Abstract
Rationale:
Variation in dietary constituents such as carbohydrate are known to alter psychostimulant function in brain. Relatively few studies have examined the reinforcing effects of psychostimulants in subjects maintained on high-fat diets. The present experiment compared the rate of acquisition of an operant response for intravenous (i.v.) cocaine infusions (0.2 mg/kg) in rats fed either a chow-pellet diet or a 35.9% (by weight) high-fat diet for 45 days prior to cocaine self-administration testing.
Results:
Rats maintained on a high-fat diet for 45 days exhibited diminished acquisition of cocaine self-administration, and this effect was not a function of dietary-induced obesity.
Conclusions:
The results suggest that prolonged exposure to a high-fat diet diminishes the efficacy of cocaine reinforcement.
Keywords: Acquisition, Autoshaping, Behavior, Dietary Fat
Nutritional status is an important modulatory factor for the acquisition and maintenance phases of cocaine self-administration (Campbell and Carroll, 2000; Carroll, 1998). Food deprivation/restriction augments the acquisition of cocaine and amphetamine self-administration (Carroll, 1998) and augments the acute locomotor response to cocaine (Bell et al., 1997). In contrast, the acquisition of drug self-administration is delayed when the rats are trained under conditions of food satiation (Campbell and Carroll, 2000; Carroll and Lac, 1998; Carroll and Meisch, 1980) or when rats are fed a chow diet spiked with a sweet flavor such as saccharin (Carroll and Lac, 1998).
Rodent chow diets ordinarily contain about 10% fat of total calories, whereas humans consume more than 30% of their daily calories as fat (Bray et al., 2002). Rats maintained on a chow pellet diet will “binge” on fat (Corwin, 2004) when such fat is offered during a brief access period (i.e., under conditions of restricted fat access). Acute consumption of fat is reinforcing, as indexed by conditioned taste preference (Sclafani and Ackroff, 2004) and by the capacity of acute consumption of fat to induce a conditioned place preference (Figlewicz et al., 2004). Acute ingestion of liquid fat (corn oil) during sham-feeding increases extracellular levels of dopamine within the nucleus accumbens (NACC) (Liang et al., 2006).
Relatively few studies have examined the impact of acute or chronic fat ingestion on drug reinforcement. Gosnell (Gosnell, 2000) reported that preference for fat, as assessed by fat consumption in a brief access test, does not predict the subsequent rate of acquisition of i.v. cocaine self-administration, whereas sucrose preference does. The Gosnell study, however, did not assess the impact of maintenance diet fat content on cocaine self-administration. Consumption of fat reduces oral consumption of amphetamine by rats (Kanarek et al., 1996), which suggests that fat consumption may diminish subsequent amphetamine reinforcement. That variation in daily fat intake may modulate reactivity to cocaine and other psychostimulants is important given the propensity of humans to consume high-fat diets (Bray et al., 2002).
The aforementioned studies suggest that chronic ingestion of a high-fat diet may diminish cocaine reinforcement. Accordingly, the present study considered the potential impact of exposure to a high-fat diet on subsequent weight gain and reactivity to cocaine. Rats were maintained on a chow diet or a high-fat diet for 45 days, implanted with a jugular catheter, and then tested for acquisition of cocaine self-administration, using the methods of Carroll and colleagues (Campbell and Carroll, 2000, 2001; Carroll, 1998).
Materials and Methods
Animals
The studies were approved by the Texas A&M University Laboratory Animal Care Committee. The subjects were adult male Sprague Dawley rats (Harlan Industries: Houston, TX) weighing approximately 285-325 g at the beginning of the study. The rats were single-housed in plastic hanging cages in a colony room maintained at 22.0 ± 1 °C under a 12hr light/dark cycle (lights on at 1200 hr) and fed standard chow pellets (or a high-fat test diet) and tap water ad libitum, except when noted below. Rats were tail-marked for ease of identification. Behavioral testing commenced at approximately 15:00 hrs, three hrs into the 12-hr light cycle.
Drugs
Cocaine hydrochloride was provided from the Research Technology Branch of the National Institute of Drug Abuse (to JRN) and was dissolved in heparinized saline.
Diets
Two maintenance diets that varied in fat content were used in the present study. The low-fat diet consisted of a pelleted rodent chow diet (Teklad 8604W). The high-fat (HIGH-FAT) diet consisted of two parts by weight of ground chow and one part melted vegetable shortening (Hill Country Farms, San Antonio TX) and was prepared fresh every third day (Wellman et al., 2005). The HIGH-FAT diet was thoroughly mixed while hot, allowed to cool and then remixed and stored at room temperature. The nutritional content of the chow diet (by weight) was 4.5% fat and 24.8% protein; with a calculated gross energy content of 3.3 Kcal/g. In contrast, the HIGH-FAT diet contained about 35.9% fat (by weight) and 16.3% protein and its calculated energy content was 5.28 Kcal/g. Each diet was also made available to the rats in the self-administration chambers during the acquisition period.
Surgical Procedures
Rats were pretreated with 0.4 mg/kg (ip) atropine sulphate and anesthetized with an IM combination of ketamine (80 mg/kg) and xylazine (20 mg/kg). Using a backplate technique, implantation of chronic indwelling jugular catheters was performed using sterile techniques as described in detail elsewhere (Nation et al., 2003; Rocha et al., 2005). The rats were allowed 5 days to recover from surgery before commencing cocaine self-administration testing. During this recovery period, each rat received in the home cage hourly intravenous (i.v.) infusions (200 μl) of a sterile saline solution containing heparin (1.25 U/ml) and penicillin potassium G (250,000 U/ml). Following recovery, animals received automated hourly infusions (213 μl) of heparinized saline over a 6.0 sec time frame in the home cage for the duration of the study. All animals received continuous access to food and water for 5 days while recovering from surgery.
Inasmuch as food restriction is known to accelerate cocaine acquisition (Campbell and Carroll, 2001), the rats were not food deprived in the present studies. Rats were fed either the chow or the HIGH-FAT diet throughout the study, including during the daily acquisition sessions. Uncontaminated water was available ad libitum throughout the study. Animals were weighed daily prior to testing. Food was continuously available in home cages following the end of each daily testing session.
Apparatus
Twelve operant conditioning chambers (Model E10-10, Coulbourn, Allentown, PA) in sound-attenuating cubicles served as the test apparatus. Each chamber had two levers and a stimulus light located above each lever. Infusion pumps (Razel Scientific Instruments; Stamford, CT) controlled drug delivery to each of the boxes. A 20-ml syringe delivered i.v. infusions (160 μl) over a 6.0 sec time frame. The system was interfaced with 2 IBM computers, each controlling drug delivery and recording data from 6 chambers. The rats were offered a 15 ml beaker of water and their food source (pellet or HIGH-FAT) in each test chamber. Food pellets were glued to plastic Petri dishes whereas the HIGH-FAT diet was offered to the rats in a 15 ml beaker glued to a plastic Petri dish. Food spillage was collected on paper pads beneath each chamber; total 6 hour food intake was measured at the end of each session.
Procedure
Autoshaping component
Each of the 6-hr experimental sessions consisted of two parts, an autoshaping, and a self-administration component. Testing was carried out seven days per week. For the first 3 hrs, during the autoshaping component, testing commenced with the retractable lever drawn outside the reach or vision of the animal. After a 90-sec time-out period, the retractable lever extended into the operant chamber at which point the animal received an i.v. cocaine infusion if it pressed the lever or after 15-sec, whichever occurred first. Once again, a 90-sec time-out period was instituted. As before, the active lever was then extended into the chamber and the animal was given 15-sec to press the lever for an immediate infusion of cocaine, or, if no response occurred the animal received a noncontingent cocaine infusion at the end of the 15-sec period. This cycle repeated for the first 20 min of each hr for 3 hrs (30 total cocaine infusions).
With the chamber house-light off, the stimulus light above the active (right) lever was lit for the 6-sec duration of the infusion and terminated immediately after. The inactive (left) lever remained extended inside the chamber throughout the study. Responses on the inactive lever, as well as responses during an infusion, were recorded but had no programmed consequences. A 0.20 mg/kg cocaine HCl infusion (.160 ml) was delivered to the animal following each lever retraction regardless of whether the action was contingent or noncontingent. After the first 20 min of each hour, following the 10 cocaine infusions, all stimulus lights were extinguished and the active lever remained retracted for a 40 min time-out session, until testing recommenced at the beginning of the next hr.
Self-administration component
For the second 3-hr component of the experiment, the retractable lever remained extended and cocaine infusions were contingent upon lever pressing under an FR-1 schedule. As before, responses on the left lever and responses during an infusion delivery were recorded, but had no programmed consequences. At the end of the 3-hr self-administration period, testing was concluded for the day.
The criterion for acquisition of cocaine self-administration was a mean of 25 infusions per day over 2 consecutive daily self-administration sessions and was based on our previous study {Rocha, 2005 #213}. The cocaine dose (0.20 mg/kg) was chosen based on data from previous studies that show this dose is marginally reinforcing, and does not produce satiation or motoric impairments (Campbell and Carroll, 2001; Rocha et al., 2005).
In order to maintain patency during acquisition training, catheters were flushed twice daily with 0.2 mls of a heparinized saline solution; once prior to and once following each daily testing session. At the end of the study, each animal received an i.v. infusion of 7.50 mg/kg sodium pentobarbital. Catheter patency was verified by rapid onset of brief anesthesia.
Statistical Analyses
Data were analyzed only for rats sustaining open catheters throughout the experiment. Animal body weights recorded during the 45 day diet exposure phase were converted to change values (day 45 weight less Day 0 weight). The differences in weight gain between chow and HIGH-FAT groups were contrasted using a student's t test. Two different approaches were taken with regard to analysis of acquisition of cocaine self-administration. In the first set of analyses, the comparative rates of acquisition of cocaine self-administration were assessed for successive 5-day periods using the Kaplan–Meier survival analysis, Breslow statistic (Rocha et al., 2005). This analytical procedure is ideally suited for determining differences in rate with respect to animals reaching a set criterion (SPSS; Chicago, Il). An additional analysis used a proportion test to compare performance patterns in which animals reach criterion at different rates (Bruning JL, 1997). Food intakes during the 6-hr daily session were recorded to the nearest 0.1 g and converted to Kcals. Difference probabilities that are ≤0.05 were deemed statistically significant.
Results
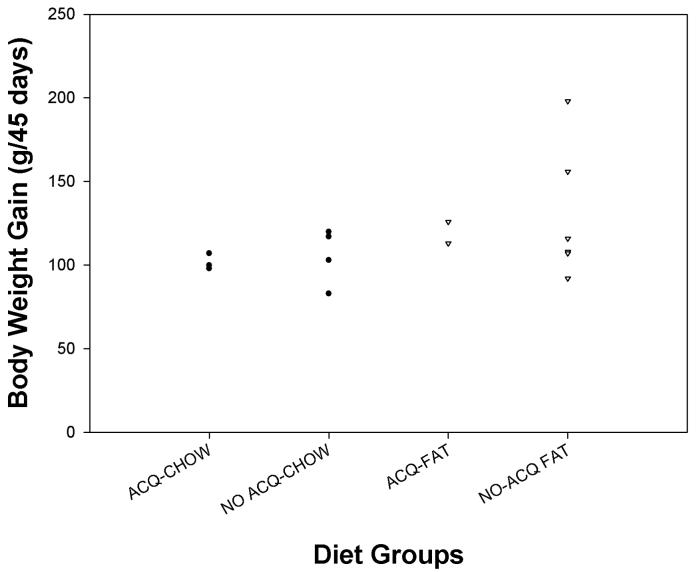
Rats fed a chow pellet diet or a high-fat diet for 45 days showed variable degrees of weight gain (see Figure 1). Comparison of average weight gains indicated that the HIGH-FAT rats gained significantly more body weight than did the chow-fed rats during the 45 day exposure period (p < 0.04). In this figure, the weight gains are displayed separately for rats fed the chow diet that either acquired (ACQ-CHOW) or did not acquire cocaine self-administration (NO ACQ CHOW) and for rats fed the high-fat diet that either acquired (ACQ-FAT) or did not acquire cocaine self-administration (NO ACQ FAT). As can be seen, the rats that acquired cocaine self-administration tended to show weight gains that were close to the average of each diet group.
Figure 1.
Changes in body (g) for rats fed either a chow-pellet diet or a 35.9% (by weight) fat diet for a 45 day period prior to self-administration surgery and testing. The groups are broken into those that acquired or did not acquire while fed a chow diet (N=7) or a high-fat diet (N=8).
Figure 2 illustrates the cumulative percentage of rats in each diet exposure condition meeting criterion for the acquisition of cocaine self-administration. Rats maintained on a chow pellet diet slowly acquired cocaine self-administration such that at the end of 25 days, 3 of 7 rats met the criterion of 25 responses/3 hour self-administration session. In contrast, 2 rats of 8 maintained on the HIGH-FAT diet acquired the self-administration of cocaine, but this did not occur until day 17 and day 25, respectively, of the 25-day acquisition period. Survival analyses indicated significant differences between the HIGH-FAT and chow groups during days 11-15, and 16-20 (p < 0.05) but not during days 1-5, 6-10, or 21-25. A proportion test indicated a significant difference (p < 0.03) between the groups on day 16 of the acquisition period. The rats were fed their respective diets during the 6 hr testing period. The chow-fed rats consumed an average of 6.9 grams of the chow diet (approximately 22.7 Kcal) over the 6-hr test period, whereas the HIGH-FAT rats consumed an average of 5.7 g (approximately 30 Kcals), a difference that was not statistically significant (p = 0.337). Food intakes were not recorded in the home cage between self-administration sessions.
Figure 2.
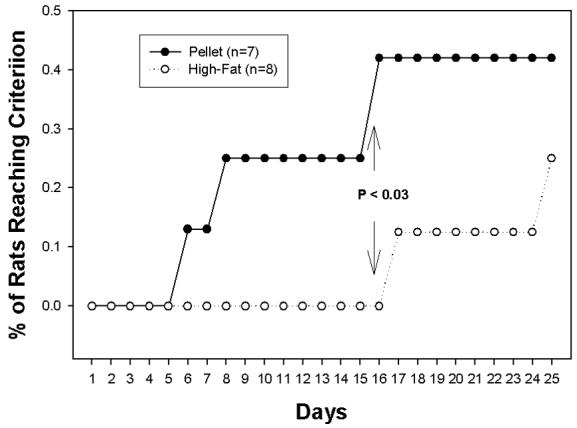
Cumulative percentage (%) of rats fed a pellet diet (Closed symbol: N=7) or a high-fat diet (open symbol: N=8) rats meeting the criterion for the acquisition of cocaine self-administration within the 25-day limit. Chow pellet rats were fed a pellet diet in the home cage whereas high-fat rats were fed a 35.9% (by weight) high-fat diet for 45 days prior to the start of cocaine testing. The cocaine hydrochloride dose was 0.2 mg/kg/infusion and the criterion was set at 25 responses during the last 3 hours on 2 consecutive days.
CONCLUSIONS
In the present experiment, rats fed a standard pellet diet showed gradual acquisition of i.v. cocaine self-administration such that 42% of the rats met the acquisition criterion by day 25. This pattern of acquisition is similar to that noted in a chow-fed control group in a recent report from this laboratory (Rocha et al., 2005). In contrast, rats fed a high-fat diet showed diminished acquisition of cocaine self-administration. One explanation for this effect is that maintenance on a high-fat diet results in differential weight gain such that high-fat fed rats gain more weight on average than do chow-fed rats (Levin, 2000). A fraction of rats fed a high-energy diet will rapidly gain weight and are termed diet-induced obese (DIO) rats, whereas another fraction, termed diet-resistant (DR) rats, maintain body weights comparable to chow-fed rats. Thus, DIO and DR rats are both exposed to a high-energy diet, but DR rats do not exhibit significant weight gain.
Obesity per se might alter drug dosing, pharmacokinetics of cocaine, or CNS reactivity to cocaine. However, in the present experiment, cocaine dosing was adjusted for body weight. Moreover, there was substantial variability in weight gain (see Figure 1) within the groups relative to that between groups. As can be seen, rats that acquired cocaine self-administration (groups ACQ-CHOW or ACQ-FAT) showed weight gains that were close to the median for the chow group and for the HIGH-FAT group. With regard to pharmacokinetic differences associated with varying degrees of body weight, male and female rats differ substantially in body weight and carcass fat content, yet show similar pharmacokinetic profiles to cocaine (Bowman et al., 1999). Body weight per se does not appear to account for the differences in acquisition of cocaine self-administration.
Nutritional status is known to modulate the acquisition of psychostimulant self-administration. It has been shown that palatable food has higher reward efficacy via increased dopamine release in NACC (Roop et al., 2002) and that food deprivation increases the rewarding effects of drugs of abuse (Carr, 2002). A common laboratory procedure is to restrict rats to a limited amount of food per day during acquisition of cocaine self-administration. Such restriction necessarily alters body weight and growth patterns. In the present study, rats were offered food at all times in the home cage as well as in the self-administration testing chambers. It is unlikely that the present effect of diminished acquisition of cocaine self administration represents a difference in calories consumed during the 6-hr session, inasmuch as there were no significant differences between the groups in terms of calories consumed. Consumption of food per se during acquisition training is known to diminish the rate of acquisition. In the present instance, it appears that feeding rats a high-fat diet diminishes the rate of acquisition of cocaine self-administration. Acute consumption of corn oil augments dopamine levels within the NACC, whereas relatively few studies have examined the impact of chronic maintenance on a high-fat diet on psychostimulant reactivity.
The diets used in the present study are dissimilar in a number of dimensions. The HIGH-FAT diet has a greasy rather than solid texture, contains more calories as fat (35.9% versus 4%), less protein (16.3% versus 24.0%) and reduced amounts of vitamins and minerals (due to dilution by the addition of fat). It should therefore be noted that these diets are not balanced with regard to protein or to vitamins and minerals and it is thus possible that the behavioral results reflects one (or more) of these diet differences. It is unlikely that the protein difference produced this profile given that the level of protein in the HIGH-FAT diet exceeds the maintenance requirement of 5-15% for adult animals (Anonymous, 1995; Bernardis and Bellinger, 1981). Future studies using protein and vitamin/mineral balanced diets will be required to confirm the present results.
Psychostimulants are also used to suppress appetite and reduce body weight (Blosser et al., 1987; Cochrane et al., 1998; Wellman, 2005) and several studies have considered the interaction between dietary fat consumption and drug-induced anorexia. Clegg et al. (2003) has shown that increased dietary fat reduces the anorectic effects of ICV injection of MTII, without change in mRNA expression of POMC, AgRP and MC4R. Elsewhere, rats fed a high-fat diet exhibit greater anorexia and weight loss in response to nicotine administration than that noted in a chow control group (Wellman et al., 2005). Rats fed a 35.9% HIGH-FAT diet exhibited a greater suppression of eating to the systemic satiety peptide cholecystokinin (CCK) than did rats fed a chow pellet diet (Torregrossa and Smith, 2003). These studies suggest that maintenance on a high-fat diet can augment or diminish the capacity of drugs to suppress appetite. The present study extends that literature to include a reduced reinforcing action of cocaine in rats fed a high-fat diet.
Acknowledgements
This research was supported by Public Health Service Grants DA13188 and MH65728 to JRN and DA017230 to PJW. We would like to express our gratitude to John Saglime and Leslie Brush for their expert technical assistance in the conduct of the investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anonymous . Nutrient Requirements of Laboratory Animals. Institute foe Laboratory Animal Research; 1995. [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology (Berl) 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. Response of growth-retarded, hypophagic-hypodipsic rats with dorsomedial hypothalamic lesions to a diet in liquid and powder forms. J Nutr. 1981;111:2142–2151. doi: 10.1093/jn/111.12.2142. [DOI] [PubMed] [Google Scholar]
- Blosser JC, Barrantes M, Parker RB. Correlation between anorectic potency and affinity for hypothalamic (+)-amphetamine binding sites of phenylethylamines. European Journal of Pharmacology. 1987;134:97–103. doi: 10.1016/0014-2999(87)90136-1. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther. 1999;290:1316–1323. [PubMed] [Google Scholar]
- Bray GA, Lovejoy JC, Smith SR, DeLany JP, Lefevre M, Hwang D, Ryan DH, York DA. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. Journal of Nutrition. 2002;132:2488–2491. doi: 10.1093/jn/132.9.2488. [DOI] [PubMed] [Google Scholar]
- Bruning JL KB. Computational Handbook of Statistics. Longman; New York: 1997. [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol. 2000;8:312–325. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Effects of ketoconazole on the acquisition of intravenous cocaine self-administration under different feeding conditions in rats. Psychopharmacology (Berl) 2001;154:311–318. doi: 10.1007/s002130000627. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME. Acquisition and reacquisition (relapse) of drug abuse: modulation by alternative reinforcers. NIDA Res Monogr. 1998;169:6–25. [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl) 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Dietary additives and the acquisition of cocaine self-administration in rats. Psychopharmacology (Berl) 1998;137:81–89. doi: 10.1007/s002130050596. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. The effects of feeding conditions on drug-reinforced behavior: maintenance at reduced body weight versus availability of food. Psychopharmacology (Berl) 1980;68:121–124. doi: 10.1007/BF00432128. [DOI] [PubMed] [Google Scholar]
- Cochrane C, Malcolm R, Brewerton T. The role of weight control as a motivation for cocaine abuse. Addict Behav. 1998;23:201–207. doi: 10.1016/s0306-4603(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake predicts rate of acquisition of cocaine self-administration. Psychopharmacology (Berl) 2000;149:286–292. doi: 10.1007/s002130000375. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Mathes WF, Przypek J. Intake of dietary sucrose or fat reduces amphetamine drinking in rats. Pharmacol Biochem Behav. 1996;54:719–723. doi: 10.1016/0091-3057(96)00012-3. [DOI] [PubMed] [Google Scholar]
- Levin BE. Metabolic imprinting on genetically predisposed neural circuits perpetuates obesity. Nutrition. 2000;16:909–915. doi: 10.1016/s0899-9007(00)00408-1. [DOI] [PubMed] [Google Scholar]
- Liang N-C, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–1239. doi: 10.1152/ajpregu.00226.2006. %R 10.1152/ajpregu.00226.2006. [DOI] [PubMed] [Google Scholar]
- Nation JR, Cardon AL, Heard HM, Valles R, Bratton GR. Perinatal lead exposure and relapse to drug-seeking behavior in the rat: a cocaine reinstatement study. Psychopharmacology (Berl) 2003;168:236–243. doi: 10.1007/s00213-003-1405-2. [DOI] [PubMed] [Google Scholar]
- Rocha A, Valles R, Cardon AL, Bratton GR, Nation JR. Enhanced acquisition of cocaine self-administration in rats developmentally exposed to lead. Neuropsychopharmacology. 2005;30:2058–2064. doi: 10.1038/sj.npp.1300729. [DOI] [PubMed] [Google Scholar]
- Roop RG, Hollander JA, Carelli RM. Accumbens activity during a multiple schedule for water and sucrose reinforcement in rats. Synapse. 2002;43:223–226. doi: 10.1002/syn.10041. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004;82:89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Torregrossa AM, Smith GP. Two effects of high-fat diets on the satiating potency of cholecystokinin-8. Physiology & Behavior. 2003;78:19–25. doi: 10.1016/s0031-9384(02)00888-0. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Curr Drug Targets. 2005;6:191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Bellinger LL, Cepeda-Benito A, Susabda A, Ho DH, Davis KW. Meal patterns and body weight after nicotine in male rats as a function of chow or high-fat diet. Pharmacol Biochem Behav. 2005;82:627–634. doi: 10.1016/j.pbb.2005.11.002. [DOI] [PubMed] [Google Scholar]