Abstract
Purpose
We recently reported that Vcsa1 is one of the most down-regulated genes in the corpora of rats in 3 distinct models of erectile dysfunction. Since gene transfer of plasmids expressing Vcsa1 or intracorporeal injection of its mature peptide product sialorphin into the corpora of aging rats was shown to restore erectile function, we proposed that the Vcsa1 gene has a direct role in erectile function. To determine if similar changes in gene expression occur in the corpora of human subjects with erectile dysfunction we identified a human homologue of Vcsa1 (hSMR3A) and determined the level of expression of hSMR3A in patients.
Materials and Methods
hSMR3A was identified as a homologue of Vcsa1 by searching protein databases for proteins with similarity. hSMR3A cDNA was generated and subcloned into the plasmid pVAX to generate pVAX-hSMR3A. pVAX-hSMR3A (25 or 100 μg) was intracorporeally injected into aging rats. The effect on erectile physiology was compared histologically and by measuring intracorporeal pressure/blood pressure with controls treated with the empty plasmid pVAX. Total RNA was extracted from human corporeal tissue obtained from patients undergoing previously scheduled penile surgery. Patients were grouped according to normal erectile function (3), erectile dysfunction and diabetes (5) and patients without diabetes but with erectile dysfunction (5). Quantitative reverse-transcriptase polymerase chain reaction was used to determine the hSMR3A expression level.
Results
Intracorporeal injection of 25 μg pVAX-hSMR3A was able to significantly increase the intracorporeal pressure-to-blood pressure ratio in aging rats compared to age matched controls. Higher amounts (100 μg) of gene transfer of the plasmid caused less of an improvement in the intracorporeal pressure-to-blood pressure ratio compared to controls, although there was histological and visual evidence that the animals were post-priapitic. These physiological effects were similar to previously reported effects of intracorporeal injection of pVAX-Vcsa1 into the corpora of aging rats, establishing hSMR3A as a functional homologue of Vcsa1. More than 10-fold down-regulation in hSMR3A transcript expression was observed in the corpora of patients with vs without erectile dysfunction. In patients with diabetes associated and nondiabetes associated erectile dysfunction hSMR3A expression was found to be down-regulated.
Conclusions
These results suggest that hSMR3A can act as a marker for erectile dysfunction associated with diabetic and nondiabetic etiologies. Given that our previous studies demonstrated that gene transfer of the Vcsa1 gene and intracorporeal injection of its protein product in rats can restore erectile function, these results suggest that therapies that increase the hSMR3A gene and product expression could potentially have a positive impact on erectile function.
Keywords: penis, diabetes, impotence, gene transfer techniques, rats, Sprague-Dawley
A National Institutes of Health consensus panel defined ED as the inability to achieve or maintain erection sufficient for satisfactory sexual performance. The development of ED is multifactorial and there are several risk factors for ED. Depending on the cause ED can be broadly classified as organic, psychogenic or mixed.1 Because of the multifactorial nature of ED, it has been difficult to identify a universal molecular marker for organic ED. Two of the most common risk factors for organic ED are diabetes and aging.2 Diabetic men are 3 times as likely to have ED as nondiabetic men and men 50 to 90 years old are at 10 times greater risk for ED than those younger than 50 years.3
Penile erection is a neurovascular process that relies on a concerted action of the nervous system, the vascular system and cavernous smooth muscle tissues. ED is attributable to inability of the cavernous smooth muscle tissue to undergo relaxation. It might be expected that different types of ED that have overlapping pathophysiological mechanisms may also have common biochemical pathways contributing to ED. However, microarray studies of different models of ED, such as diabetes4 and post-radical prostatectomy models,5 only serve to highlight that ED involves changes in a diverse set of molecular pathways that do not overlap. However, we and others recently reported that the Vcsa1 transcript (variable coding sequence a1 gene, also known as a submandibular rat 1 gene) is one of the most down-regulated genes in the corpora of rats in 3 distinct models of ED, including diabetic, age related and neurogenic (bilaterally ligated cavernous nerve) ED models.5,6 These reports led to the suggestion that Vcsa1 is a potential marker for ED.
Vcsa1 encodes a precursor protein that gives rise to 3 peptide products, including an undecapeptide, a hexapeptide and a pentapeptide.7 The final mature peptide is the pentapeptide, named sialorphin. There are several lines of evidence that sialorphin has a role in male rat sexuality since there is 100 to 500 times greater circulating sialorphin peptide levels in adult male rats than in females and dorsal tail injection of sialorphin modulates male rat sexual behavior.8,9 In addition, when Vcsa1 was intracorporeally injected into aging rats, there was improved erectile function at lower doses and priapism occurred at higher doses, leading to the suggestion that the Vcsa1 gene product has a direct role in erectile function. Indeed, it was subsequently shown that the mature peptide product of Vcsa1, sialorphin, can also restore erectile function in the aging rat, mediated through smooth muscle tissue relaxation.10
Homologues with close identity to the Vcsa1 gene were reported in mice (mSG1, mSG2 and mSMR2), cows (bovine P-B) and humans (hSMR3A).11,12 The human homologue hSMR3A has 34% identity with Vcsa1 over the entire amino acid sequence and 55% identity in the first 38 amino acids of the protein, which encodes the functional mature peptide sialorphin. The similarity of the sequences suggests that the 2 proteins may perform similar physiological roles. Therefore, we determined if hSMR3A is a functional homologue of Vcsa1, and if patients with ED have decreased hSMR3A expression. hSMR3A might then serve as a marker for organic ED in patients and potentially as a target for its treatment.
MATERIALS AND METHODS
Sequence Analysis and Comparison
The Basic Local Alignment Search Tool, available from the National Center of Biotechnology, National Institutes of Health, was used to search for gene and protein sequences with similarity to Vcsa1. Sequences were aligned using MultiAlin,13 available on-line from Institut National de la Recherche Agronomique.
Cloning of hSMR3A and Construction of pVAX-hSMR3A
We PCR amplified the full length gene from human corporeal cell cDNA using the primers SMR3AF (5′-ggatgaaat-cactgacttggatc-3′) and SMR3AR (5′-gtatttagggtgcaggag-taggg-3′), and cloned hSMR3A into the pPCR-4-TOPO vector. After sequencing the insert to confirm the correct sequence we subcloned hSMR3A into the pVAX vector (Invitrogen™) to create pVAX-hSMR3A.
Measurement of ICP/BP
A total of 17 Sprague-Dawley retired breeder rats at ages 9 to 10 months weighing greater than 500 gm were used to determine the effect of intracorporeal injection of pVAX-hSMR3A or the empty vector pVAX on erectile physiology, essentially as previously described.6 All study protocols were approved by the Animal Use Committee at the Albert Einstein College of Medicine.
For gene transfer experiments vectors/plasmids were microinjected into the rat corporeal tissue, essentially as previously described.6 Briefly, the rats were anesthetized with pentobarbital sodium (35 mg/kg intraperitoneally). An incision was made through the perineum, the corpus spongiosum was identified and a window was made in the corpus spongiosum to identify the corpus cavernosum. Using an insulin syringe all microinjections consisted of a bolus injection of naked plasmid DNA into the corporeal tissue. The final volume of all microinjections was 150 μl.
For cavernosometry determining the ICP response to CN stimulation the rats were anesthetized with pentobarbital sodium (35 mg/kg intraperitoneally). An incision was made in the perineum and a window was made in the ischiocavernosus muscle to expose the corpus cavernosum. The CNs were identified adjacent to the prostate gland. The CN was directly electrostimulated with a delicate stainless steel bipolar hook electrode attached to a multijointed clamp. Each probe was 0.2 mm in diameter and the 2 poles were separated by 1 mm. Monophasic rectangular pulses were delivered by a signal generator that was custom made with a built-in constant current amplifier. Stimulation parameters were frequency 20 Hz, pulse width 0.22 milliseconds, duration 1 minute, and current 0.75 and 4 mA. Changes in ICP and systemic BP were recorded at each intensity of stimulation. Mean ± SD ICP/BP and ANOVA were calculated for each treatment group. Significant differences between treatment groups were determined by Student’s t test.
Patient Samples
Human corporeal tissue was procured from several patients during penile prosthetic implant surgery according to protocols approved by the AECOM/Montefiore Hospital Internal Review Board. Table 1 lists the conditions and ages of patients 1 to 10. Tissue samples were immediately flash frozen after removal in liquid nitrogen and stored at –70C until RNA and cDNA preparation.
Table 1.
Patient information
| Failure
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Pt—Age | Phosphodiesterase-5 | Intracavernous Injection |
MUSE® | Diabetes Mellitis |
Other Disease | Smoker | Previous Pelvic Surgery | Prosthesis |
| 0A—26 | No | No | No | |||||
| 0B—35 | No | No | No | |||||
| 0C—51 | No | No | No | |||||
| 1—62 | Yes | No | No | Yes | Hypertension | No | Radical retropubic prostatectomy 3 colon | Semirigid |
| 2—66 | No | No | No | Yes | Hypertension, congestive heart failure, hypercholesterol | No | No | Semirigid |
| 3—64 | Yes | No | No | Yes | Benign prostatic hyperplasia, gastroesophageal reflux disease | No | Prostate Ca brachytherapy | Inflatable |
| 4—65 | No | No | No | Yes | Hypertension, peripheral vascular disease | No | No | Semirigid |
| 5—68 | Yes | Yes | Yes | Yes | No | No | No | Semirigid |
| 6—72 | No | No | No | No | Peripheral vascular disease, osteoporosis | Yes | No | Inflatable |
| 7—45 | No | No | No | No | Hypercholesterol | Yes | No | Inflatable |
| 8—79 | No | No | No | No | Hypothyroidism | Yes | No | Inflatable |
| 9—72 | No | No | No | No | Gout, no family history | No | Penile revascularization | Inflatable |
| 10—59 | Yes | No | No | No | Prostate Ca | No | Laparoscopic radical prostatectomy | Inflatable |
Automobile accident, sex change surgery and penile cancer surgery in patients 0A, 0B and 0C, respectively.
Isolation of Patient RNA and Quantitative RT-PCR
Total RNA was extracted from frozen tissue with TRIzol® according to manufacturer instructions. Briefly, approximately 50 mg tissue were added to 1 ml TRIzol reagent and homogenized using a Polytron™ homogenizer for 30 seconds. Homogenized tissues were incubated for 5 minutes at room temperature, followed by the addition of 200 μl chloroform. After mixing, the aqueous phases were separated by centrifugation at 12,000 × gravity for 15 minutes at 4C and they were then transferred to a clean tube. RNA was precipitated from the aqueous phase by the addition of isopropyl alcohol and pelleted by centrifugation at 12,000 × gravity for 15 minutes at 4C, washed once with 75% ethanol and again pelleted at 12,000 × gravity for 15 minutes. Ethanol was aspirated and the RNA pellet was dried and then dissolved in sterile water.
Total RNA (1 μg) was reverse transcribed to first strand cDNA primed with oligo(deoxythymidine) using the Superscript™ First-Strand Synthesis System for real-time PCR. RNA was denatured for 5 minutes at 65C and immediately cooled on ice. RNA was then combined with Superscript II RT, 40 U RNaseOUT™ recombinant ribonuclease inhibitor and RT reaction buffer. cDNA synthesis was then performed for 50 minutes at 42C. RT products were amplified using SYBR® Green 2X PCR Master Mix. Real-time quantitative PCR analysis was performed using a 7300 real-time PCR system (Applied Biosystems™). The primers for hSMR3A were forward 5′-CTATGGTCCAGGGAGATTTCC-3′ and reverse 5′-GAGGAGGAAGAGAGTGTGATTG-3′. GAPDH (forward primer 5′-GCCGCCTGCTTCACCACCTTCT-3′ and reverse primer 5′-GCATGGCCTTCCGTGTTCCTACC-3′) served as an endogenous control. PCR reactions for all samples were performed in 96-well plates with 1 μl cDNA, 100 nM of each primer and 12.5 μl SYBR® Green in a 25 μl reaction volume. Cycling conditions were SYBR Green DNA polymerase activation at 95C for 10 minutes, 40 cycles of denaturation at 95C for 15 seconds and annealing/extension at 60C for 1 minute. Real-time PCR results are presented as threshold cycles normalized to that of the GAPDH gene according to a previously described method.6 The relative quantified value for each target gene is expressed as 2− (Ct−Cc), where Ct and Cc represent mean threshold cycle differences after normalizing to GAPDH. Transcript expression was analyzed using the comparative Ct method, also known as the 2−δ δCt method. This method was applicable because the efficiency of the SMR3A primers for generating products was found to be close to that of the housekeeping gene GAPDH, which was used to normalize samples.
RESULTS
DNA and Sequence Analysis
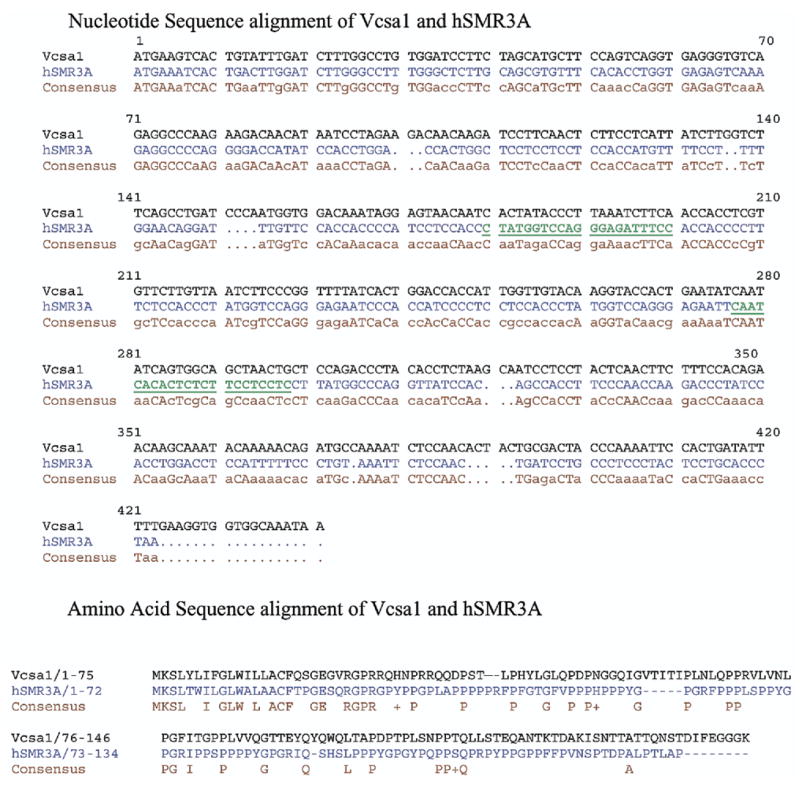
We searched GenBank™ for human proteins with the greatest similarity to Vcsa1. The closest human gene with homology to Vcsa1 was identified as hSMR3A, which has 34% identity with Vcsa1 at the protein level (fig. 1). In the first 38 amino acids of the protein, which encodes the functional mature peptide sialorphin, there is 55% identity. This level of identity suggests that the proteins perform similar physiological roles.
Fig. 1.
Nucleotide sequence comparison of Vcsa1 and hSMR3A with consensus sequence and amino acid sequence comparison of Vcsa1 and hSMR3A with consensus sequence. Bold, green and underlined text indicates primers used to specifically amplify hSMR3A gene.
Effect of Intracorporeal pVAX-hSMR3A Injection Into Retired Breeders on Erectile Physiology
We previously reported that pVAX-Vcsa1 injection into retired breeder rats can improve erectile physiology when 25 μg are injected intracorporeally but higher amounts of plasmid results in priapism.6 To confirm that hSMR3A is a functional homologue of Vcsa1 these experiments were repeated to determine if hSMR3A has comparable physiological effects on the penis.
When 25 μg pVAX-hSMR3A were intracorporeally injected into retired breeder rats, there was significant improvement in the erectile response, as indicated by an increased ICP-to-BP ratio compared with that in control rats treated with the empty vector pVAX (table 2). The values obtained were similar to those in published experiments, in which the effect of gene transfer of pVAX-Vcsa1 into the corpora of retired breeders was investigated for an effect on erectile function.6
Table 2.
ICP/BP measurements in retired breeder rats after pVAX or pVAX-hSMR3A gene transfer and CN electrostimulation
| Mean ± SD ICP/BP
|
||||
|---|---|---|---|---|
| Intracorporeal Injection (dose) | No. Rats | Baseline | 0.75 mA | 4 mA |
| Control pVAX (100 μg) | 8 | 0.063 ± 0.02 | 0.22 ± 0.12 | 0.33 ± 0.06 |
| pVAX-hSMR3A: | ||||
| 25 μg | 3 | 0.153 ± 0.06 | 0.28 ± 0.07* | 0.61 ± 0.02* |
| 100 μg | 5 | 0.102 ± 0.05 | 0.13 ± 0.07 | 0.39 ± 0.01* |
Significantly different vs control (Student’s t test p <0.05).
Also similar to reported experiments for Vcsa1,6 higher doses of the pVAX-hSMR3A plasmid resulted in only slight improvement in ICP-to-BP ratios (table 2), although there was visible and histological evidence of a priapitic episode. The histological appearance of 4 of the 5 animals treated with pVAX-hSMR3A showed visible indications of edema, which is a possible indication of a vasocongested state, whereas in untreated control animals corporeal morphology appeared normal. Histological examination and comparison to control animals also suggested that SMR3A causes changes in penile morphology, which might have been a result of the vasocongested (priapism-like) state (fig. 2). The dorsal vein was greatly enlarged. This would occur if there was increased blood flow or post-penile obstruction, which was not observed. In addition, there was evidence of sinusoidal congestion of blood in animals treated with hSMR3A but not in control animals. Overall the occurrence of vaso-congestion (a priapism-like state) has not been observed in the history of animal experiments at our department in which vasodilating drugs or genes were injected into the corpora.
Fig. 2.

Histological comparison of distal sections of normal penis and that of rat treated with 100 μg pVAX-hSMR3A. Reduced from ×4.
The sequence and functional similarity of Vcsa1 to hSMR3A suggests that they are indeed the homologues of each other. Since we proposed that Vcsa1 is a marker for ED in rats,6 we performed analysis of human corporeal samples to determine if hSMR3A is present in patients with no reported ED and down-regulated in patients with ED.
Detection of SMR3A in Human Corpora
Although the rat Vcsa1 gene was originally isolated from the rat submandibular gland (hence, the original designation SMR1) and it is highly expressed in that tissue, it appears to be expressed in various other tissues.12 We recently reported its presence in rat corpus cavernosum tissue. Therefore, we determined if hSMR3A is similarly present in human corporeal tissue. Corporeal samples were available from patients 0A, 0B and 0C, who did not report ED (table 1). In these 3 patients hSMR3A was clearly detectable using quantitative RT-PCR. To our knowledge this is the first demonstration that the gene is expressed in human corporeal tissues.
Decreased hSMR3A Expression in Patients With ED Compared to That in Patients Without ED
We recently reported that the rat homologue of Vcsa1 was down regulated in animals with ED.6 We determined hSMR3A levels in patients undergoing prosthetic implant surgery (table 1 and fig. 3). hSMR3A expression in the patients was normalized to GAPDH and the expression level was compared to that in patient 0A without ED. In patients with ED there were significantly lower levels of expression compared to those in the 3 control patients (more than a 10-fold decrease, Student’s t test p <0.5), suggesting that, as in the rat model, hSMR3A is a marker for erectile function. We also grouped the patients with ED into those with and without diabetes. The 2 groups had significantly lower levels of hSMR3A expression compared to control patients (Student’s t test p <0.5). However, compared to the control mean age of 37 years the median age in the 2 ED groups with and without diabetes was higher (each mean 65). Therefore, in these groups of patients it was not possible to distinguish if the reported ED was a result of diabetes or age. However, overall down-regulation of hSMR3A expression is a marker for ED caused by several factors.
Fig. 3.
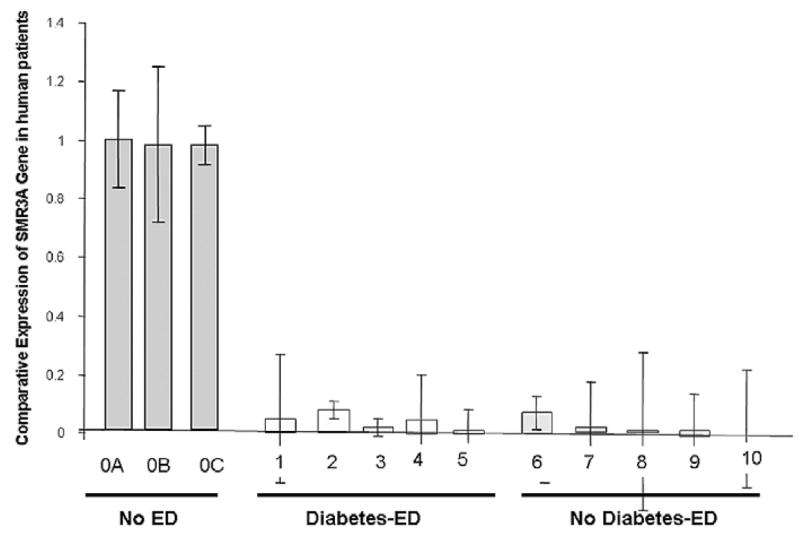
hSMR3A transcript expression was analyzed using comparative Ct method, also known as 2−δδCt method, which was applicable because SMR3A primer efficiency for generating products was close to that of housekeeping gene GAPDH used to normalize samples. Expression level is compared to that in patient 0A.
DISCUSSION
We provide evidence that hSMR3A is the human homologue of the Vcsa1 gene. This conclusion is based on sequence comparison and gene transfer of hSMR3A by intracorporeal injection into an aging rat model. Similar to Vcsa1, hSMR3A can improve erectile function when 25 μg are intracorporeally injected and it can cause priapism at higher amounts.6,10 To our knowledge we report for the first time that hSMR3A is expressed in human corpora tissue and it is down-regulated in patients with ED. Down-regulation of hSMR3A is highly significant despite the small number of control patient samples that could be obtained for this study. The addition of more control patients may have to wait for a less invasive assay, for example an immunoassay, in which the protein product of hSMR3A is measured in the bloodstream. However, the current study indicates that hSMR3A acts as a marker for human ED.
Although in the rat Vcsa1 and hSMR3A appear to have a direct role in erectile function since intracorporeal injection of plasmids expressing the gene can improve erectile function in an aging model of ED, down-regulation of the gene could be a cause or an effect of ED. In the rat Vcsa1 gene expression is regulated by androgens, which can cause 100 to 200-fold enhancement of Vcsa1 in the acinar cells of rat submandibular glands during puberty.9,14 To our knowledge it remains to be determined if the regulation of hSMR3A expression is also under hormonal regulation.
We recently noted that the mature peptide product of Vcsa1, sialorphin, was able to directly improve erectile function in the aging rat.10 Sialorphin acts as an inhibitor of rat membrane bound NEP.15 We proposed that the ability of sialorphin to prolong the activity of agonists that are normally broken down by NEPs causes heightened smooth muscle relaxation in the corpora cavernosa, leading to penile erection. Given the similarity of the amino acid sequence and the fact that SMR3A and Vcsa1 are down-regulated with ED, it is likely that hSMR3A also gives rise to peptide products that act as NEP inhibitors and, thereby, cause human corporeal smooth muscle tissue relaxation. Recently PROL1, another member of the Vcsa1 gene family found in humans, was shown to give rise to a protein product called opiorphin, which is secreted in saliva. This protein also acts as an NEP inhibitor, suggesting that the physiological effect of this family of proteins is mediated through NEP inhibition.16
The identification of hSMR3A as a marker for ED has applications as a diagnostic tool for organic ED and in the development of novel therapies. In the era of agents that are noninvasive and successful for treating ED the quest to establish an etiological diagnosis has been downplayed. However, the potential ability to suggest to the patient that the condition is reversible, ie psychogenic if the level is normal, with an accurate but invasive test (diagnostic corporeal biopsy) or with the development of a noninvasive immunoassay would be of significance to the physician and patient, particularly young men who are convinced that they have a nonreversible physical problem, as well as for reimbursement issues for therapy by insurance companies.17 In addition, it is increasingly recognized that ED is an important marker of vascular disease and there is growing evidence that ED and cardiovascular disease share common mechanisms of development through vascular endothelial dysfunction.18 Indeed, it has been recommended that patients with ED should be investigated for cardiovascular disease.19 The development of a test for organic ED in men who approach physicians for treatment represents an enormous potential for prescreening and the prevention of more serious vascular complications. In conclusion, given that our previous studies demonstrated that gene transfer of the Vcsa1 gene and its protein product in rats can restore erectile function,6,10 these results suggest that therapies that increase the expression of the hSMR3A gene or other genes in the Vcsa1 gene family, resulting in peptide products that act as NEP inhibitors, could have a positive impact on erectile function.
Acknowledgments
Supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants P01-DK060037 and K01-DK67270 (KPD).
Abbreviations and Acronyms
- BP
blood pressure
- CN
cavernous nerve
- Ct
crossing threshold
- ED
erectile dysfunction
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- hSMR3A
human homologue of SMR1
- ICP
intracorporeal pressure
- NEP
neutral endopeptidase
- PCR
polymerase chain reaction
- RT
reverse transcriptase
- SMR1
submandibular rat 1 gene
- Vcsa1
variable coding sequence a1 gene
Footnotes
Study received approval from the Albert Einstein College of Medicine Animal Use Committee and the AECOM/Montefiore Hospital Internal Review Board.
References
- 1.Lizza EF, Rosen RC. Definition and classification of erectile dysfunction: report of the Nomenclature Committee of the International Society of Impotence Research. Int J Impot Res. 1999;11:141. doi: 10.1038/sj.ijir.3900396. [DOI] [PubMed] [Google Scholar]
- 2.Korenman SG. Epidemiology of erectile dysfunction. Endocrine. 2004;23:87. doi: 10.1385/ENDO:23:2-3:087. [DOI] [PubMed] [Google Scholar]
- 3.Shabsigh R, Perelman MA, Lockhart DC, Lue TF, Broderick GA. Health issues of men: prevalence and correlates of erectile dysfunction. J Urol. 2005;174:662. doi: 10.1097/01.ju.0000165389.73148.d1. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan CJ, Teal TH, Luttrell IP, Tran KB, Peters MA, Wessells H. Microarray analysis reveals novel gene expression changes associated with erectile dysfunction in diabetic rats. Physiol Genomics. 2005;23:192. doi: 10.1152/physiolgenomics.00112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.User HM, Zelner DJ, McKenna KE, McVary KT. Micro-array analysis and description of SMR1 gene in rat penis in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003;170:298. doi: 10.1097/01.ju.0000060882.75475.5a. [DOI] [PubMed] [Google Scholar]
- 6.Tong Y, Tar M, Davelman F, Christ G, Melman A, Davies KP. Variable coding sequence protein A1 as a marker for erectile dysfunction. BJU Int. 2006;98:396. doi: 10.1111/j.1464-410X.2006.06247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rougeot C, Rosinski-Chupin I, Njamkepo E, Rougeon F. Selective processing of submandibular rat 1 protein at dibasic cleavage sites. Salivary and bloodstream secretion products. Eur J Biochem. 1994;219:765. doi: 10.1111/j.1432-1033.1994.tb18556.x. [DOI] [PubMed] [Google Scholar]
- 8.Messaoudi M, Desor D, Nejdi A, Rougeot C. The endogenous androgen-regulated sialorphin modulates male rat sexual behavior. Horm Behav. 2004;46:684. doi: 10.1016/j.yhbeh.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Rosinski-Chupin I, Huaulme JF, Rougeot C, Rougeon F. The transcriptional response to androgens of the rat VCSA1 gene is amplified by both binary and graded mechanisms. Endocrinology. 2001;142:4550. doi: 10.1210/endo.142.10.8428. [DOI] [PubMed] [Google Scholar]
- 10.Davies KP, Tar M, Rougeot C, Melman A. Sialorphin (the mature peptide product of Vcsa1) relaxes corporal smooth muscle tissue and increases erectile function in the ageing rat. BJU Int. 2007;99:431. doi: 10.1111/j.1464-410X.2006.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isemura S, Saitoh E. Molecular cloning and sequence analysis of cDNA coding for the precursor of the human salivary proline-rich peptide P-B. J Biochem (Tokyo) 1994;115:1101. doi: 10.1093/oxfordjournals.jbchem.a124464. [DOI] [PubMed] [Google Scholar]
- 12.Isemura S, Watanabe S, Fujiwara S, Sanada K. Tissue distribution and nucleotide sequence of bovine mRNA for salivary proline-rich protein P-B. Arch Oral Biol. 2004;49:881. doi: 10.1016/j.archoralbio.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosinski-Chupin I, Tronik D, Rougeon F. High level of accumulation of a mRNA coding for a precursor-like protein in the submaxillary gland of male rats. Proc Natl Acad Sci U S A. 1988;85:8553. doi: 10.1073/pnas.85.22.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rougeot C, Messaoudi M, Hermitte V, Rigault AG, Blisnick T, Dugave C, et al. Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci U S A. 2003;100:8549. doi: 10.1073/pnas.1431850100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wisner A, Dufour E, Messaoudi M, Nejdi A, Marcel A, Ungeheuer MN, et al. Human opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc Natl Acad Sci U S A. 2006;103:17979. doi: 10.1073/pnas.0605865103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melman A, Fogarty J, Hafron J. Can self-administered questionnaires supplant objective testing of erectile function? A comparison between the International Index of Erectile Function and objective studies. Int J Impot Res. 2006;18:126. doi: 10.1038/sj.ijir.3901361. [DOI] [PubMed] [Google Scholar]
- 18.Muller A, Mulhall JP. Cardiovascular disease, metabolic syndrome and erectile dysfunction. Curr Opin Urol. 2006;16:435. doi: 10.1097/01.mou.0000250284.83108.a6. [DOI] [PubMed] [Google Scholar]
- 19.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294:2996. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]