Abstract
Sphingolipid signaling is thought to regulate apoptosis via mechanisms that are dependent on the concentration of ceramide relative to that of sphingosine-1-phosphate (S1P). This study reports defects in reproductive structures and function that are associated with enhanced apoptosis in Drosophila Sply05091 mutants that lack functional S1P lyase and thereby accumulate sphingolipid long chain base metabolites. Analyses of reproductive structures in these adult mutants unmasked multiple abnormalities, including supernumerary spermathecae, degenerative ovaries, and severely reduced testes. TUNEL assessment revealed increased cell death in mutant egg chambers at most oogenic stages and in affected mutant testes. These reproductive abnormalities and elevated gonadal apoptosis were also observed, to varying degrees, in other mutants affecting sphingolipid metabolism. Importantly, the reproductive defects seen in the Sply05091 mutants were ameliorated both by a second site mutation in the lace gene that restores long chain base levels towards normal and by genetic disruption of the proapoptotic genes reaper, hid and grim. These data thus provide the first evidence in Drosophila that accumulated sphingolipids trigger elevated levels of apoptosis via the modulation of known signaling pathways.
Keywords: Sphingolipid, Ceramide, S1P, Apoptosis, Cell death, Drosophila, Oogenesis, Spermatogenesis, Reproduction, Germ cells
INTRODUCTION
Sphingolipids comprise a complex and ubiquitous class of membrane lipids involved in numerous cellular processes, including cell proliferation, differentiation, migration and apoptosis (Dbaibo and Hannun, 1998; Pyne and Pyne, 2000; Yang et al., 2004). Certain sphingolipid metabolites such as sphingosine-1-phosphate (S1P) are recognized to promote cell survival whereas others such as ceramide and sphingosine have been implicated in cell death (Cuvillier, 2002; Pettus et al., 2002; Spiegel and Milstein, 2003). Cell fate has, thus, been proposed to be dependent, in part, on the dynamic balance between sphingolipid modulators that are cytoprotective and those that elicit a death response. A growing body of work has also drawn attention to the involvement of sphingolipid signaling in developmental processes at the organismal level (Acharya and Acharya, 2005; Oskouian and Saba, 2004) and to diseases associated with disruption of the metabolic pathway (Herr et al., 2003; Riley et al., 2001). For instance, the human hereditary sensory and autonomic neuropathy (HSAN1) has been linked to mutations in the SPTLC1 gene required for de novo sphingolipid synthesis (Dawkins et al., 2001; McCampbell et al., 2005), Niemann-Pick types A and B are characterized by defects in acid sphingomyelinase (Kolodny, 2000), and, most recently, pulmonary emphysema has been shown to involve ceramide upregulation (Petrache et al., 2005)- all of which underscore the involvement of sphingolipid metabolic defects in human disease.
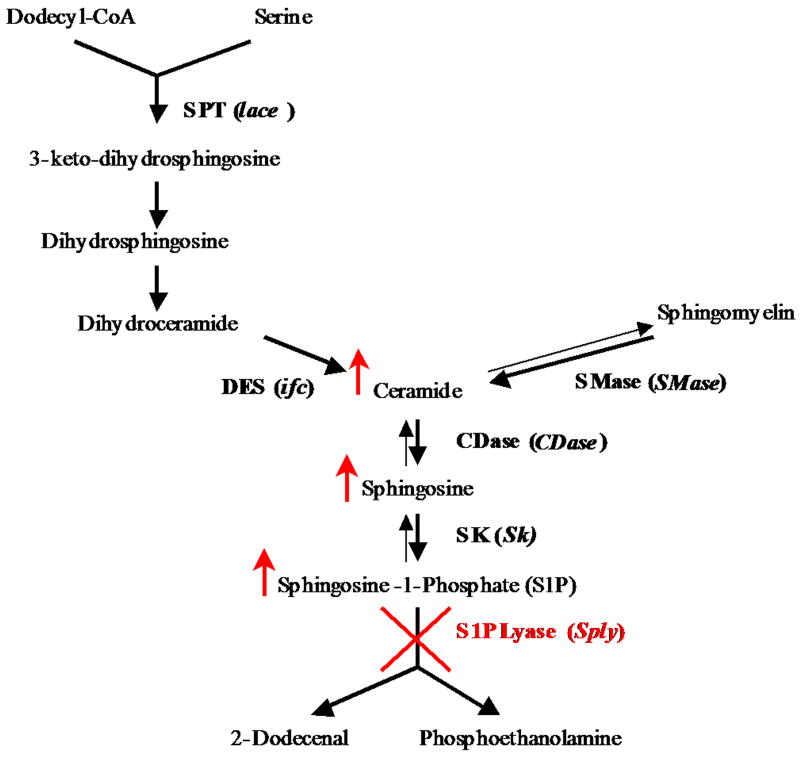
The enzyme responsible for the catalysis of the final degradative step in the sphingolipid metabolic pathway, SIP lyase, is a key regulator of the pathway (Figure 1) since inhibiting its function leads to accumulation of long chain sphingolipid bases and higher order ceramides (Herr et al., 2003). Early studies performed in S. cerevisiae showed that S1P lyase null mutants exhibited thermotolerance and altered response to nutrient deprivation as well as stationary phase viability (Gottlieb et al., 1999). In Dictyostelium, disruption of the enzyme resulted in slime mold mutants with abnormal fruiting bodies and defective slug migration (Li et al., 2001). Inhibition of SIP lyase using RNAi in the nematode led to reduced feeding and defective food pumping, delayed growth, sluggish movement and underdeveloped distal gonads (Mendel et al., 2003). Most recently, it has been shown in the mouse that S1P lyase has a critical role in vivo in regulating S1P concentration. Inhibition of S1P lyase led to marked accumulation of S1P and disruption of lymphocyte egress from the thymus (Schwab et al., 2005). In Drosophila, we have shown that SIP lyase (Sply05091) null mutants exhibit elevated apoptosis during embryogenesis, impaired muscle development and integrity, reduced viability and diminished fecundity (Herr et al., 2003).
Figure 1. Sphingolipid metabolic pathway in Drosophila.
An overview of the enzymes responsible for the generation and degradation of sphingolipid signaling molecules. Disruption of the sphingosine-1-phosphate lyase gene causes accumulation of key sphingolipid metabolites (red arrows).
Previous work has identified sphingolipid signaling to be involved in regulating the survival of germ cells, and hence the development of the gonads. For example, mice that lacked functional acid sphingomyelinase, a ceramide-generating enzyme, exhibited oocyte hyperplasia due to decreased apoptosis in the oocytes (Morita et al., 2000). This proliferative phenotype was recapitulated in wild-type ovaries upon administration of S1P, a pro-mitogenic sphingolipid metabolite that also protects oocytes from radiation-and chemotherapy-induced cell death in vivo (Morita et al., 2000; Perez et al., 1997). Additionally, S1P was shown to abrogate doxorubicin-induced death of cultured murine oocytes. The ability of S1P to preserve ovarian function and fertility, however, is dependent on its concentration relative to that of ceramide since high levels of S1P reportedly induces apoptosis in ovarian cancer cells (Hong et al., 1999), presumably due to its conversion into ceramide. The effects of S1P and ceramide on male germ cell apoptosis were recently reported in the human testis (Suomalainen et al., 2003). Increased testicular ceramide was found to immediately precede caspase-3 activation and DNA fragmentation, indicative of apoptosis. Exogenous S1P partially inhibited this in vitro-induced testicular cell death.
Many aspects of Drosophila gonadal development are similar to that of mammals. Oogenesis in the fly is asynchronous and begins with the development of the germ cells in the germarium, a region in the apical tip of each of the 15–20 ovarioles that make up an ovary (Spradling, 1993). Each ovariole contains sequentially developed egg chambers (also called egg follicles) comprising 15 nurse cells and one oocyte surrounded by an envelope of somatic epithelial follicle cells. During oogenesis defective egg follicles or superfluous cells are eliminated via apoptosis so as to ensure the maturation of the healthy oocytes. Apoptosis is a regulated process in Drosophila ovarian development that typically occurs at three specific stages: 1) early oogenesis in region 2a/2b of the germarium, 2) mid-oogenesis (stages 7–8) immediately prior to vitellogenesis (yolk production) and 3) late oogenesis after which the nurse cells have transferred their cytoplasmic contents to the surviving oocyte (Cavaliere et al., 1998; Giorgi and Deri, 1976; III et al., 2002). Expressions and activities of two of the four known Drosophila effector caspases -Dcp-1 and drice- have been found to be significantly elevated during nurse cell degeneration (McCall and Stellar, 1998; Peterson et al., 2003).
Cell death also plays an important role in spermatogenesis by removing defective or abnormal spermatozoa that are unable to complete meiosis (Ashley, 2000). Studies in mice revealed spermatogenic defects linked to loss-or gain- of function mutations in genes that encode members of the Bcl-2 family, including the pro-apoptotic Bax (Knudson et al., 1995) and the pro-survival Bcl-w (Ross et al., 1998).
In the fly, spermatogenesis occurs at the apical tip of the testes when the 5–9 germline stem cells and the somatic stems cells asymmetrically divide to generate spermatogenic cysts that mature into large cells, each containing bundles of 64 spermatids with needle-shaped nuclei and long flagellar tails (Fuller, 1993). Differentiation of individual sperm occurs when the bulk of the cytoplasm is gathered caudally down the spermatid tail into a ‘cystic bulge’ that is eventually shed into a waste bag so as to allow the emergence of free motile sperm (Noguchi and Miller, 2003). Recently, it was reported that multiple apoptotic regulators are needed for this final step because loss-of-function mutations in several caspases resulted in improper formation of free-swimming spermatozoa (Arama et al., 2003; Huh et al., 2004).
Since there is a remarkable conservation of the apoptotic machinery among species, Drosophila is an ideal model organism for examining the role of sphingolipids in gonadal cell death. Given a requirement for Sply05091 in normal reproductive function (Herr et al., 2003) and a dramatic accumulation of both free and phosphorylated sphingoid bases in Sply05091 null mutants, we wished to asses the extent of apoptosis that is modulated by sphingolipids and the relationship between sphingolipid metabolism and Drosophila gametogenesis. In the present report, we provide evidence of severe morphological defects and enhanced cell death in structures affecting reproduction in Sply05091 null homozygotes. These phenotypes, moreover, are recapitulated to varying degrees in several other mutants affecting sphingolipid metabolism. Notably, through genetic interactions, we demonstrate that these distinct phenotypes in Sply05091 mutants can be suppressed by a second-site mutation in lace or by H99, a deficiency that disrupts the proapoptotic genes reaper, grim and hid.
MATERIALS AND METHODS
Drosophila Stocks and Fly Husbandry
Wild-type Canton-S (BL-1), Sply05901 (BL-11393), ifc4 (BL-1549), lacek05305 (BL-12176), lace2 (BL-3156), hid (also referred to as Wrinkled1) (BL-2398) and Df(3L)H99 (BL-1576) fly lines were obtained from the Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN). The Sk2KG050894 P-element insertion line was kindly received from the Bellen/Rubin/Spradling laboratories and its molecular characterization has been reported previously (Herr et al., 2004). Flies were cultured at 22°C on standard sucrose media supplemented with yeast.
Analysis of Genital Discs
Wandering third instar larvae were filleted in 1X PBS and briefly stained with acridine orange to enhance visualization of the genital discs. Images were obtained using a CV-View digital camera and dissecting microscope. All other imaginal discs, including wing, leg and haltere were viewed with a Leica DMIRBE fluorescence microscope and photographed with a MagnaFire digital camera. Dimensions of the imaginal discs were measured, analyzed and compared using the ImageJ software program downloaded from NIH.
Analysis of Ovaries and Testes
Gonads from adult flies of 4–6 days old were dissected in 1X PBS on ice. Pictures of freshly removed ovaries and testes were taken using a CV-View digital camera mounted on a dissecting microscope.
Histology
Gonadal tissue from 4–6 days-old adult flies were dissected on 1X PBS on ice and subsequently fixed in 4% paraformaldehyde for 20 minutes at room temperature. Intact ovaries, egg chambers and testes were then permeabilized in 1X PBT (1X PBS containing 0.1% Triton-X) at room temperature for 10 minutes each wash on a rotator. Tissues were then either incubated with TUNEL reagent for 4 hours at 37°C for detection of apoptosis (In situ cell death detection kit, Roche) or co-stained with 1:20 dilution DAPI and 1:40 rhodamine-phalloidin for 30 minutes at room temperature for assessment of nucleus and filamentous actin, respectively. After washing three times in 1X PBS, tissues were mounted in anti-fade Vectashield (Vector Labs, Burlingame, CA). Incorporation of fluorescence was viewed on Leica DMIRBE fluorescence microscope and images of gonads were captured with a MagnaFire digital camera.
Single Pair Mating
A pair of virgin female and young male of the indicated fly strain was placed in a 3-inch high plastic chamber atop a plate of grape agar mixture containing a smear of yeast paste. Each pair was allowed to acclimate over a 24-hour period, with the first count of laid eggs conducted the next day. Each old grape plate was exchanged for a new grape plate everyday over 5 days for easier counting of the eggs deposited. For each of the fly strain, 20 chambers consisting a pair were set up and the average number of eggs laid was calculated.
RESULTS
Sply05091 mutant larvae have reduced genital disc size
In an earlier assessment of apoptosis in null Sply05091 mutants, a cluster of apoptotic nuclei was localized distinctly to the A8 to A10—abdominal segments from which the genital disc would derive (Herr et al., 2003). In order to determine whether genital disc morphology was affected in the mutants, an analysis was undertaken to assess for changes in disc size. Sply05091 genital discs were reduced in size by 39% when compared to those of the wild-type controls (compare Fig. 2A with 2B). Precise excision (Sply14a) of the P-element underlying the Sply05091 mutation completely restored the reduced-size phenotype (Fig. 2C). This significant reduction in size appears to be specific to the genital disc since none of the wing, leg or haltere discs were reduced in the mutants (Fig. 2D). While the genital disc develops into internal structures that connect to the adult ovaries and testes, it is the gonadal tissue that actually differentiates into the mature gonads. Similar to a reduction seen in the mutant genital disc size, a marked area decrease (74.6%) was observed in gonads dissected from Sply05091 larvae (Fig. 2E).
Figure 2. Sply05091 mutant genital disc and larval gonads are reduced.
(A–C) Filleted third-instar larvae were stained with acridine orange to better visualize the genital discs (arrowhead). (A) Canton-S. (B) Sply05091. (C) Sply14a revertant. (D–E) The areas of the imaginal discs and gonads were determined using Image J. For each tissue group, area was normalized to wild-type Canton-S. A size change is only observed for the genital discs and gonads of homozygous Sply05091, which are reduced 39% and 75%, respectively compared to Canton-S. Bar, 100 μm. * indicates p<0.001
Sply05091 mutant females express gross internal reproductive morphological defects
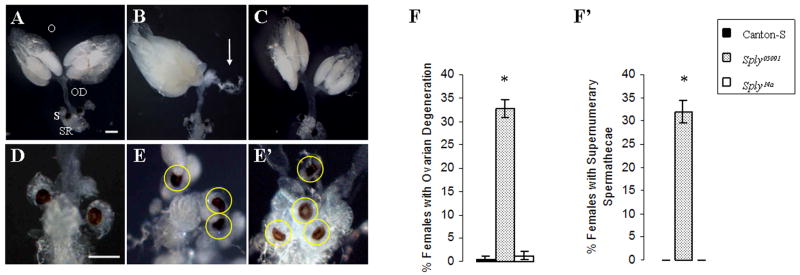
Since the larval genital disc and gonad are responsible for development of the external and internal genitalia in the adult, an examination of their reproductive structures was undertaken. Analysis of adult ovaries revealed that 32.8% of the homozygous Sply05091 mutant females exhibited grossly degenerative or missing ovaries (Fig. 3B and 3E). Moreover, both ovaries were absent in one third of the affected mutant females examined (data not shown). It was also not unusual to find a single degenerative ovary paired with an ovary replete with mature eggs, a phenotype that bears resemblance to an egg retention phenotype recently described for sphingosine kinase 2 mutants (Herr et al., 2004). In contrast, wild-type and Sply14a revertant ovaries showed minimal, if any, signs of degeneration, remaining intact and connected to the common oviduct (Fig. 3A and 3C, respectively). At the distal end of the Drosophila common oviduct are genital disc-derived structures that are normally required for storage of spermatozoae: a single seminal receptacle and a pair of spermathecae. Surprisingly, a third and sometimes fourth spermathecae was found in 31.9% of the Sply05091 mutant females (Fig. 3E and 3E′, respectively). The significance of this rare supernumerary phenotype is not clearly understood, with the only other account of the phenomenon previously reported for the homeotic protein engrailed (enspt). This mutation is temperature-sensitive since at high temperature females have fused spermathecal ducts while at low temperature they exhibit 3 spermatheca, each originating from separate branches of one duct (Chase and Baker, 1995).
Figure 3. Adult ovaries are degenerative in Sply05091 mutant.
(A–C) Ovaries (O) from 4-day old female flies were dissected and photographed (along with the common oviducts (OD), seminal receptacles (SR) and spermathecae (S)). (A) Canton-S. (B) Sply05091. (C) Sply14a revertant. Sply05091 mutant females exhibit (B) degenerative ovaries (arrow) and (E and E′) supernumerary spermathecae (circles). (F) Degenerative ovaries and (F′) supernumerary spermathecae are observed for 32.8% (N=186) and 31.5% (N=177), respectively, of the Sply05091 mutant females and rarely, if ever, for Canton-S and Sply14a revertants. Bar, 200 μm. * indicates p<0.001.
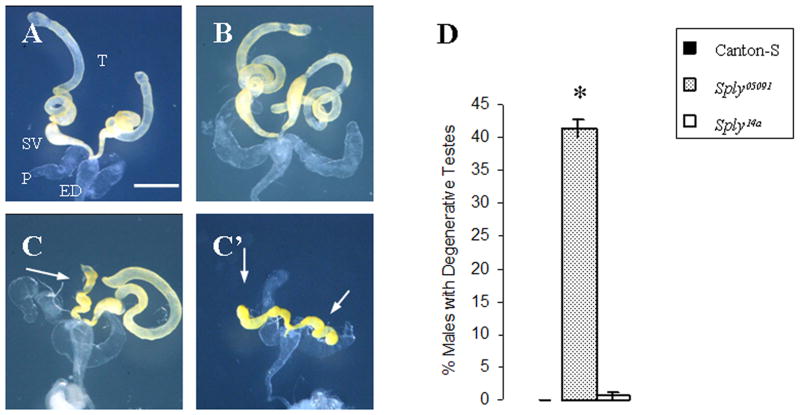
Spy05091 mutant males exhibit severely reduced testes
Evidence of reproductive structural defects was not limited to the Sply05091 mutant females as 41.4% of their male counterparts also expressed drastically reduced, shriveled testes with patches of cellular debris. Compared to the typical set of long, spiraling testes found in wild-type control and Sply14a revertant males (Fig. 4A and 4B, respectively), this degenerative phenotype affected either singular or paired mutant testes (Fig. 4C and 4C′, respectively). Although normal testicular morphology can be influenced by age, the frequency of reduced testes remained relatively constant among those examined from 2-, 9-, 16- and 23- day old male flies (data not shown). In males, the genital disc gives rise to both the external and internal genitalia, including the genital arch, lateral plates, claspers, penis apparatus, sperm pump, ejaculatory duct, paragonia and vas deferens. The female genital disc differentiates into the external and internal genitalia, comprising the bristled vaginal plates, dorsal and ventral vulva, uterus, seminal receptacle, parovaria, spermathecae and oviduct (Chen and Baker, 1997). Thus, the reduction in size of both the mutant genital disc and gonads in larvae is consistent with the presence of abnormal phenotypes seen in the mutant ovaries, testes, and some associated tissues in adults, such as the spermathecae, oviducts, paragonia, and vas deferens. Taken together, the reproductive structural defects shown both in the mutant females and mutant males are likely to have contributed significantly to the previously reported diminished reproductive capacity exhibited in the Sply05091 homozygotes (Herr et al., 2003). In order to formally address this issue, egg-laying behavior was analyzed in single pair mating involving all combinations of wild-type Canton-S (CS) and Sply05091 individuals (Table 1). Either Sply05091 female or male individuals mated with a Canton-S partner resulted in a significant delay in the onset of egg-laying and a reduced number of eggs.
Figure 4. Adult testes are reduced in Sply05091 male homozygotes.
(A–C′) Photomicrographs of dissected testes (T), seminal vesicles (SV), paragonia (P), and ejaculatory ducts (ED) from 4-day old male flies. (A) Canton-S and (B) Sply14a revertant with normal testes. Sply05091 mutant male with (C) one reduced testis (arrow) and (C′) two reduced testes (arrows). (D) Reduced testicular phenotype is seen in 41.4% (N=170) of the Sply05091 mutant males and rarely, if ever, in Canton-S and Sply14a revertants. Bar, 400 μm. * indicates p<0.001.
Table 1.
Reproductive proficiencies of wild-type Canton-S, Sply05091 homozygotes, and their reciprocal counterparts
| Mating Pairs | Number of Eggs Laid | ||
|---|---|---|---|
| Day 1 | Day 3 | Day 5 | |
| CS female X CS male | 14.4±3.09 | 32.3±3.97 | 41.1±5.1 |
| 77.8%* | 100%* | 100%* | |
| Sply05091 female X Sply05091 male | 1.63±1.63 | 7.44±5.49 | 19.8±7.96 |
| 5.26%* | 22.2%* | 83.3%* | |
| CS female X Sply05091 male | 8.21±2.43 | 23.9±4.42 | 26.1±5.15 |
| 52.6%* | 87.5%* | 87.5%* | |
| Sply05091 female X CS male | 5.47±2.10 | 22.2±5.33 | 29.5±6.98 |
| 47.1%* | 75.0%* | 81.8%* | |
Single mating between the indicated fly strains were performed in 20 pairs over the course of 5 days, up until which the death of many of the Sply05091 mutants prevented further examination. Day 1 of egg counting corresponds to the second day subsequent to introduction of virgin female to male flies. The asterisk represents the percentage of females depositing eggs on the specified day.
Apoptosis is elevated in the Sply05091 mutant reproductive structures
In order to assess whether apoptotic signaling was involved in the degeneration of the adult gonads, we used the TUNEL technique whereby damaged DNA nick ends are selectively labeled with a fluorescein-tagged dUTP by the terminal transferase enzyme. In contrast to wild-type females (Fig. 5A and 5D), Sply05091 mutant females exhibited enhanced TUNEL-positive nuclei within both their spermathecae and ovaries (Fig. 5B and 5E, respectively). The ring of apoptotic nuclei observed in the spermathecae appears to be of spermathecal origin since mutant female virgins also exhibit this phenotype (data not shown). Excessive levels of cell death were seen in Sply05091 egg chambers of almost all stages, including pre-vitellogenic and mature chambers, in affected mutant ovaries (Fig. 5H). Apoptosis was prevalent in the follicle cells as well as the germ cells, and in some instances, entire ovarioles were completely decimated. In contrast, the minimal apoptosis that was observed in the egg chambers of wild-type control ovaries occurred only during mid-oogenesis and late-oogenesis and affected primarily the nurse cells and polar cells (Fig. 5G). The observation of enhanced cell death within the structures affecting normal reproduction in the females highlights the significance of tightly regulated sphingolipid metabolism in the normal development of the Drosophila oocyte.
Figure 5. Apoptosis is elevated in Sply05091 reproductive structures.
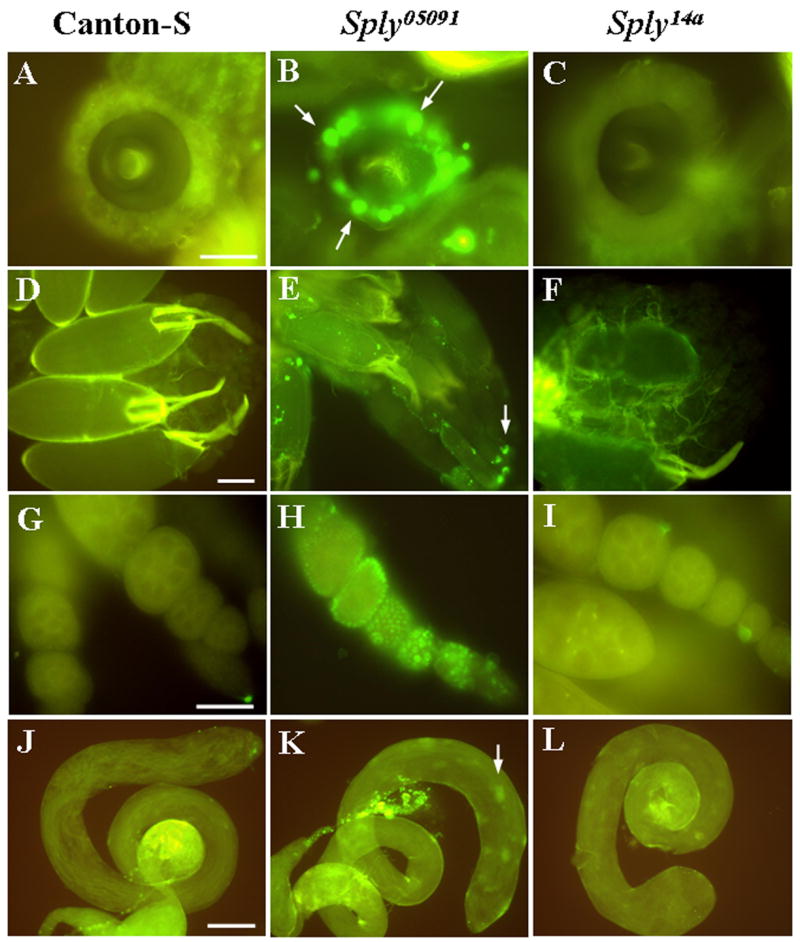
(A–L) Reproductive organs from 4-day old flies analyzed for cell death using TUNEL staining. TUNEL-positive nuclei (arrows) are increased in the spermathecae (A–C), ovaries (D–F), egg follicles (G–I) and testes (J–L) of homozygous Sply05091 mutants (middle panel) compared to Canton-S and Sply14a revertants. Bars: (A–C and G–I), 50 μm; (D–F and J–L), 100 μm.
Similarly, TUNEL-positive nuclei were also present in the Sply05091 mutant testes, although at moderate levels when compared to wild-type control testes (compare Fig. 5K to 5J, respectively). However, in the case where both testes were severely reduced, an intense apoptotic signal was observed that coincided with areas of cellular debris. Further investigation is underway in our laboratory to address the cell types and stages in spermatogenesis that are affected.
Sply05091 egg chambers exhibit nuclear condensation and loss of actin structures
One of the hallmark characteristics of apoptosis is nuclear condensation leading to membrane blebbing and apoptotic bodies. In order to confirm the TUNEL-staining results, the nuclear stain DAPI was used to visualize pyknotic nuclei that are indicative of apoptosis. Basal patterns of apoptosis were detected in the wild-type control egg follicles (Fig. 6A) as well as in the Sply14a egg chambers (Fig. 6C). In contrast, a striking increased frequency of condensed nuclei and apoptotic bodies was observed in many of the Sply05091 mutant egg chambers (Fig. 6B). In addition, co-staining with anti-phalloidin revealed an absence of filamentous actin structures such as the ring canals that normally provide physical support among the developing nurse cells (Fig. 6B′). This loss of structural integrity is a consistent event that parallels apoptosis in dying egg chambers (Peterson et al., 2003).
Figure 6. Sply05091 egg chambers exhibit pronounced nuclear and actin degeneration.
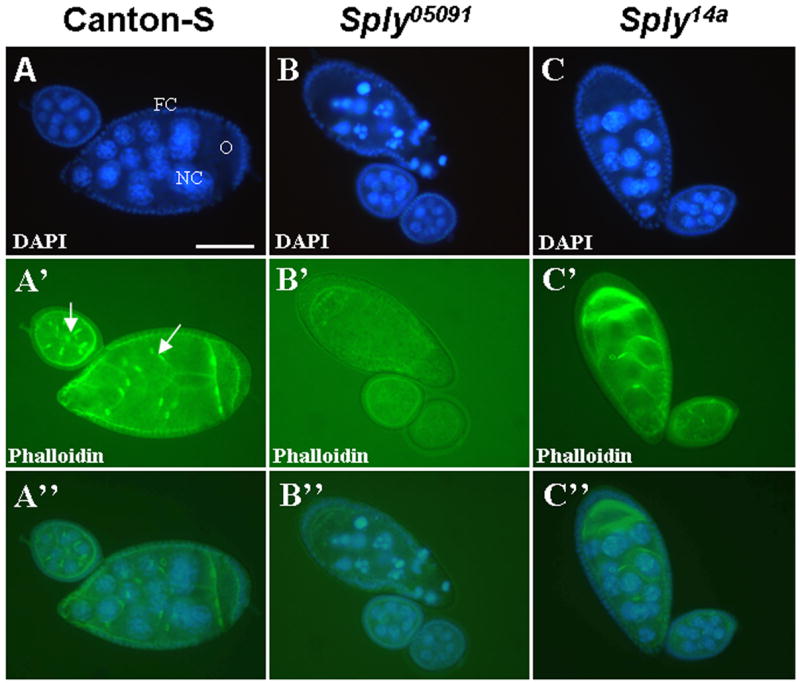
Dissected ovarioles containing egg chambers from 4-day old female flies co-stained with DAPI and phalloidin for detection of the nuclei and actin structures, respectively. (A–C) DAPI staining reveals increased membrane blebbing and nuclear condensation of Sply05091 mutant nurse cells (NC), oocyte (O) and enveloping follicle cells (FC) and minimal cell death of Canton-S and Sply14a revertant egg chambers. (A′–C′) Phalloidin staining shows loss of actin filaments such as the ring canals (arrow). (A″–C″) Overlay of the co-stained images. Bar, 50 μm.
Other mutants affecting sphingolipid regulation also exhibit abnormal reproductive phenotypes and elevated levels of apoptosis
In order to enhance our understanding of the requirement for tight regulation of sphingolipid metabolism and reproductive function, we examined other mutants that disrupt the pathway (Figure 1). The ifc (infertile crescent) gene encodes the 4-dihydroceramide desaturase, an enzyme apparently required for cell cycle control during Drosophila spermatogenesis. Previous studies have reported that various null alleles of the ifc mutation cause male sterility, primarily due to the blockage of cell cycle and spermatid differentiation at the start of the first meiotic division (Basu and Li, 1998; Endo et al., 1996). Our study confirms these phenotypes, providing morphological evidence of shortened testes in 100% of the ifc4 male homozygotes (Fig. 7C). This testicular reduction, however, differed from that observed in the Sply05091 mutant males in that the truncated testes were still full and distended, rather than shriveled and wrinkled (compare Fig. 7C with Fig. 7A). Furthermore, in some instances, the testes were round and circular in shape and not attached to the seminal vesicles, which also appeared diminutive (data not shown). Although previous studies reported no defects in the ifc female mutants, the present study has uncovered numerous abnormalities in the ovaries of these mutant females (Fig. 7E–J). These defects included malformed mature oocytes, follicle degeneration, egg retention and supernumerary spermathecae, many of which are shared by Sply05091 females, although the degree of apoptosis-induced degeneration of egg chambers (Fig. 7K) and the duplication of the spermathecae (40–50%; Table 2) were greater in ifc4 mutant females. In addition, mature ifc4 mutant oocytes –some of which occasionally had missing or short dorsal appendages -were incorrectly oriented with their dorsal appendages facing the common oviduct (Fig. 7E). Despite these phenotypes, however, ifc4 female homozygotes are fertile, albeit at a 50% reduced level (data not shown).
Figure 7. Reproductive defects are observed in other sphingolipid metabolic mutants.
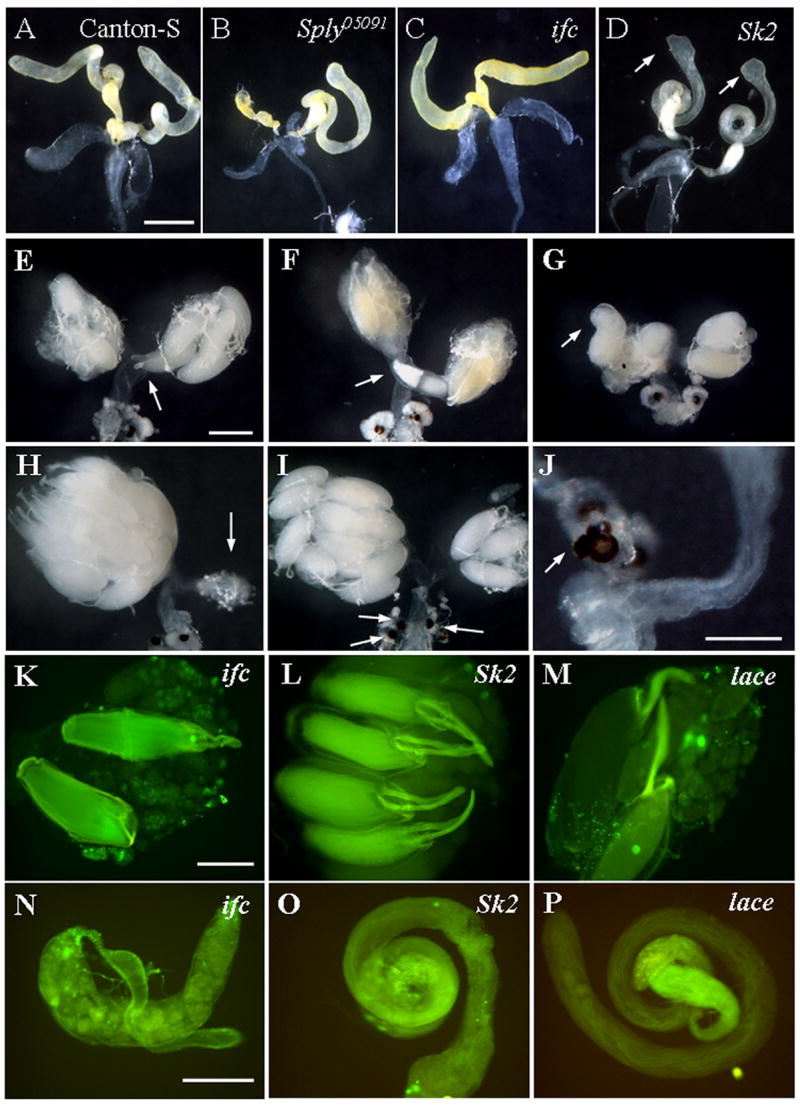
(A–D) Testes from indicated representative fly lines were dissected and photographed. (C) Ifc4 mutant testes are reduced, though not shriveled like (B) Sply05091 mutant testes. (D) Sk2 mutant testes have enlarged apical ends (arrows), but no obvious degeneration. (E–J) Ovarian phenotypes of ifc4 mutants: E and F) disoriented oocytes; G) malformed mature oocytes; H) egg retention and ovarian degeneration; I) supernumerary spermathecae; J) blebbing of spermathecae. Apoptosis assessment of (K–M) ovaries and (N–P) testes dissected from each indicated fly line. (K and N) ifc4 homozygotes exhibit elevated apoptosis in their ovaries and testes while (L and O) Sk2 homozygotes do not. Cell death is seen in (M) lace mutant ovaries but not in (P) mutant testes. Bars: (A–I), 400 μm; (J–P), 200 μm.
Table 2.
Reproductive phenotypes exhibited in mutants affecting sphingolipid metabolism
| Strain | Degenerative Ovaries | Supernumerary Spermathecae | Egg Retention | Misoriented Oocyte | Degenerative Testes | Enlarged Testis Tips |
|---|---|---|---|---|---|---|
| lace | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| ifc4 | 46.2±1.93 | 40.1±4.92 | 30.4±2.68 | 27.8±4.05 | 100±0.00 | 0.00±0.00 |
| Sk2KG05094 | 0.00±0.00 | 0.00±0.00 | 41.4±1.61 * | 0.00±0.00 | 0.00±0.00 | 42.3±4.66 |
| Sply05091 | 32.8±1.84 | 31.9±2.44 | 28.0±1.15 | 0.00±0.00 | 41.4±1.61 | 0.33±0.33 |
The average frequencies of the above phenotypes were obtained from three to four separate examinations of the gonads in the adult flies of the indicated strains. Except for the lace transheterozygotes, all other mutant fly lines each had a sample size exceeding at least 30 individuals per examination.
We also analyzed the gonads of flies with a loss-of-function mutation in the sphingosine kinase 2gene (Sk2) that encodes one of the two Drosophila sphingosine kinases that convert the metabolite sphingosine into S1P. Sk2KG05894 female mutants have previously been shown to exhibit a 60% reduction in egg-laying capacity, characterized by an egg-retention phenotype (Herr et al., 2004). Assessment of apoptosis with TUNEL, however, revealed minimal levels of cell death in the ovaries and egg chambers (Fig. 7L). Likewise, Sk2KG05894 male homozygotes showed no observable degeneration of testes or an increase in TUNEL-positive nuclei (Fig. 7D and Fig 7O). Nevertheless, compared to the testes of wild-type Canton-S, Sk2KG05894 male mutants exhibited testes with enlarged, misshapen apical ends, many of which appeared to be twice the circumference of the testis itself (Fig. 7D).
The lace gene suppresses the severe degeneration seen in Sply05091 ovaries and testes
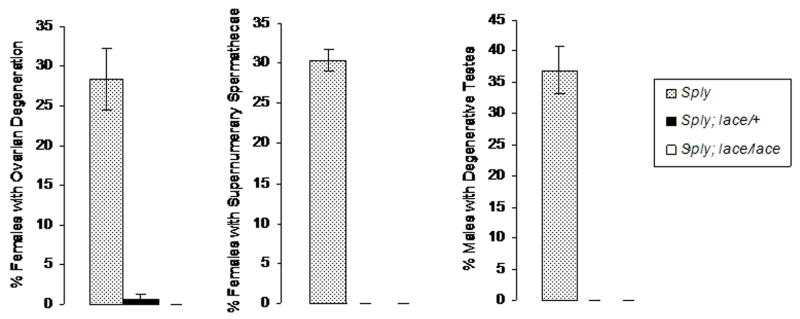
Sphingolipids can be synthesized de novo in a pathway that requires the serine palmitoyltransferase enzyme that is encoded by the lace gene. Studies have previously shown severe developmental and morphological phenotypes, along with decreased viability, in Drosophila adults carrying lace mutations. (Adachi-Yamada et al., 1999; Herr et al., 2003) We examined the morphology of the reproductive structures of hypomorphic lace mutant transheterozygotes and found that, in contrast to the gonadal degeneration that was common to Sply05091 and ifc4 mutants, there were no obvious morphological defects (data not shown). Despite having normal morphologies, however, some lace mutant ovaries exhibited enhanced levels of TUNEL-positive nuclei (Fig. 7M). Interestingly, introduction of either a single copy or double copies of the lace mutation into the Sply05091 background ameliorated the reproductive defects seen in Sply05091 female and male homozygotes as none of the double mutants exhibited degeneration of the ovaries or testes (Fig. 8). These data, clearly, demonstrate the important inter-relationship between the Sply and lace genes in the proper regulation of sphingolipid metabolism and its importance to gonadal development and function in Drosophila.
Figure 8. Lace rescues the gonadal degeneration exhibited by Sply05091.
One copy of lace is sufficient to completely suppress the phenotypes of ovarian degeneration, additional spermathecae, and testicular reduction seen in Sply05091 homozgous mutants. * indicates p<0.001.
Degeneration of the reproductive structures observed in Sply05091 is suppressed by hid and H99
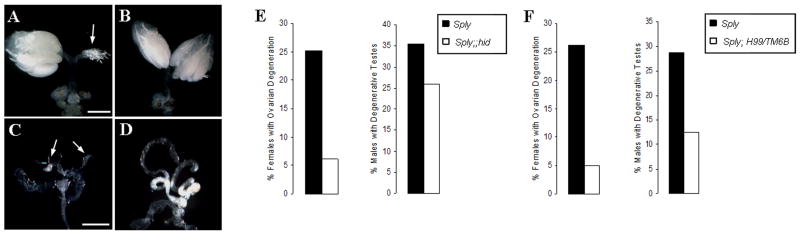
To address the involvement of apoptotic genes in the gonadal degeneration associated with the Sply05091 loss-of-function mutation, double mutants were generated between Sply05091 and a mutant form of hid, one of several Drosophila pro-apoptotic genes. In contrast to the 25.3% of Sply05091 homozygous mutants that exhibited degenerative ovaries, only 6.1% of Sply05091;hid female mutants exhibited this phenotype (compare Fig. 9A with 9B; Fig. 9E). Defective testes were also observed in fewer Sply05091;hid male mutants (25.9%) than in the Sply05091 homozygotes (35.4%) (compare Fig. 9C with 9D; Fig. 9E), although this reduction was not as dramatic as that seen in the ovaries. The substantial rescue demonstrates that hid is involved in the apoptotic events leading to ovarian degeneration (and to a lesser degree, testes degeneration) that are precipitated by disruption of the sphingolipid pathways. However, since the specific hid allele used was a gain-of-function mutant and availability of a null allele of hid was limited, we sought another line defective in cell death to verify our findings. Hid is closely linked to two other apoptosis activators, reaper and grim on the left arm of chromosome 3, of which a small deletion results in the deficiency Df(3L)H99 (Chen et al., 1996; White et al., 1994). While previous work has shown that H99 genes are not required for nurse cell death nor developmental apoptosis during oogenesisis (Foley and Cooley, 1998; Peterson, 2007), we found that removal of hid, reaper and grim was able to rescue the Sply05091 gonadal defects, with ovarian degeneration seen in only 5% Sply05091;H99/TM6B females and testicular reduction in 12.5% of their male counterparts (Fig. 9F).
Figure 9. The severity of the reproductive defects Sply05091 is partially alleviated by hid and H99.
Ovaries from (A) Sply05091 and (B) Sply05091;hid females and testes from (C) Sply05091 and (D) Sply05091;hid males were dissected and photographed. (E) Ovarian degeneration (arrow) is observed in 6.08% (N=35) of the Sply05091;hid females compared to 25.3% (N=73) of the Sply05091 females. Testicular reduction (arrows) is seen in 25.9% (N=39) of the Sply05091;hid males compared to 35.4% (N=127) of the Sply05091 counterparts. (F) Degeneration of the ovaries and testes is seen in 5% Sply05091;H99/TM6B females and 12.5% Sply05091;H99/TM6B males, respectively. Bar, 400 μm.
DISCUSSION
Sphingolipid homeostasis is essential for the normal development and function of a variety of tissues since disruption of this metabolic pathway can lead to a number of pathological consequences, including neurodegeneration (Cutler et al., 2004; Osuchowski et al., 2005), hepatotoxicity and nephrotoxicity (Riley et al., 2001), and various lysosomal storage disorders (Sillence and Platt., 2003). Mounting evidence has implicated ceramide, the sphingolipid metabolite that is derived either by de novo synthesis or generated from membrane sphingomyelin, in the signaling of somatic cell death and germline apoptosis in various mammalian systems (Kolesnick and Kronke, 1998; Morita, 2000; Suomalainen et al., 2003). From a clinical perspective, extensive inappropriate cell death, at least in the case of ovarian development, can lead to premature menopause and infertility (Tilly and Kolesnick, 2002).
Here we report reproductive defects in Drosophila Sply mutants in which S1P lyase, a key enzyme catalyzing the final degradative step of sphingolipid metabolism is disrupted. These reproductive anomalies are shown to involve cell death as indicated by elevated levels of apoptosis in affected structures, including the ovaries, spermathecae and testes. The degeneration of gonads in both mutant females and males harboring Sply05091 loss-of-function alleles appears to account, in large part, for their poor reproductive function – egg deposition is reduced by 70% in mutant females compared to wild-type Canton-S (Herr et al., 2003).
The normal pattern of apoptosis during Drosophila oogenesis is stage-specific and distinctly regulated; developmental cell death of nurse cells occurs only during late oogenesis. Degeneration of entire defective egg follicles can also occur during early and mid-oogenesis when induced in response to poor nutritional state, impaired ecdysone signaling, or stress (Drummond-Barbosa and Spradling, 2001; McCall, 2004). Sply05091 null ovaries, on the other hand, exhibit a pattern of apoptosis that is altogether atypical because cell death occurs during most, if not all, oogenic stages, and is most pronounced between the germarium and mid-oogenesis. We speculate that the accumulation of sphingolipid metabolites may initiate an apoptotic response, thereby disrupting the normally intact follicle cells and leading to the collapse of the entire egg chamber and subsequent degeneration of the ovaries. However, the precise molecular mechanism downstream of sphingolipid accumulation remains unclear. Previous work has shown that when induced with the pro-apoptotic inducer of apoptosis genes, reaper and hid, the follicle layer can show increased sensitivity and consequent ectopic death (Chao and Nagoshi, 1999). Subsequent to the disruption of the follicle layer, nurse cells underwent rapid, stage-specific apoptosis and exhibited alterations in nuclear morphology, disorganization of the ring canals and degradation of actin filaments. Stage-specific degradation of nurse cells, however, is not dependent on either reaper or hid, even though the nurse cells themselves express both genes beginning at stage 9 (Foley and Cooley, 1998). Moreover, it was recently reported that removal of reaper, hid and grim has no effect on normal apoptosis during oogenesis (Peterson, 2007). In contrast, our study shows that the H99 locus seems to be involved in the apoptotic events associated with perturbation in sphingolipid signaling since substantial suppression of ovarian degeneration is observed in Sply05091;H99/TM6B mutants. Despite this, however, H99 does not seem to fully restore the reduced fertility in Sply05091 homozygotes (data not shown). It is possible that other apoptotic pathways are involved (e.g. JNK) and/or that additional signaling events (i.e. those associated with environmental conditions and lipid regulation) are contributing to the gonadal phenotypes when sphingolipids are disrupted.
Flies that are deprived of nutrients exhibit specific cell death within the germarium at the transitional region 2a/2b since degenerating germline cysts are seen in 70% of flies reared on a poor food source (Drummond-Barbosa and Spradling, 2001). Similarly, flies grown on sugar alone exhibit degenerative egg chambers due to premature apoptosis at stage 8/9 (Terashima and Bownes, 2004). The decision for oogenic cells to either progress through normal development or commit to apoptosis under nutritional challenge appears to be regulated by expression of Broad Complex (Br-C), an early target gene in ecdysone signaling. Elevated levels of the steroid hormone ecdysone are found in underfed flies leading to reduced egg production (Bownes, 1989); thus, the number of eggs laid is regulated in accord with the availability of food. We do not believe that the increased premature cell death observed in Sply05091 female homozygotes was a reflection of malnutrition since all flies examined, including wild-type and Sply05091 null mutants, were raised on the same standard laboratory media containing sucrose and yeast paste (see Materials and Methods). More importantly, the high levels of apoptosis that were seen in the Sply05091germaria and not in their normal counterparts nor in the reverted fly strain, Sply14a, were ameliorated in Sply05091;lace mutants in which the accumulated sphingolipids in Sply05091 homozygotes are normalized to near wild-type levels (Herr et al., 2003). Thus, we attribute the elevation of gonadal cell death to the accumulated sphingolipid metabolites. Nevertheless, we can not exclude the possibility that loss-of-function of Sply05091 activity may also be coupled with disturbances in other pathways affecting ecdysone signaling and/or other aspects of intermediary metabolism. In this regard, mutations in the Drosophila insulin receptor substrate protein, chico, result in increased ovarian degeneration and blockage of entry into vitellogenesis (Drummond-Barbosa and Spradling, 2001) while insulin receptor mutants exhibit a reduction in ecdysteroid levels (Tu et al., 2002). It would be intriguing to profile the ecdysteroid levels in the Sply05091 mutant ovaries, in particular the hormone, 20-hydroxyecdysone (20E) which has been reported to induce apoptosis during mid-oogenesis (Soller et al., 1999). The BrC gene that modulates 20E-induced cell death, interestingly, regulates transcription of several Drosophila apoptotic genes, including reaper, hid and the caspase dronc (Cakouros et al., 2002; Jiang et al., 2000). Recently, it was shown that the receptor for ecdysone, EcR-Usp, is also required for regulation of dronc by binding directly to its promoter (Cakouros, 2004). While EcR is expressed in both nurse cells and follicle cells at all oogenic stages, its response genes are predominantly expressed during mid-oogenesis (Buszczak et al., 1999). It would then seem plausible that a disruption of sphingolipid metabolism could lead to altered levels of ecdysone, which in turn modulates the expression of genes involved in targeting cell death in the ovary. Disrupted lipid composition in Drosophila leading to impaired oogenesis has been observed in diacyglycerol acytransferase mutants (midway), which exhibit premature nurse cell death and egg chamber degeneration (Buszczak, 2002).
Caspases have also been shown to have a role in Drosophila ovarian development, and their expression and activity levels exhibit stage-dependent variability during oogenesis. For instance, mutations in Dcp-1 appears to hinder nurse cell death during mid-oogenesis but not during late oogenesis (Laundrie et al., 2003). Furthermore, while caspase inhibition by Diap-1 protects cells in mid-oogenesis (Rodriguez et al., 2002) its overexpression does not prevent late nurse cell death (Peterson et al., 2003). We hope to assess caspase involvement in the degeneration of Sply05091ovaries in future experiments to gain a better understanding of the apoptotic mechanisms that are elicited by sphingolipid dysregulation during Drosophila oogenesis.
In contrast to the marked elevation of apoptosis induced in the Sply05091 ovaries, cell death levels observed in the mutant testes were not as dramatic, although certainly more pronounced than in wild-type control testes. This may be explained, in part, by the recent observation that in fly testes, components of the apoptotic machinery also have non-apoptotic roles such as those involved in spermatid differentiation and individualization (Arama et al., 2003; Huh et al., 2004). Expression of the Drosophila initiator caspase, dronc, and presence of the activated form of the effector caspase, Ice, were detected throughout the length of the elongated spermatids, which when targeted with caspase inhibitors resulted in thick spermatids and male sterility (Arama et al., 2003). Loss-of-function mutation in Cyt-c-d, one of two Drosophila cytochrome-c genes required for activation of Drice in the spermatids, also led to sterile males with no obvious defects in apoptosis, viability or development. The activity of dronc can be initiated when inducers such as hid, reaper and grim disrupt its interaction with the caspase inhibitor Diap-1, thus permitting it to be activated by Ark (Drosophila homolog of mammalian death-activating adaptor, Apaf-1) (Salvesen and Abrams, 2004). Ark- and hid-dependent activation of dronc, along with the caspase Dredd and the adaptor dFADD, were found to be central in spermatid individualization (Huh et al., 2004). Hence, accumulated levels of sphingolipid metabolites may lead to increased expression and activities of apoptotic regulators that, in the testes, direct cell differentiation more so than cell death as in the ovaries. In support of this, only partial inhibition of germ cell apoptosis by S1P was reported in human testes undergoing apoptosis (Suomalainen et al., 2003) whereas complete suppression was observed in death-induced ovaries (Morita et al., 2000). Although we have yet to determine the apoptotic levels in Sply05091;hid double homozygotes or Sply05091;H99/Tm6B mutants, morphological assessment reveals that there is a significantly greater rescue of ovarian degeneration than that of the testes. In Drosophila spermatogenesis, hid is only one of many interactive cell death genes needed for proper sperm development. Thus, it is likely that other downstream apoptotic regulators, such as caspases, or combinations of regulators have a more involved role in the testicular defects observed in Sply05091 male homozygotes. Sply05091 mutant seminal vesicles contain a substantial reduction in free-swimming sperms (unpublished data), a sterile phenotype that is paradoxically described for many of the above-mentioned loss-of-function mutations in Drosophila caspases. While decreased caspase activity may be responsible for individualization blockage in the spermatid cysts, over-activation of caspases can perhaps lead to excessive and/or early spermatid differentiation and individualization events that result in the premature production of spermatozoa, which may not be readily transferred to the seminal vesicles, and, therefore, destroyed. Consistent with this notion, “patches” of apoptotic cells are observed throughout Sply05091 mutant testes that appear to be only partially degenerative while those having more extensive degeneration exhibit larger areas of punctated nuclei (unpublished observation). The paucity of motile sperm in the mutant seminal vesicles offers an explanation for the partial male sterility seen in Sply05091 homozygotes.
The degenerative gonadal phenotypes seen in Sply05091 mutants are also seen to varying degrees in other mutants disrupting sphingolipid metabolism. Loss-of-function ifc4 homozygotes, in particular, exhibit all of the gonadal defects observed in Sply05091mutants, with even greater severity during gametogenesis, leading to complete sterility in males, and malformed egg chambers possessing mis-oriented oocytes in the females. This commonality in phenotypes between ifc4 and Sply05091 mutants suggests that ceramide may not be the only predominant inducer of apoptosis as previously thought, but that dihydroceramide and/or upstream metabolites, that are predicted to be preferentially elevated in the ifc4 mutants, may be relatively more potent with regard to the development of the gonads. Precise analysis of lipid profiles will be required to establish the relative levels of each metabolite and their changes in the mutant gonads. What seems clear, however, is that accumulated levels of sphingolipids are contributing to the degeneration of the ovaries and testes since a hypomorphic allelic combination of the lace gene, a mutation that is characterized by gross depletion of sphingolipids, is able to substantially rescue the Sply05091 gonadal phenotype. In the double mutant combinations, the highly elevated sphingolipid concentrations that are characteristic of Sply05091 are normalized near to their wild-type levels. Moreover, these double mutants have restored number and function of the indirect flight muscles, which are fewer and appear to be degenerating in Sply05091adults, in a manner similar to that observed for the gonadal tissues (Herr et al., 2003). While Sk2KG05894 mutants do not show the same degenerative and apoptotic phenotypes as Sply05091 homozygotes, the accumulated levels of upstream sphingolipids are greatly reduced compare to Sply05091 mutants (Herr et al., 2004). Presumably, the presence of a second sphingosine kinase encoded by the Sk1 gene may be providing a reasonable level of compensation for the loss of Sk2KG05894. Thus, although we have yet to explore the specific signaling pathways that lead ultimately to apoptosis, the activation of those pathways appears to be due to elevated levels of pro-apoptotic sphingolipids such as ceramide and dihydroceramide.
We have shown that the regulation of sphingolipid metabolism is essential during Drosophila gametogenesis in vivo. Moreover, a link between sphingolipid dysregulation and the activation of the proapoptotic genes grim, reaper and hid has been established. Further studies using this model system will allow us to gain a greater understanding of the molecular mechanisms underlying sphingolipid signaling as well as the relationship between sphingolipid-induced apoptosis and gonadal function.
Acknowledgments
We thank Steve Barlow from the SDSU EM facility for help with fluorescence microscopy and Kevin Holleman for technical assistance. This work was supported by NIH grants 5R21HL80811 (G.L.H.), 1R01GM066954 (J.D.S), and 5R25GM58907 (SDSU-Minority Biomedical Research for Students).
Abbreviation used in this paper
- S1P
sphingosine-1-phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acharya U, Acharya J. Enzymes of sphingolipid metabolism in Drosophila melanogaster. Cell Mol Life Sci. 2005;62:128–142. doi: 10.1007/s00018-004-4254-1. [DOI] [PubMed] [Google Scholar]
- Adachi-Yamada T, et al. De novo synthesis of sphingolipids is required for cell survival by down-regulating c-Jun N-terminal kinase in Drosophila imaginal discs. Molecular and Cellular Biology. 1999;19:7276–7286. doi: 10.1128/mcb.19.10.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arama E, et al. Caspase activity and a specific cytochrome c are required for sperm differentiation in Drosophila. Developmental Cell. 2003;4:687–697. doi: 10.1016/s1534-5807(03)00120-5. [DOI] [PubMed] [Google Scholar]
- Ashley T. An integration of old and new perspectives of mammalian meiotic sterility. Results Probl Cell Differ. 2000;28:131–173. doi: 10.1007/978-3-540-48461-5_6. [DOI] [PubMed] [Google Scholar]
- Basu J, Li Z. The Des-1 protein, required for central spindle assembly and cytokinesis, is associated with mitochondria along the meiotic spindle apparatus and with the contractile ring during male meiosis in Drosophila melanogaster. Mol Gen Genet. 1998;259:664–673. doi: 10.1007/s004380050861. [DOI] [PubMed] [Google Scholar]
- Bownes M. The roles of juvenile hormone, ecdysone, and the ovary in the control of Drosophila vitellogenesis. J Insect Physiol. 1989;35:409–413. [Google Scholar]
- Buszczak M, et al. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126:4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Lu X, Segraves W, Chang T, Cooley L. Mutations in the midway gene disrupt a Drosophila acyl coenzyme A: diacylglycerol acytransferase. Genetics. 2002;160:1511–1518. doi: 10.1093/genetics/160.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakouros D, et al. Ecdysone-induced expression of the caspase Dronc during hormone-dependent programmed cell death in Drosophila is regulated by Broad-Complex. Journal of Cell Biology. 2002;157:985–995. doi: 10.1083/jcb.200201034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakouros D, Daish T, Kumar S. Ecdysone receptor directly binds the promoter of the Drosophila caspase Dronc, regulating its expression in specific tissues. Journal of Cell Biology. 2004;165:631–640. doi: 10.1083/jcb.200311057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere V, et al. Apoptosis of nurse cells at the late stages of oogenesis. Dev Genes Evol. 1998;208:106–112. doi: 10.1007/s004270050160. [DOI] [PubMed] [Google Scholar]
- Chao S, Nagoshi R. Induction of apoptosis in the germline and follicle layer of Drosophila egg chambers. Mechanisms of Development. 1999;88:159–172. doi: 10.1016/s0925-4773(99)00183-5. [DOI] [PubMed] [Google Scholar]
- Chase B, Baker B. A genetic analysis of intersex, a gene regulating sexual differentiation in Drosophila melanogaster females. Genetics. 1995;139:1649–1661. doi: 10.1093/genetics/139.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Baker B. Compartmental organization of the Drosophila genital imaginal discs. Development. 1997;124:205–218. doi: 10.1242/dev.124.1.205. [DOI] [PubMed] [Google Scholar]
- Chen P, et al. Grim, a novel cell death gene in Drosophila. Genes Dev. 1996;10:1773–1782. doi: 10.1101/gad.10.14.1773. [DOI] [PubMed] [Google Scholar]
- Cutler R, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. PNAS. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvillier O. Sphingosine in apoptosis signaling. Biochimica et Biophysica Acta. 2002;1585:153–162. doi: 10.1016/s1388-1981(02)00336-0. [DOI] [PubMed] [Google Scholar]
- Dawkins J, et al. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type 1. Nature Genetics. 2001;3:309–312. doi: 10.1038/85879. [DOI] [PubMed] [Google Scholar]
- Dbaibo G, Hannun Y. Signal transduction and the regulation of apoptosis: roles of ceramide. Apoptosis. 1998;5:317–334. doi: 10.1023/a:1009668802718. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling A. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Developmental Biology. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Endo K, et al. Degenerative spermatocyte, a novel gene encoding a transmembrane protein required for the initiation of meiosis in Drosophila spermatogenesis. Molecular General Genetics. 1996;253:157–165. doi: 10.1007/s004380050308. [DOI] [PubMed] [Google Scholar]
- Foley K, Cooley L. Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development. 1998;125:1075–1082. doi: 10.1242/dev.125.6.1075. [DOI] [PubMed] [Google Scholar]
- Fuller M, editor. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. [Google Scholar]
- Giorgi F, Deri P. Cell death in ovarian chambers of Drosophila melanogaster. Journal of Embryology Experimental Morphology. 1976;35:521–533. [PubMed] [Google Scholar]
- Gottlieb D, et al. The DPL1 gene is involved in mediating the response to nutrient deprivation in Saccharomyces cerevisiae. Molecular Cellular Biology Research Communication. 1999;1:66–71. doi: 10.1006/mcbr.1999.0109. [DOI] [PubMed] [Google Scholar]
- Herr D, et al. Characterization of the Drosophila sphingosine kinases and requirement for Sk2 in normal reproductive function. The Journal of Biological Chemistry. 2004;279:12685–12694. doi: 10.1074/jbc.M310647200. [DOI] [PubMed] [Google Scholar]
- Herr D, et al. Sply regulation of sphingolipid signaling molecules is essential for Drosophila development. Development. 2003;130:2443–2453. doi: 10.1242/dev.00456. [DOI] [PubMed] [Google Scholar]
- Hong G, et al. Sphingosine-1-phosphate modulates growth and adhesion of ovarian cancer cells. FEBS. 1999;460:513–518. doi: 10.1016/s0014-5793(99)01400-3. [DOI] [PubMed] [Google Scholar]
- Huh J, et al. Multiple apoptotic caspase cascades are required in nonapoptotic roles for Drosophila spermatid individualization. PLOS Biology. 2004;2:43–53. doi: 10.1371/journal.pbio.0020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JS, et al. Daughterless coordinates somatic cell proliferation, differentiation and germline cyst survival during follicle formation in Drosophila. Development. 2002;129:3255–3267. doi: 10.1242/dev.129.13.3255. [DOI] [PubMed] [Google Scholar]
- Jiang C, et al. A steroid-triggered transcriptional hierarchy controls salivary gland cell death during Drosophila metamorphosis. Molecular Cell. 2000;5:445–455. doi: 10.1016/s1097-2765(00)80439-6. [DOI] [PubMed] [Google Scholar]
- Knudson C, et al. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- Kolesnick R, Kronke M. Regulation of ceramide production and apoptosis. Annual Review Physiology. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Kolodny E. Niemann-Pick disease. Curr Opin Hematol. 2000;7:48–52. doi: 10.1097/00062752-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Laundrie B, et al. Germline cell death is inhibited by P-element insertions in the Dcp-1/Pita nested gene pair in Drosophila. Genetics. 2003;165:1881–1888. doi: 10.1093/genetics/165.4.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, et al. Sphingosine-1-phosphate lyase has a central role in the development of Dictyostellium discoideum. Development. 2001;128:3473–3483. doi: 10.1242/dev.128.18.3473. [DOI] [PubMed] [Google Scholar]
- McCall K. Eggs over easy: cell death in the Drosophila ovary. Developmental Biology. 2004;274:3–14. doi: 10.1016/j.ydbio.2004.07.017. [DOI] [PubMed] [Google Scholar]
- McCall K, Stellar H. Requirement for dcp-1 caspase during Drosophila oogenesis. Science. 1998;279:230–234. doi: 10.1126/science.279.5348.230. [DOI] [PubMed] [Google Scholar]
- McCampbell A, et al. Mutant SPTC1 dominantly inhibits serine palmitoyltransferase activity in vivo and confers an age-dependent neuropathy. Human Molecular Genetics. 2005;14:3507–3521. doi: 10.1093/hmg/ddi380. [DOI] [PubMed] [Google Scholar]
- Mendel J, et al. Sphingosine phosphate lyase expression is essential for normal development in Caenorhabditis elegans. Journal of Biological Chemistry. 2003;278:22341–22349. doi: 10.1074/jbc.M302857200. [DOI] [PubMed] [Google Scholar]
- Morita Y, Tilly J. Sphingolipid regulation of female gonadal cell apoptosis. Annals New York Academy of Sciences. 2000;905:209–220. doi: 10.1111/j.1749-6632.2000.tb06551.x. [DOI] [PubMed] [Google Scholar]
- Morita Y, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nature Medicine. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Miller K. A role for actin dynamics in individualization during spermatogenesis in Drosophila melanogaster. Development. 2003;130:1805–1816. doi: 10.1242/dev.00406. [DOI] [PubMed] [Google Scholar]
- Oskouian B, Saba J. Death and taxis: what non-mammalian models tell us about sphingosine-1-phosphate. Seminars in Cell and Developmental Biology. 2004;15:529–540. doi: 10.1016/j.semcdb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Osuchowski M, et al. Fumonisin B1-induced neurodegeneration in mice after intracerebroventricular infusion is concurrent with disruption of sphingolipid metabolism and activation of proinflammatory signaling. NeuroToxicology. 2005;26:211–221. doi: 10.1016/j.neuro.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Perez G, et al. Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nature Medicine. 1997;3:1228–1332. doi: 10.1038/nm1197-1228. [DOI] [PubMed] [Google Scholar]
- Peterson J, Bass B, Jue D, Rodriguez A, Abrams J, McCall K. Noncanonical cell death pathways act during Drosophila oogenesis. Genesis. 2007;45:396–404. doi: 10.1002/dvg.20306. [DOI] [PubMed] [Google Scholar]
- Peterson J, et al. Stage-specific regulation of caspase activity in Drosophila oogenesis. Developmental Biology. 2003;260:113–123. doi: 10.1016/s0012-1606(03)00240-9. [DOI] [PubMed] [Google Scholar]
- Petrache I, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nature Medicine. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus B, et al. Ceramide in apoptosis: an overview and current perspectives. Biochimica et Biophysics Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- Pyne S, Pyne N. Sphingosine-1-phosphate signaling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R, et al. Sphingolipid perturbations as mechanisms of fumonisin carcinogenesis. Environmental Health Perspectives. 2001;109:301–308. doi: 10.1289/ehp.01109s2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, et al. Unrestrained caspase-dependent cell death caused by loss of Diap1 function requires the Drosophila Apaf-1 homolog, Dark. EMBO. 2002;21:2189–2197. doi: 10.1093/emboj/21.9.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A, et al. Testicular degeneration in Bclw-deficient mice. Nature Genetics. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- Salvesen G, Abrams J. Caspase activation- stepping on the gas or releasing the brakes? Lessons from humans to flies. Oncogene. 2004;23:2774–2784. doi: 10.1038/sj.onc.1207522. [DOI] [PubMed] [Google Scholar]
- Schwab S, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Sillence D, Platt F. Storage diseases: new insights into sphingolipid functions. TRENDS in Cell Biology. 2003;13:195–203. doi: 10.1016/s0962-8924(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Soller M, et al. Control of oocyte maturation in sexually mature Drosophila females. Developmental Biology. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstein S. Sphingosine-1-phosphate: an enigmatic signaling lipid. Nature Reviews Molecular Cell Biology. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Spradling A. The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. [Google Scholar]
- Suomalainen L, et al. Sphingosine-1-phosphate in inhibition of male germ cell apoptosis in the human testis. Journal of Clinical Endocrinological Metabolism. 2003;88:5572–5579. doi: 10.1210/jc.2003-030776. [DOI] [PubMed] [Google Scholar]
- Terashima J, Bownes M. Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics. 2004;167:1711–1719. doi: 10.1534/genetics.103.024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly J, Kolesnick R. Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochimica et Biophysica Acta. 2002;1585:135–138. doi: 10.1016/s1388-1981(02)00333-5. [DOI] [PubMed] [Google Scholar]
- Tu M, et al. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell. 2002;1:158–160. doi: 10.1046/j.1474-9728.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- White K, et al. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. Ceramide and other sphingolipids in cellular responses. Cell Biochemistry and Biophysics. 2004;40:323–350. doi: 10.1385/CBB:40:3:323. [DOI] [PubMed] [Google Scholar]