical efficacy in chronic obstructive pulmonary disease (COPD). Results from in vitro studies with roflumilast indicate that it has anti‐inflammatory properties that may be applicable for the treatment of COPD.
Methods
In a crossover study, 38 patients with COPD (mean (SD) age 63.1 (7.0) years, post‐bronchodilator forced expiratory volume in 1 s (FEV1) 61.0 (12.6)% predicted) received 500 μg roflumilast or placebo once daily for 4 weeks. Induced sputum samples were collected before and after 2 and 4 weeks of treatment. Differential and absolute cell counts were determined in whole sputum samples. Markers of inflammation were determined in sputum supernatants and blood. Spirometry was performed weekly.
Results
Roflumilast significantly reduced the absolute number of neutrophils and eosinophils/g sputum compared with placebo by 35.5% (95% CI 15.6% to 50.7%; p = 0.002) and 50.0% (95% CI 26.8% to 65.8%; p<0.001), respectively. The relative proportion of sputum neutrophils and eosinophils was not affected by treatment (p>0.05). Levels of soluble interleukin‐8, neutrophil elastase, eosinophil cationic protein and α2‐macroglobulin in sputum and the release of tumour necrosis factor α from blood cells were significantly reduced by roflumilast compared with placebo treatment (p<0.05 for all). Post‐bronchodilator FEV1 improved significantly during roflumilast compared with placebo treatment with a mean difference between treatments of 68.7 ml (95% CI 12.9 to 124.5; p = 0.018).
Conclusion
PDE4 inhibition by roflumilast treatment for 4 weeks reduced the number of neutrophils and eosinophils, as well as soluble markers of neutrophilic and eosinophilic inflammatory activity in induced sputum samples of patients with COPD. This anti‐inflammatory effect may in part explain the concomitant improvement in post‐bronchodilator FEV1.
Chronic obstructive pulmonary disease (COPD) is characterised by progressive airflow limitation which is not fully reversible1 and is associated with an abnormal inflammatory response in the airways.2 The inflammatory infiltrate within the airway lumen in COPD consists mainly of neutrophils.3 Airway neutrophilia is reflected by sputum neutrophilia and is also indicative of systemic inflammation.4,5 Furthermore, in patients with COPD, elevated levels of pro‐inflammatory cytokines related to neutrophil activity are evident in sputum and the systemic circulation, including interleukin (IL)‐86 (associated with enhanced neutrophil chemotaxis), neutrophil elastase7 (involved in connective tissue degradation), tumour necrosis factor (TNF)α8 (involved in inflammatory cell activation) and circulating adhesion molecule E‐selectin9 (important in rolling of leucocytes to the endothelium).
At present, treatment options for COPD are limited and consist of bronchodilators alone or in combination with inhaled corticosteroids.1 Treatment with inhaled steroids only slightly ameliorates the accelerated decline in forced expiratory volume in 1 s (FEV1) in these patients10 but, in combination with long‐acting bronchodilators, reduces inflammatory cell numbers in the bronchial wall and sputum.11
A novel approach for therapeutic intervention in COPD is through inhibition of phosphodiesterase 4 (PDE4) activity. PDE4 is an intracellular enzyme involved in the degradation of the second messenger cyclic adenosine monophosphate (cAMP).12,13 Raised levels of this second messenger relax airway smooth muscle and modulate inflammatory cell activity.14 PDE4 is expressed in inflammatory cells and airway smooth muscle, while basophils, mast cells, monocytes/macrophages, T lymphocytes and smooth muscle cells also express PDE3.14 Roflumilast is a targeted PDE4 inhibitor which is under investigation for the treatment of COPD and bronchial asthma. It ameliorates the activity of various inflammatory cells in vitro15 and reduces pulmonary inflammation in complex in vivo animal models.16,17 Furthermore, roflumilast has clinical effects in patients with COPD. Treatment for 24 weeks improves lung function and reduces the number of mild exacerbations.18 However, at present it is unknown whether roflumilast has anti‐inflammatory properties in patients with COPD.
A study was therefore undertaken to examine the efficacy of oral roflumilast treatment (500 μg once daily for 4 weeks) compared with placebo on the reduction in the percentage of sputum neutrophils in patients with COPD. Secondary end points examined were the effect of roflumilast versus placebo on FEV1, absolute numbers of neutrophils in sputum, other inflammatory cell numbers and percentages in sputum, and markers of activation of inflammatory cells (IL‐8, neutrophil elastase, lactoferrin and eosinophil cationic protein (ECP)) and markers of microvascular leakage (α2‐macroglobulin) in sputum supernatant. In addition, the effect of oral roflumilast on systemic markers of inflammation was studied by measurement of TNFα production by whole blood cultures following lipopolysaccharide (LPS) stimulation and soluble E‐selectin levels in serum.
Methods
Patients
Patients with a history of COPD1 for at least 1 year were invited to participate in the study and had to fulfil the following criteria: age 45–75 years, current smokers or ex‐smokers (stable for ⩾6 months) with a smoking history ⩾10 pack‐years, pre‐bronchodilator FEV1/FVC ⩽70%, post‐bronchodilator FEV1 35–75% predicted, reversibility in FEV1 <12% or <200 ml from the pre‐bronchodilator value, sputum neutrophilia (⩾45% non‐squamous cells) and no exacerbation or upper respiratory tract infection during the 4 weeks before the start of the study. Short‐acting bronchodilators were allowed during the study whereas long‐acting bronchodilators, theophylline (2 weeks), inhaled and/or oral corticosteroids (4 weeks) were discontinued prior to inclusion. The study was approved by the medical ethics committee of the Leiden University Medical Center and performed according to the Declaration of Helsinki.2 All patients gave written informed consent.
Design
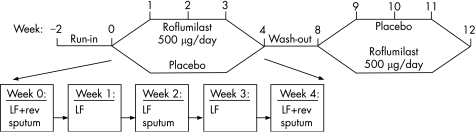
This randomised, double‐blind, placebo‐controlled, crossover study consisted of two treatment periods of 4 weeks with a washout period of 4–6 weeks between treatments (fig 1). Following inclusion, patients entered a run‐in period of 2 weeks during which placebo tablets were used. Patients compliant to the study medication (>70% of tablets used) and with lung function and sputum within the criteria of inclusion were randomised to receive either roflumilast 500 μg or placebo once daily for 4 weeks by means of a concealed computer‐generated randomisation list.
Figure 1 Following a 2‐week run‐in period, patients were randomised to receive roflumilast (500 μg once daily) or matching placebo for a period of 4 weeks. Four to 6 weeks after washout of the medication of the first treatment period, patients crossed over to the alternative treatment. During each treatment period, patients visited the department on a weekly basis. LF, lung function measurement by spirometry; rev, reversibility testing of forced expiratory volume in 1 s (FEV1) with 400 μg salbutamol.
During each treatment period, patients visited the laboratory at weekly intervals for measurement of lung function, compliance check and sampling of blood for determination of E‐selectin and whole blood stimulation assay with LPS for measuring TNFα. Blood samples were drawn at weeks 0 and 8 before administration of medication (total volume 49 ml), but at all other visits blood was drawn approximately 1 h after the intake of study medication (total volume 34.5 ml). Sputum samples were collected at inclusion in the study, at the start of each treatment period, and after 2 and 4 weeks of treatment. All patients were supplied with a salbutamol metered dose inhaler (100 μg/puff) for on‐demand symptom relief. Patients withheld salbutamol, smoking and caffeine‐containing beverages for 6 h and anticholinergic agents for 8 h before lung function measurements. ECG, vital signs and clinical laboratory parameters were performed at inclusion in the study and at the beginning and end of each treatment period. Adverse events were monitored throughout the study.
Measurements
FEV1 was recorded from maximal expiratory flow‐volume curves on a calibrated pneumotachograph according to standards.19,20 Reversibility of FEV1 was determined 30 min after inhalation of 400 μg salbutamol.
Sputum induction was performed by inhalation of 4.5% hypertonic saline aerosols.21 Whole sputum samples were processed.22 Differential cell counts were expressed as the percentage of non‐squamous cells. Absolute cell numbers were calculated as (% cell × total cell count)/sputum weight. IL‐8, lactoferrin, α2‐macroglobulin23 and neutrophil elastase were measured by enzyme‐linked immunosorbent assays (ELISA) and ECP was measured by fluoroimmunoassay. E‐selectin concentrations in serum were measured by ELISA. TNFα release was assessed in whole blood cultures of heparinised blood stimulated with LPS, essentially as described.24
Safety
This trial involved extensive safety and tolerability assessments including vital signs, clinical, laboratory and adverse event monitoring.
Statistical analysis
Demographic data are presented as mean (SD) or median (minimum, maximum). Non‐normally distributed data were log‐transformed before analysis. The primary end point of the study was the reduction in the percentage of sputum neutrophils. Further analyses included FEV1, absolute number of sputum neutrophils, other inflammatory cell numbers and percentages in sputum, markers of activation of inflammatory cells (IL‐8, neutrophil elastase, lactoferrin and ECP), markers of microvascular leakage (α2‐macroglobulin) in sputum supernatant, and systemic markers of inflammation including TNFα production by whole blood cultures following LPS stimulation and soluble E‐selectin levels in serum. The sample size was calculated using a two‐sided α of 5% and a power of 80%, together with a conservative estimation of the correlation between paired observations (0.5). It was calculated that, with a sample size of 32 (evaluable) patients, we would be able to detect a treatment effect with a standard deviation of 1.9 times the mean difference. Longitudinal data were analysed by repeated measures analysis with a correction for period effects (ANCOVA). Effect estimates between treatments were calculated from linear mixed models and presented as point estimates with 95% confidence intervals. Confidence intervals of point estimates not including 1 for log‐transformed data and not including 0 for normally distributed data were interpreted as statistically significant. A p value of <0.05 was considered significant for the latter analyses.
Results
Participants
Forty‐four patients with COPD were included in the study between January 2001 and March 2002, 41 of whom were randomised. In two patients FEV1 reversibility criteria were not met and in one patient the neutrophilia criteria were not met. These patients were excluded from the analyses. Data from a total of 38 patients were therefore used for the efficacy analysis. Six patients dropped out of the study during treatment for the following reasons: withdrawal of consent (n = 1), exacerbation of COPD requiring additional treatment (n = 2), and adverse events to the study medication (n = 3, see below). The baseline characteristics of the patients who contributed to the efficacy analysis are shown in tables 1 and 2.
Table 1 Demographic data of patients in the efficacy analysis.
| Sex (M:F)* | 29:9 |
| Age (years) | 63.1 (7.0) |
| Smoking history (pack‐years)† | 40.0 (10–90) |
| Smoking status (current:ex)* | 18:20 |
| Pre‐bronchodilator FEV1 (l) | 1.79 (0.60) |
| Pre‐bronchodilator FEV1 (% predicted) | 57.9 (12.9) |
| Post‐bronchodilator FEV1 (% predicted) | 61.0 (12.6) |
| Reversibility (% from pre‐bronchodilator FEV1) | 6.0 (6.0) |
| Pre‐bronchodilator FEV1/FVC (%) | 54.4 (10.1) |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Data are mean (SD) unless otherwise indicated.
*Numbers.
†Median (minimum − maximum).
Table 2 Inflammatory markers in sputum and blood at baseline of patients in the efficacy analysis.
| % | ×105 cells/g sputum | Concentration | |
|---|---|---|---|
| Induced sputum | |||
| Total non‐squamous cells | – | 18.0 (2.0–87.1)† | |
| Squamous cells | 10.7 (0–74.4) | – | |
| Neutrophils | 74.4 (46.4–95.4) | 13.0 (1.7–84)† | |
| Macrophages | 20.5 (2.6–47.6) | 3.2 (0.2–21.1)† | |
| Eosinophils | 0.8 (0.02–8.0)† | 0.14 (0.001–4.2)† | |
| Lymphocytes | 1.4 (0–4.6) | 0.24 (0.024–1.87)† | |
| Markers in sputum supernatant | |||
| IL‐8 (ng/ml) | 12.5 (0.49–179.9)† | ||
| Neutrophil elastase (μg/ml) | 3.61 (0.14–120.3)† | ||
| Lactoferrin (μg/ml) | 73.5 (0.8–512.9)† | ||
| ECP (μg/l) | 170.8 (15.6–5767.5)† | ||
| α2‐macroglobulin (ng/ml) | 1345.7 (20.1–15063.04)† | ||
| Blood | |||
| E‐selectin (ng/ml) | 51.7 (33.5–94.6)† | ||
| TNFα (ng/ml) | 8.1 (1.6–37.0)† |
IL, interleukin; ECP, eosinophil cationic protein; TNFα, tumour necrosis factor α.
Data are median or †geometric mean (minimum − maximum).
Lung function
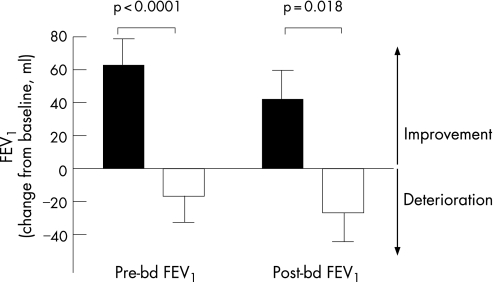
Pre‐ and post‐bronchodilator FEV1 improved significantly during roflumilast treatment (fig 2). The change in both pre‐ and post‐bronchodilator FEV1 was significantly different between roflumilast and placebo treatment (fig 2, p<0.0001 and p = 0.018, respectively). The point estimate of difference between the treatments was 79.5 ml (95% confidence interval (CI) 54.0 to 105.1) for pre‐bronchodilator FEV1 and 68.7 ml (95% CI 12.9 to 124.5) for post‐bronchodilator FEV1.
Figure 2 Change in pre‐ and post‐bronchodilator (bd) forced expiratory volume in 1 s (FEV1) during roflumilast (black bars) and placebo (white bars) treatment for 4 weeks.
Inflammatory cells in sputum
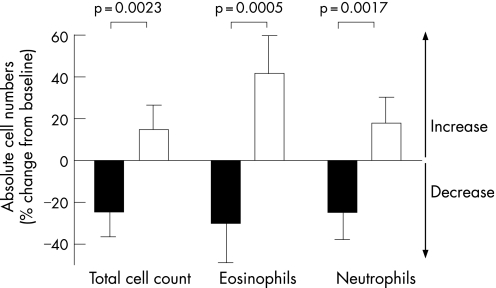
At baseline the total inflammatory cell count comprised primarily neutrophils (74%). The inflammatory cell load in the airways, as reflected by the total non‐squamous cell count in sputum samples, decreased during roflumilast treatment but increased during placebo treatment (difference between treatments p = 0.002, fig 3). Overall, the total cell count decreased significantly by 34% during roflumilast treatment compared with placebo (table 3). In particular, roflumilast treatment was associated with a significant decrease in neutrophil numbers in sputum (p = 0.002, fig 3, table 3). In addition, roflumilast treatment reduced sputum eosinophil (p<0.001, fig 3, table 3), macrophage (p = 0.067, table 3) and lymphocyte numbers (p = 0.022, table 3). Sputum weight was not affected by roflumilast or placebo treatment (p = 0.68, table 3).
Figure 3 Change in total cell count and numbers of neutrophils and eosinophils in sputum during roflumilast (black bars) and placebo (white bars) treatment for 4 weeks.
Table 3 Mean overall difference between roflumilast and placebo treatment.
| Mean difference (95% CI) | p Value | |
|---|---|---|
| Sputum characteristics | ||
| Total cell count (×105 cells/g)† | −33.6% (−48.8 to −13.8) | 0.002 |
| Weight (g)* | 0.15 (−0.56 to 0.86) | 0.68 |
| Viability (%)* | −2.0 (−4.4 to 0.3) | 0.091 |
| Squamous cell contamination (%)* | −1.8 (−6.0 to 2.5) | 0.41 |
| Cellular inflammation in sputum | ||
| Absolute number of neutrophils†‡ | −35.5% (−50.7 to −15.6) | 0.002 |
| Absolute number of eosinophils†‡ | −50.0% (−65.8 to −26.8) | <0.001 |
| Absolute number of macrophages†‡ | −24.4% (−43.9 to 2.0) | 0.067 |
| Absolute number of lymphocytes†‡ | −34.8% (−54.7 to −6.1) | 0.022 |
| % Neutrophils* | −1.6 (−4.8 to 1.6) | 0.31 |
| % Eosinophils† | −25.2% (−44.3 to 0.3) | 0.052 |
| % Macrophages* | 1.8 (−0.9 to 4.6) | 0.19 |
| % Lymphocytes† | −4.1% (−23.8 to 20.7) | 0.72 |
| Markers of inflammation in sputum supernatant | ||
| IL‐8 (ng/ml)† | −25.9% (−44.7 to −0.8) | 0.044 |
| Neutrophil elastase (μg/ml)† | −30.6% (−49.8 to −4.1) | 0.028 |
| Lactoferrin (μg/ml)† | −29.0% (−57.3 to 17.9) | 0.18 |
| ECP (μg/l)† | −34.3% (−53.1 to −7.9) | 0.015 |
| Markers of microvascular leakage in sputum supernatant | ||
| α2‐Macroglobulin (ng/ml)† | −40.8% (−55.0 to −22.3) | <0.001 |
| Systemic markers of inflammation | ||
| E‐selectin (ng/ml)† | −1.7% (−4.5 to 1.1) | 0.23 |
| TNFα (pg/ml)† | −10.4% (−19.7 to −0.1) | 0.047 |
| Lung function | ||
| Pre‐bronchodilator FEV1 (ml)* | 79.5 ml (54.0 to 105.1) | <0.001 |
| Post‐bronchodilator FEV1 (ml)* | 68.7 ml (12.9 to 124.5) | 0.018 |
IL, interleukin; ECP, eosinophil cationic protein; TNFα, tumour necrosis factor α; FEV1, forced expiratory volume in 1 s.
*Mean difference between treatments represents “absolute” difference between roflumilast and placebo treatment for sputum weight, viability, squamous cell contamination, percentage of neutrophils and percentage of macrophages in sputum, and pre‐ and post‐bronchodilator FEV1.
†”Relative” difference (mean ratio of change from baseline) for total cell count, number of neutrophils, eosinophils, macrophages and lymphocytes, percentage of eosinophils, percentage of lymphocytes, IL‐8, neutrophil elastase, lactoferrin, ECP, α2‐macroglobulin, E‐selectin and TNFα since these parameters were log‐transformed prior to analyses.
‡Unit of absolute cells in sputum ×105 cells/g sputum.
In contrast to absolute cell numbers, differential cell counts for neutrophils, macrophages and lymphocytes, expressed as a percentage of total non‐squamous cells, were not affected by the treatments (table 3). The percentage of eosinophils tended to be reduced by roflumilast treatment compared with placebo (p = 0.052, table 3).
Markers of inflammation and microvascular leakage in sputum supernatant
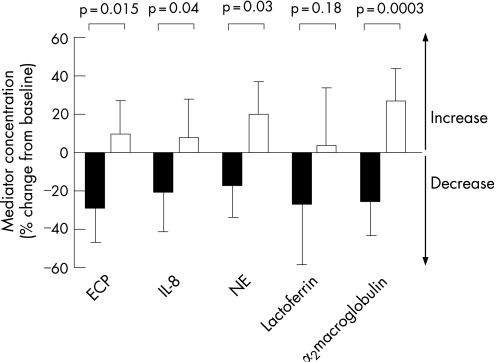
The levels of the neutrophil chemoattractant IL‐8 and the neutrophil degranulation product neutrophil elastase decreased significantly during roflumilast treatment compared with placebo (p<0.05, fig 4, table 3). In contrast to neutrophil elastase, levels of lactoferrin (a marker of neutrophil activity but also produced by submucosal glands) were not affected during treatment (p = 0.18, fig 4, table 3). Eosinophil activity, as reflected by ECP levels in sputum, was significantly reduced by roflumilast treatment compared with placebo (p = 0.015, fig 4, table 3). Roflumilast significantly reduced microvascular leakage, as measured by α2‐macroglobulin, compared with placebo (p<0.001, fig 4, table 3).
Figure 4 Changes in eosinophil cationic protein (ECP), interleukin (IL)‐8 and neutrophil elastase (NE), lactoferrin and α2‐macroglobulin levels in sputum supernatants during roflumilast (black bars) and placebo (white bars) treatment for 4 weeks.
Markers of inflammation in the circulation
TNFα secretion by whole blood cultures following ex vivo stimulation by LPS was significantly reduced during roflumilast treatment compared with placebo (p = 0.047, table 3). In contrast, E‐selectin levels in the circulation were not different between the two treatments (p = 0.23, table 3).
There was no carry‐over effect from the first to the second treatment period in any statistical analysis presented.
Adverse events
Thirty‐three patients (87%) reported at least one adverse event during roflumilast treatment compared with 26 patients (67%) during placebo treatment (table 4). These adverse events were of mild or moderate intensity and transient. No serious adverse events were reported. The most frequent adverse events with roflumilast were diarrhoea and headache, which are part of the known side effect profile of this drug. Three patients discontinued the study owing to adverse events, which were all during roflumilast treatment. Their adverse events resolved upon withdrawal of the study medication. There were no relevant findings regarding laboratory, ECG and vital signs.
Table 4 Number (%) of patients with adverse events during treatment periods.
| Roflumilast | Placebo | |
|---|---|---|
| Body as a whole (flu syndrome, abdominal pain, malaise, back pain) | 17 (45) | 10 (26) |
| Digestive system (diarrhea, nausea, abnormal stool, vomiting, dyspepsia) | 17 (45) | 4 (10) |
| Nervous system (headache, dizziness) | 17 (45) | 2 (5) |
| Respiratory system (increased cough, dyspnoea, upper respiratory tract infection, rhinitis, increased sputum production) | 16 (42) | 15 (39) |
| Cardiovascular system (ECG abnormal, chest pain, palpitation, thrombophlebitis) | 4 (11) | 1 (3) |
Adverse events with incidence ⩾1% are listed.
Discussion
This crossover placebo controlled study is the first to examine the anti‐inflammatory and pulmonary effects of the oral PDE4 inhibitor roflumilast in patients with COPD. Our results show that roflumilast treatment reduces the inflammatory activity in induced sputum, a surrogate for airway inflammation. The absolute number of sputum inflammatory cells (including neutrophils, eosinophils and lymphocytes) were reduced by 35–50% during the 4‐week treatment with roflumilast compared with placebo, with a similar tendency for the number of macrophages. As a result, the total cell count was significantly decreased by 34%. Furthermore, this was accompanied by a reduction in the levels of chemoattractant stimuli for neutrophils, cellular activity of eosinophils and neutrophils in sputum, as well as microvascular leakage. Concomitant with these changes in sputum inflammation were a decrease in TNFα release in the blood and an improvement in pulmonary function as assessed by FEV1. We conclude that roflumilast has anti‐inflammatory effects in patients with COPD which is accompanied by an improvement in lung function.
Our data extend the findings of Gamble et al who showed that treatment with the PDE4 inhibitor cilomilast for 12 weeks significantly reduced inflammatory cell numbers in bronchial biopsy specimens from patients with COPD.25 In the present study, as well as in the study of Gamble et al, treatment with a PDE4 inhibitor did not change the differential cell counts in sputum samples. This may imply that absolute cell numbers rather than relative cell counts should be assessed to monitor anti‐inflammatory effects of treatment in patients with COPD, as has been suggested previously.26 Furthermore, we have shown that roflumilast significantly attenuates cellular activity within the airways of patients with COPD, as reflected by reductions in the level of IL‐8, neutrophil elastase and ECP, and reduces microvascular leakage. Such anti‐inflammatory effects in patients with COPD have previously been shown for the PDE4 inhibitor BAY 19‐8004, although treatment with this compound was not associated with an effect on cellular inflammation.27 Lactoferrin levels were not significantly changed by roflumilast. However, the effect size was of the same order as for the other inflammatory parameters studied, which may indicate that the signal to noise ratio was too large for lactoferrin in our study with a relatively small sample size.
In addition to anti‐inflammatory activity, we have shown that roflumilast improves both pre‐ and post‐bronchodilator FEV1 in the same patients with COPD. This finding confirms recently reported data that roflumilast provides improvement in FEV1 of about 100 ml together with a 34% reduction in the number of exacerbations in patients with lung function that is poorly reversible to bronchodilators.18 This improvement in FEV1 may in part be explained by the parallel reduction in the number and activation status of neutrophils and eosinophils in induced sputum samples of these patients with COPD.
A large proportion of patients experienced adverse events during roflumilast and placebo treatment, which is in line with previous studies.18 The most frequently encountered adverse events during roflumilast treatment were diarrhoea and headache, which are typically PDE4‐related and part of the known side effect profile of roflumilast. All adverse events were transient and none of the patients experienced a serious adverse event. Although three patients discontinued the study because of adverse events during roflumilast treatment, these events resolved on withdrawal of the study medication. Whether roflumilast could be tolerated by these patients using a gradual increase in the dose was not investigated in this study.
The present study has potential limitations. First, patients with sputum neutrophils ⩾45% were included in the study to ensure that there would be room for improvement in sputum neutrophils during treatment. Ninety‐five percent of ex‐smokers with COPD had sputum neutrophilia of this magnitude and only two patients were excluded from participating in the study because of a lower percentage of neutrophils. It is therefore likely that our results can be generalised to most patients with COPD. Second, it has been suggested recently that a window of at least 6 months should be considered to evaluate the effect of treatment in COPD.28 Remarkably, even though our patients were treated for only 4 weeks, the present results show a clear treatment effect within this short time, as has been demonstrated previously for roflumilast.18 The treatment effect was corrected for variation during placebo treatment. During placebo treatment, a relative worsening of inflammation was observed as opposed to a relative attenuation during roflumilast treatment, resulting in a mean overall difference between roflumilast and placebo which was significant for various cellular and soluble markers of inflammation. Furthermore, the statistical analyses showed that there was no carry‐over effect from the first period to the second period of treatment. Finally, the effect of possible confounders—such as smoking status and previous inhaled steroid usage—on roflumilast treatment could not be assessed because of the limited sample size. In order to investigate these effects and to examine whether the anti‐inflammatory effect of roflumilast is causally related to the improvement in lung function, prospective trials with a larger sample size should be performed.
How can the present results be explained? Roflumilast is a new oral once‐daily administered inhibitor targeting PDE4. In patients with COPD, systemically available treatment may interact with pulmonary as well as systemic inflammation, both of which are present in COPD.1,4 Systemic effects of roflumilast associated with the initiation of inflammation can therefore be anticipated. TNFα is a major product of mononuclear leucocytes in the circulation which is regulated by cAMP15 and is involved in upregulation of the adhesion molecule E‐selectin on vascular endothelium.29 Selectin‐mediated adhesion of leucocytes to the vascular endothelium is a key early event in the initiation of inflammatory responses.29 Interestingly, increased levels of E‐selectin in serum have been found in patients with COPD.9 In the present study, PDE4 inhibition by roflumilast was associated with a slight reduction in TNFα release following ex vivo stimulation of whole blood cells. However, E‐selectin levels in serum were unchanged during roflumilast treatment. This may indicate either that the reduction in TNFα was too small to exert an effect on the expression of adhesion molecules or that the ex vivo release of TNFα by whole blood cells does not predict E‐selectin release.
Microvascular leakage is regulated through the formation and closure of intercellular gaps between endothelial cells which facilitate the extravasation of fluid, macromolecules and cells.30 Increased levels of cAMP decrease intercellular gap formation and permeability, thereby restoring the integrity of the pulmonary endothelial barrier.30 In pulmonary endothelial monolayers cultured in vitro, induced hyperpermeability can be reduced by PDE3 and PDE4 inhibitors.31 The finding that α2‐macroglobulin levels in sputum supernatant, a marker of microvascular leakage,32 were reduced by roflumilast suggest that in vivo inhibition of PDE4 also leads to restoration of the endothelial barrier. Apparently, this resulted in non‐differential attenuation of cell extravasation from the circulation, as shown by a reduction in absolute cell counts for neutrophils, eosinophils, macrophages and lymphocytes in sputum without an effect on cell differential counts. These effects are not observed only in sputum samples, but also in bronchial biopsy specimens.25 Several studies have shown that the level of inflammation within the airways is associated with lung function in patients with COPD.33,34 It is likely that attenuation of airways inflammation by roflumilast is one of the mechanisms underlying the observed improvement in lung function. Although a direct effect of PDE4 inhibition on smooth muscle relaxation cannot be excluded,12 this does not seem a likely explanation for the observed improvement in lung function because it has been shown that a single dose of PDE4 inhibitor does not provide bronchodilation.27,35
Extensive in vitro and in vivo studies in animals have shown that inhibition of PDE4 increases the intracellular concentration of cAMP, leading to broad anti‐inflammatory and immunomodulatory effects.15,16 Indeed, in our study, the activity of several types of inflammatory cells was suppressed following roflumilast, as shown by large reductions in the levels of IL‐8, neutrophil elastase and ECP. Although lactoferrin levels were not significantly reduced by roflumilast, the effect size was of the same order as for the other soluble markers. Alternatively, it could be speculated that PDE4 inhibition selectively affects the degranulation of azurophilic granules in neutrophils containing neutrophil elastase rather than in the specific granules containing lactoferrin.36 Because the submucosal glands, as well as neutrophils, are a major cellular source of lactoferrin production, an inhibitory effect of treatment on lactoferrin release by neutrophils may have been masked.
Our data have important clinical implications. At present the recommended treatment for COPD consists of bronchodilators for symptom relief with the addition of inhaled corticosteroids for more severe disease.1,37 Inhaled steroids have only limited effects on airway inflammation and lung function (decline) in patients with COPD.10,38,39 Roflumilast treatment in COPD improves not only lung function and health status,18 but also reduces the rate of mild exacerbations as judged from an increase in bronchodilator use and symptoms.18 This may be associated with reduced airway inflammation, as observed in our study.
In summary, our results support the hypothesis that roflumilast treatment has anti‐inflammatory effects in patients with COPD. Whether such treatment also reduces airway remodelling, the progressive decline in FEV1 and the associated mortality risk in patients with COPD remains to be examined in long‐term follow‐up studies.
Acknowledgements
The authors thank Dr Kathy B Thomas and Dr Angela Schilling (Medical Writing, ALTANA Pharma AG, Konstanz, Germany) for helpful suggestions during the preparation of the article.
Abbreviations
COPD - chronic obstructive pulmonary disease
ECP - eosinophil cationic protein
FEV1 - forced expiratory volume in 1 s
FVC - forced vital capacity
IL - interleukin
LPS - lipopolysaccharide
PDE - phosphodiesterase
TNFα - tumour necrosis factor α
Footnotes
This study was financially supported by ALTANA Pharma AG, Konstanz, Germany. The study was designed by DCG, PJS, DB, TDB, PSH and KFR. Data collection was performed by DCG, SAG and RMV. Altana monitored all collected data. Statistical analysis was performed by DCG, PJS, PSH and KFR and confirmed by an independent statistician (Dr G Rippin, Omnicare Clinical Research, Biometrics International). DCG, PJS, PSH and KFR interpreted the data and wrote the manuscript, which was subsequently discussed with and approved by ALTANA. All investigators and ALTANA agreed upon submission of the manuscript to Thorax.
Competing interests: DCG, SAG and RMV have no declared conflict of interest. The Department of Pulmonology, and thereby PJS (staff member), PSH (staff member) and KFR (head of the department), has received grants from ALTANA Pharma, Novartis, Bayer, AstraZeneca, Pfizer, MSD, Exhale Therapeutics, Boehringer Ingelheim, Roche and GSK in the years 2001–6. PSH has participated as a speaker in various meetings co‐financed by various pharmaceutical companies. KFR has been a consultant, participated in Advisory Board Meetings and received lecture fees from AstraZeneca, ALTANA Pharma, MSD and GSK. KFR holds no stock or other equities in pharmaceutical companies. JJH, DB and TDB are employees of ALTANA Pharma.
References
- 1.Rabe K F, Hurd S, Anzueto A.et al Global initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007176532–555. [DOI] [PubMed] [Google Scholar]
- 2.Hogg J C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004364709–721. [DOI] [PubMed] [Google Scholar]
- 3.Keatings V M, Barnes P J. Granulocyte activation markers in induced sputum: comparison between chronic obstructive pulmonary disease, asthma, and normal subjects. Am J Respir Crit Care Med 1997155449–453. [DOI] [PubMed] [Google Scholar]
- 4.Wouters E F, Creutzberg E C, Schols A M. Systemic effects in COPD. Chest 2002121(5 Suppl)127–30S. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson G C, Seemungal T A, Patel I S.et al Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 20051281995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keatings V M, Collins P D, Scott D M.et al Differences in interleukin‐8 and tumor necrosis factor‐a in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med 1996153530–534. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto K, Kubo K, Yamamoto H.et al Eosinophilic inflammation in the airway is related to glucocorticoid reversibility in patients with pulmonary emphysema. Chest 1999115697–702. [DOI] [PubMed] [Google Scholar]
- 8.Gan W Q, Man S F, Senthilselvan A.et al Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta‐analysis. Thorax 200459574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riise G C, Larsson S, Lofdahl C G.et al Circulating cell adhesion molecules in bronchial lavage and serum in COPD patients with chronic bronchitis. Eur Respir J 199471673–1677. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland E R, Allmers H, Ayas N T.et al Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 200358937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes N C, Qiu Y S, Pavord I D.et al Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med 2006173736–743. [DOI] [PubMed] [Google Scholar]
- 12.Grootendorst D C, Rabe K F. Selective phosphodiesterase inhibitors for the treatment of asthma and chronic obstructive pulmonary disease. Curr Opin Allergy Clin Immunol 2002261–67. [DOI] [PubMed] [Google Scholar]
- 13.Lipworth B J. Phosphodiesterase‐4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet 2005365167–175. [DOI] [PubMed] [Google Scholar]
- 14.Essayan D M. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol 2001108671–680. [DOI] [PubMed] [Google Scholar]
- 15.Hatzelmann A, Schudt C. Anti‐inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther 2001297267–279. [PubMed] [Google Scholar]
- 16.Bundschuh D S, Eltze M, Barsig J.et al In vivo efficacy in airway disease models of roflumilast, a novel orally active PDE4 inhibitor. J Pharmacol Exp Ther 2001297280–290. [PubMed] [Google Scholar]
- 17.Wollin L, Bundschuh D S, Wohlsen A.et al Inhibition of airway hyperresponsiveness and pulmonary inflammation by roflumilast and other PDE4 inhibitors. Pulm Pharmacol Ther 200619343–352. [DOI] [PubMed] [Google Scholar]
- 18.Rabe K F, Bateman E D, O'Donnell D.et al Roflumilast—an oral anti‐inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005366563–571. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 19951521107–1136. [DOI] [PubMed] [Google Scholar]
- 20.Quanjer P, Tammeling G J, Cotes J E.et al Lung volumes and forced ventilatory flows. Eur Respir J 19936(Suppl 16)5–40. [DOI] [PubMed] [Google Scholar]
- 21.in't Veen J, De Gouw H, Smits H H.et al Repeatability of cellular and soluble markers in induced sputum from patients with asthma. Eur Respir J 199692441–2447. [DOI] [PubMed] [Google Scholar]
- 22.Grootendorst D C, van den Bos J W, Romeijn J J.et al Induced sputum in adolescents with severe stable asthma. Safety and the relationship of cell counts and eosinophil cationic protein to clinical severity. Eur Respir J 199913647–653. [DOI] [PubMed] [Google Scholar]
- 23.Schoonbrood D, Lutter R, Habets F.et al Analysis of plasma‐protein leakage and local secretion in sputum from patients with asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 19941501519–1527. [DOI] [PubMed] [Google Scholar]
- 24.Smits H H, Grunberg K, Derijk R H.et al Cytokine release and its modulation by dexamethasone in whole blood following exercise. Clin Exp Immunol 1998111463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamble E, Grootendorst D C, Brightling C E.et al Anti‐inflammatory effects of the phosphodiesterase 4 inhibitor cilomilast (Ariflo) in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003168976–982. [DOI] [PubMed] [Google Scholar]
- 26.Grootendorst D C, Gauw S A, van der Veen H.et al The need of analysing induced sputum absolute cell counts in addition to cell percentages in COPD [abstract]. Eur Respir J 200424(Suppl 48)306s [Google Scholar]
- 27.Grootendorst D C, Gauw S A, Benschop N.et al Efficacy of the novel phosphodiesterase‐4 inhibitor BAY 19‐8004 on lung function and airway inflammation in asthma and chronic obstructive pulmonary disease (COPD). Pulm Pharmacol Ther 200316341–347. [DOI] [PubMed] [Google Scholar]
- 28.Celli B. COPD, inflammation and its modulation by phosphodiesterase 4 inhibitors: time to look beyond the FEV1. Chest 20061295–6. [DOI] [PubMed] [Google Scholar]
- 29.Romano S J. Selectin antagonists: therapeutic potential in asthma and COPD. Treat Respir Med 2005485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore T M, Chetham P M, Kelly J J.et al Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am J Physiol 1998275L203–L222. [DOI] [PubMed] [Google Scholar]
- 31.Suttorp N, Ehreiser P, Hippenstiel S.et al Hyperpermeability of pulmonary endothelial monolayer: protective role of phosphodiesterase isoenzymes 3 and 4. Lung 1996174181–194. [DOI] [PubMed] [Google Scholar]
- 32.van Rensen E L J, Hiemstra P S, Rabe K F.et al Assessment of microvascular leakage via sputum induction. The role of substance P and neurokinin A in patients with asthma. Am J Respir Crit Care Med 20021651275–1279. [DOI] [PubMed] [Google Scholar]
- 33.di Stefano A, Capelli A, Lusuardi M.et al Decreased T lymphocyte infiltration in bronchial biopsies of subjects with severe chronic obstructive pulmonary disease. Clin Exp Allergy 200131893–902. [DOI] [PubMed] [Google Scholar]
- 34.di Stefano A, Capelli A, Lusuardi M.et al Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med 19981581277–1285. [DOI] [PubMed] [Google Scholar]
- 35.Grootendorst D C, Gauw S A, Baan R.et al Does a single dose of the phosphodiesterase 4 inhibitor, cilomilast (15 mg), induce bronchodilation in patients with chronic obstructive pulmonary disease? Pulm Pharmacol Ther 200316115–120. [DOI] [PubMed] [Google Scholar]
- 36.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect 200351317–1327. [DOI] [PubMed] [Google Scholar]
- 37.Siafakas N M, Vermeire P, Pride N B.et al Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J 199581398–1420. [DOI] [PubMed] [Google Scholar]
- 38.Gizycki M J, Hattotuwa K L, Barnes N.et al Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissue. Thorax 200257799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hattotuwa K L, Gizycki M J, Ansari T W.et al The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease: a double‐blind, placebo‐controlled biopsy study. Am J Respir Crit Care Med 20021651592–1596. [DOI] [PubMed] [Google Scholar]