Abstract
One factor, which may contribute to slowed movement in dystonia, is impairment in controlling the voluntary rate of motor output. This study examined the ability of patients with focal hand dystonia to rapidly turn force on and off at the wrist and elbow joints. Dystonic patients were slower than controls in rapidly turning on force from rest at both joints, passively relaxing force and rapidly reversing force output from a steady-state flexion contraction. Adding a preload did not improve the ability of dystonic subjects to rapidly turn on force. These results support the idea that dystonia is a disorder of impaired motor cortical activation, possibly due to basal ganglia dysfunction.
Keywords: Writer’s cramp, Isometric, Motor control, Basal ganglia
1. Introduction
Dystonia is classically defined as a movement disorder characterized by involuntary sustained muscle contractions that cause twisting movements or abnormal postures of affected body parts [1]. It is thought to involve dysfunction of the basal ganglia [2,3]. Patients with dystonia also have deficits in voluntary movement control, including movements that do not evoke dystonic muscle spasms and at joints that are clinically asymptomatic. For example, patients with writer’s cramp have been shown to have an average strength deficit of approximately 20% at the wrist and elbow joints of the clinically involved arm compared to healthy subjects [4]. Dystonic patients have also been shown to be slow at the wrist [5] and elbow joints [6] during single degree of freedom movement and during whole arm movement [7-10]. These deficits have been shown to be associated with decreased muscle activity and could not be explained by co-contraction of antagonist muscles [4,5]. These reported behavioral deficits are similar to those observed for patients with Parkinson’s disease (PD), a movement disorder caused by dysfunction of the basal ganglia [11-13]. It is possible that, much like patients with PD, one of the factors contributing to slowed movement in dystonia is an impairment in controlling the voluntary rate of motor output, specifically the ability to turn force on and off rapidly.
Neuroimaging studies have shown that voluntary movements that do not induce dystonic muscle spasms in patients with dystonia are associated with decreased activity in regions of the frontal cortex that are considered to be involved in the generation of a motor command, specifically the supplementary motor area (SMA) and primary motor cortex [14,15]. These same regions have also been implicated in the control of voluntary muscle relaxation [16,17]. These findings suggest that the control of muscle contraction during force generation and muscle relaxation may be impaired in dystonia. The purpose of this paper is to examine the ability of patients with dystonia to control their motor output when asked to rapidly turn force on and also to turn it off.
We performed two experiments testing four hypotheses. Although patients with dystonia have previously been shown to be slow when performing discrete movement, it is not clear whether this deficit extends to include the control of isometric force. The first hypothesis was that patients with dystonia would have deficits in their ability to turn on isometric force production quickly from a state of rest. In order to examine whether this dysfunction was unique to the dystonic joint, we examined torque production at two joints. We chose the wrist joint since it was a clinically involved joint in our patient group with focal hand dystonia (FHD), and also the elbow joint, which did not show evidence of dystonic symptoms. Examination of the elbow joint therefore allowed us to examine the spread of motor deficits in muscles of the same limb, which were not yet manifesting clinical symptoms.
Based on previous studies, which examined slowness of movement in patients with dystonia when movements are initiated from a resting state [5-7,9,10], we expected that hypothesis one would be supported and that patients would be slower than controls turning on force quickly from rest. It is not known however if this impairment remains or is exacerbated when the requirement for increased force output is superimposed on pre-activated muscle. Our second hypothesis explored whether dystonic patients could improve their ability to turn on force quickly with pre-activation of force output. Slowness in turning force on in the dystonic group could be due to reduced excitability of spinal or descending motor pathways leading to increased threshold for initiation of action potentials, a reduced size of the descending corticospinal volley (decreased drive), or perhaps poor spatial and temporal summation of the volleys. Pre-activating force output provides a method to examine the rate of rise of force when the motor unit and corticospinal thresholds have been lowered. Any slowness due to a higher threshold should be reduced in the presence of pre-activation. However, if the behavioral deficits in dystonia were instead due to reduced motor drive then we would not expect pre-activation to affect rise time in the patient group. Reduced motor drive in the dystonic group would then be consistent with global basal ganglia dysfunction since it is similar to findings in patients with PD [11-13], a disorder of basal ganglia origin.
It has previously been shown that the response time of muscle relaxation is impaired in patients with dystonia [18]. However, the response time reported in that study included both the reaction time and the time to shut the muscle off, therefore it was not clear if both parts of the response time were impaired. Our third hypothesis was that patients with dystonia would take longer than control subjects to shut off their force output when asked to relax. This slowness would extend across both flexor and extensor muscle force production, and both joints tested.
As well as being slow during discrete movement, patients with FHD have been shown to be slow when performing sequential arm movements and when switching from one movement to another [7]. We examined the ability of patients with FHD to rapidly reverse force output from a steady-state contraction, which is the isometric equivalent of switching movement direction. Our fourth hypothesis was that patients with FHD would be slow when actively reversing torque output.
2. Methods
2.1. Subjects
Eighteen patients with writer’s cramp participated in the first experiment (S1-18), and fifteen patients participated in the second experiment (S2-5, S7, S9, S11-13, S15-20). Table 1 shows a summary of the characteristics of dystonic subjects. A corresponding number of age, height, weight, and gender matched neurologically healthy subjects also participated in each study. Two-tailed t-tests showed no significant differences between patients and controls in age, height or weight in each experiment (p’s>.05). Patients were recruited from two surrounding medical centers. Eighteen of these patients (S1-S18) had previously participated in a separate study conducted in our lab that demonstrated muscle weakness in the patient group [4]. Inclusion criteria for participation included a diagnosis of writer’s cramp, an age range between 20 and 65 years, no history of other neurological problems or injury involving the arms, and at least a 4 month time period since receiving any botulinum toxin injection treatment for dystonia. The most clinically affected hand was tested, which for all patients, except S19 was the dominant side. Dominance and hand tested were matched across control subjects. Informed consent was obtained from each subject prior to testing, and the Institutional Review Board of the University of Illinois at Chicago approved the protocol for use. It is important to note that this task did not induce dystonic posturing in any of the patients except for patient S16 who was the most impaired subject. Results from this patient, however, were similar to the other patients.
Table 1.
Patient characteristics
| Patient | Age (yr) | Ht (m) | Wt (kg) | Gender | Handa | Involved side | Symptom duration (yrs) | Dxb | Duration since treatment with botulinum toxin | Movement scale severity (arm)c | Pattern of symptoms during writingd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 57 | 66 | 74.8 | F | R | R | 14 | DC | n/a | 2 | Thumb ext, F2-4 flex |
| S2 | 57 | 65.5 | 68.0 | F | L | L | 7 | DC | n/a | 6 | Wrist, thumb, and F1-2 flex, F2 abd |
| S3 | 61 | 62 | 56.2 | F | R | B | 17 | WC | 13 months | 9 | F2-3 flex, wrist UD |
| S4 | 63 | 65 | 55.8 | F | R | R | 17 | DC | n/a | 9 | Wrist flex and UD, F5 finger abd |
| S5 | 24 | 67 | 59.0 | F | R | R | 16 | WC | n/a | 4 | Wrist flex and UD, F2 flex |
| S6 | 53 | 64 | 68.0 | F | R | B | 41 | DC | n/a | 9 | Wrist flex and UD, F2-5 flex |
| S7 | 57 | 63 | 60.8 | F | L | L | 6 | WC | n/a | 2 | Wrist, thumb and F2 flex |
| S8 | 53 | 62.5 | 54.4 | F | R | R | 12 | WC | n/a | 2 | Wrist flex and F2 ext |
| S9 | 45 | 62.8 | 68.0 | F | R | R | 2.5 | WC | n/a | 2 | Wrist UD, thumb and F2 flex |
| S10 | 51 | 72 | 87.1 | M | R | R | 6 | DC | 4 months | 6 | Wrist flex |
| S11 | 47 | 69 | 83.9 | M | R | R | 10 | WC | n/a | 2 | Wrist ext, F2 abd and fle |
| S12 | 48 | 70.5 | 77.1 | M | L | L | 2.5 | DC | 6 months | 4 | Wrist UD, F5 flex |
| S13 | 56 | 73 | 82.6 | M | L | L | 5 | WC | n/a | 2 | Wrist ext, F2 flex |
| S14 | 53 | 70 | 70.3 | M | R | R | 5 | WC | 4 months | 2 | Thumb and F2 ext |
| S15 | 42 | 70 | 88.5 | M | R | R | 7 | WC | 14 months | 2 | Wrist ext, F2 flex |
| S16 | 45 | 72 | 108.9 | M | R | B | 23 | DC | 13 months | 12 | Wrist UD and flex, thumb and F2-3 ext |
| S17 | 33 | 73 | 77.1 | M | R | R | 5 | WC | 37 months | 2 | Wrist ext, F1-2 ext, thumb abd |
| S18 | 44 | 72 | 88.5 | M | R | R | 5 | WC | n/a | 2 | Thumb ext |
| S19 | 54 | 72 | 88.5 | M | R | L | 11 | DC | n/a | 6 | F2 flex, thumb add |
| S20 | 63 | 66 | 81.6 | F | R | R | 24 | DC | 6 months | 6 | F2 ext |
Hand dominance.
Diagnosis of WC = writer’s cramp (only writing affected); DC = dystonic cramp (more than one task involved) [31].
arm sub-scale from the Burke-Fahn-Marsden rating scale [32] with higher scores indicating more severe arm involvement (maximum score = 16).
ext = extension, flex = flexion, UD = ulnar deviation, abd = abduction, add = adduction, F2-5 = fingers 2-5.
2.2. Experimental tasks
Manipulanda devices were used for testing at the wrist and elbow joints (Fig. 1a). The wrist joint was held in a neutral position in regards to flexion or extension, and the elbow joint was fixed at 90° of elbow flexion (0° = full elbow extension). Subjects grasped a vertical handle. Joint torque is the rotary effect of force, and was measured by a torque transducer mounted on a shaft at the axis of rotation.
Fig. 1.
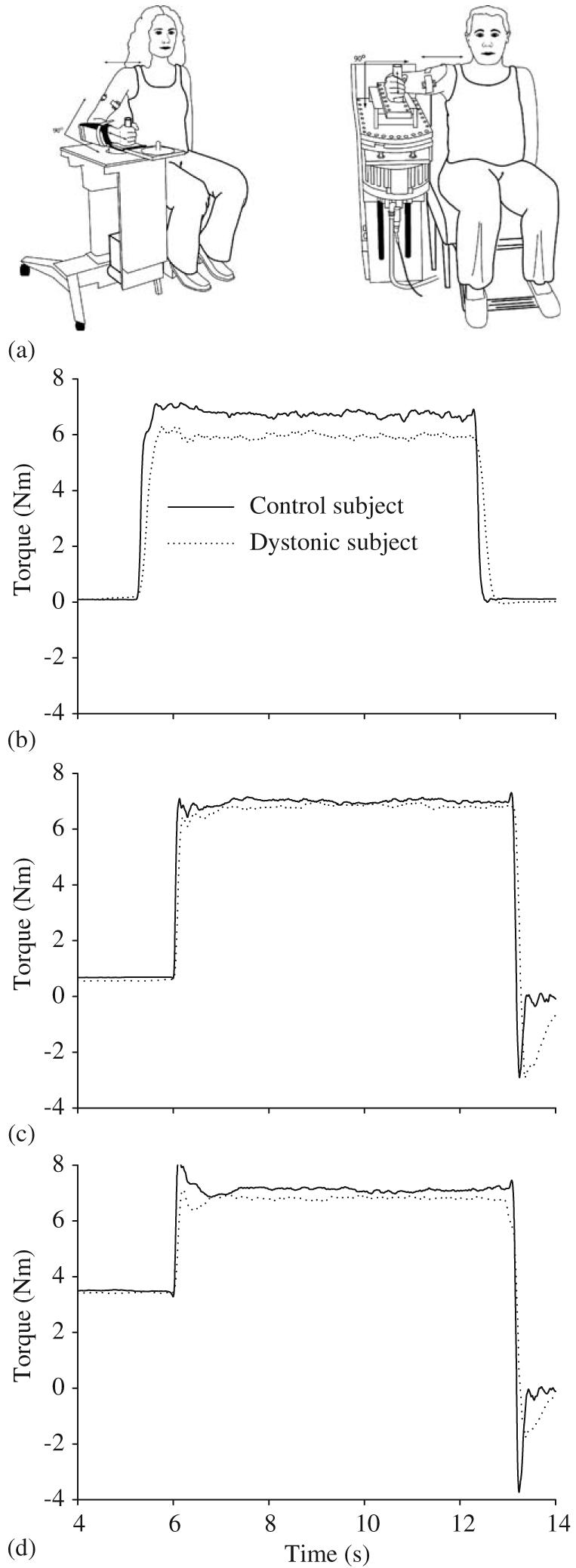
(a) Experimental setup at the wrist (left) and elbow (right). Torque records of a single trial from one dystonic subject (D18, dotted line) and one matched control subject (solid line) under the passive return condition (b), and the active return conditions at 5% MVC preload (c) and 25% preload (d) at the wrist joint.
In order to determine the appropriate target torque for each subject, maximum voluntary contraction (MVC) at each joint was first measured. Maximum torque obtained during the steady-state phase of each trial was identified as the peak torque at each joint. The average of three 6 s trials at the wrist and elbow joints was used to calculate the 50% MVC target at each of the joints used in the subsequent experiments. During the force control experiments, torque was displayed on a computer screen in front of the subject as a vertical marker. A second stationary marker bar on the screen represented target location. Target location was always set to 50% of each subject’s wrist or elbow flexion or extension MVC. The width of the target always corresponded to 0.1 N m of torque. A computer generated preparatory tone alerted the subject to prepare for a trial. A second “go” tone served as a signal to the subject to begin the trial under the instruction to move the torque marker as fast as possible to the target, and to hold it there for 7 s until they heard the final tone which signaled the subject to end the trial.
In the first experiment, we examined the ability of subjects to rapidly turn on force output from rest to either a flexion or extension target at each joint (hypothesis 1), and then to relax this force output (hypothesis 3) (Fig. 1b). Subjects were required to reach the target as quickly as possible, hold the torque at the target, then to simply relax at the final tone. We will refer to the relaxation phase of this task as the passive return condition since the subject was required to passively return the torque to baseline. Eight trials were collected for flexion and extension at each joint. The testing order of joint (wrist or elbow) and direction (flexion or extension) was randomized across subjects in each experiment, but was the same between each dystonic patient and their matched control. There was a 20 s inter-trial interval between all trials.
In the second experiment, we examined the effect of adding a preload on the ability of subjects to rapidly turn on force to a flexion target (hypothesis 2), and their ability to rapidly reverse force output to baseline (hypothesis 4). For the low pre-load, the subject was asked to produce a 5% MVC torque steady-state contraction at the preparation tone and to hold it at the prescribed level until the go tone (Fig. 1c). This was repeated for the high preload, which was set at 25% MVC torque level (Fig. 1d). At the go tone, subjects were instructed to reach the target as quickly as possible and to hold it there until the final tone. The instruction for the final tone across all preload levels was to return the torque to baseline as quickly as possible (Figs. 1c and d). We will refer to this as the active return condition since the torque is returned actively at the end of the trial. When returning to baseline, torque overshoots were encouraged in this condition so that subjects would generate force as quickly as possible and not be constrained by a speed-accuracy trade off. Active return was also tested under a no preload condition to allow comparison with torque onset times from the first experiment. Ten trials were collected in each preload condition at each joint.
2.3. Data analysis
Data were initially processed in Labview (National Instruments) using custom written software. Each trial record was visually inspected prior to data analysis. Trials were rejected if subjects failed to complete the task as instructed (range 0-3 trials per condition). A custom written algorithm (Matlab, The MathWorks, Inc.) was run on the data to identify torque onset and offset during the rising and falling phases of the torque signal using the absolute value of the first derivative of torque. The following measures were calculated: (1) Torque rise time: the duration of time between the onset and offset of the rising phase of the torque signal, (2) passive torque relaxation time: the duration of time between the onset and offset of the falling phase of the torque signal under the passive return instruction and (3) active torque return time: the duration of time between the onset and offset of the falling phase of the torque signal under the active return condition instruction [19].
To calculate torque rise time, we first identified torque onset and offset times during the rising phase of the torque signal. Torque onset was identified by searching backwards from the time of the peak of the first derivative during the rise phase. The onset time is equal to the first time point when the absolute value of the first derivative of torque fell below 1% of the peak value. Torque offset was identified by searching forward from the time of the peak of the absolute value of the first derivative of torque during the rise phase. The offset time is equal to the first time point to fall below 0.01% of peak value of the first derivative. A conditional statement was used to ensure that this offset time point was within 10% of the target force.
To calculate active return time and passive relaxation time we first identified the onset and offset times of the falling phase of the torque signal, which is the phase where torque is returned to zero. The onset of the falling phase of the torque signal was identified by searching backward from the peak of the absolute value of the first derivative during the fall phase. The return onset time is equal to the first time point when the absolute value of the first derivative of torque fell below 1% of the peak value of the first derivative. Return offset was determined by searching forward from the peak of the absolute value of the first derivative during the fall phase. The return offset time is equal to the first time point when the absolute value of the first derivative of torque fell below 1% of the peak value of the first derivative.
The dependent variables described above were analyzed using repeated measures analysis of variance (ANOVA) with one between-subjects factor for group in each experiment and two within subject factors (joint and direction) in the first experiment and one within factor for joint in the second experiment using a Statistica statistical package (version 6.1, StatSoft Inc.). Each ANOVA was interpreted as being significant when there was less than a 5% chance of making a Type I error (i.e. p<0:05).
3. Results
3.1. Turning torque on quickly from a state of rest
Patients with dystonia took significantly longer to achieve the target torque level than healthy control subjects (Fig. 2, Table 2). Across joint and direction, dystonic subjects took an average of 62 ms longer than controls to produce torque to the target. None of the other main effects were significant, and neither were any of the other interactions.
Fig. 2.
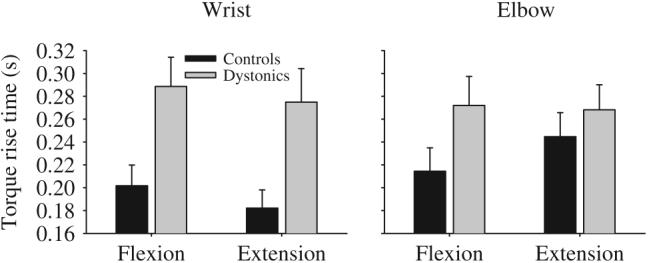
Torque rise time for targets requiring a steady-state flexor torque production and a steady-state extensor torque production at the wrist (left panel) and elbow (right panel) in healthy control subjects (black bars) and dystonic subjects (gray bars). Data are the mean and standard error.
Table 2.
Statistical results from the three-way ANOVA on torque rise time
| F | p | |
|---|---|---|
| Group | 7.60 | 0.009* |
| Joint | 0.58 | 0.450 |
| Muscle | 0.03 | 0.869 |
| Joint × Group | 2.12 | 0.155 |
| Muscle × Group | 0.47 | 0.498 |
| Joint × Muscle | 3.32 | 0.078 |
| Joint × Muscle × Group | 1.49 | 0.231 |
Degrees of freedom are 1,34.
p<0.05.
3.2. Effect of preload on turning torque on quickly
Addition of a preload did not eliminate the difference in rise time between groups (Table 3, Fig. 3 left panel). With a 5% preload, dystonic subjects were slower than controls. On average dystonic subjects took 71 ms longer at the wrist and 75 ms longer than controls at the elbow to reach the target. The joint by group interaction was not significant. Similarly, a 25% preload did not eliminate the difference between dystonic and control groups (Fig. 3 right panel). Dystonics took 41 ms longer at the wrist and 32 ms longer than controls at the elbow to reach the target. The joint by group interaction was not significant. There were significant main effects for joint at both 5% and 25% preload with longer torque rise times at the wrist compared to the elbow joint.
Table 3.
Statistical results from the two-way ANOVA of the effect of preload on torque rise time
| 5% preload |
25% preload |
|||
|---|---|---|---|---|
| F | p | F | p | |
| Group | 14.71 | <0.001* | 7.56 | 0.010* |
| Joint | 7.95 | 0.009* | 14.95 | <0.001* |
| Joint × Group | 0.07 | 0.791 | 0.71 | 0.406 |
Degrees of freedom are 1,28.
p<0.05.
Fig. 3.
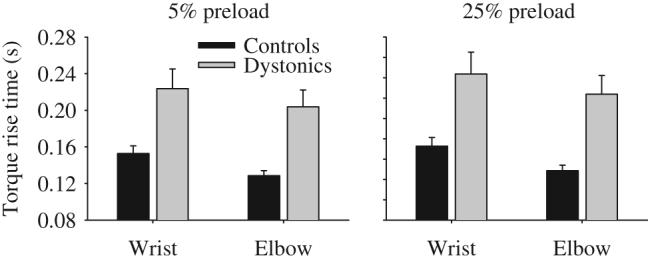
Effect of adding a 5% preload (left panel) and a 25% preload (right panel) on torque rise time in healthy control subjects (black bars) and dystonic subjects (gray bars) at the wrist and elbow joints. Data are the mean and standard error.
3.3. Passive relaxation of torque output
Under the instruction to simply “just relax” at the third tone, patients with dystonia showed significantly longer relaxation times than healthy control subjects (Table 4). On average, dystonic subjects took 68 ms longer than controls to shut off their torque output at the wrist and 67 ms longer at the elbow (Fig. 4). There was also a significant main effect for joint with increased relaxation time at the elbow compared to the wrist. None of the interactions were significant.
Table 4.
Statistical results from the three-way ANOVA on torque relaxation time
| F | p | |
|---|---|---|
| Group | 4.53 | 0.041* |
| Joint | 24.61 | <0.001* |
| Muscle | 0.06 | 0.801 |
| Joint × Group | 0.00 | 0.996 |
| Muscle × Group | 1.91 | 0.176 |
| Joint × Muscle | 2.47 | 0.125 |
| Joint × Muscle × Group | 0.07 | 0.797 |
Degrees of freedom are 1,34.
p<0.05.
Fig. 4.
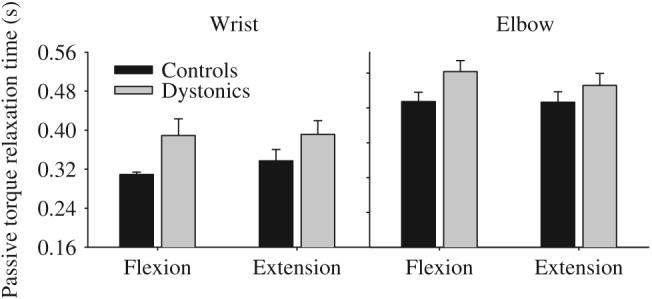
Passive torque relaxation time for targets requiring a steady-state flexor torque production and a steady-state extensor torque production at the wrist (left panel) and elbow (right panel) in healthy control subjects (black bars) and dystonic subjects (gray bars). Data are the mean and standard error.
3.4. Active reversal of torque output
In the no preload condition (Fig. 5), active torque return time was significantly longer in the dystonic group compared to the control group (Table 5). Adding either a 5% preload or a 25% preload did not alter the finding that dystonic subjects were slower to actively return torque from a steady-state flexion contraction compared to controls (Fig. 5). There was no significant main effect for joint, and none of the interactions were significant at any of the preload levels. Across all preload levels, dystonic subjects were on average 22 ms slower at the wrist and 28 ms slower at the elbow than control subjects.
Fig. 5.
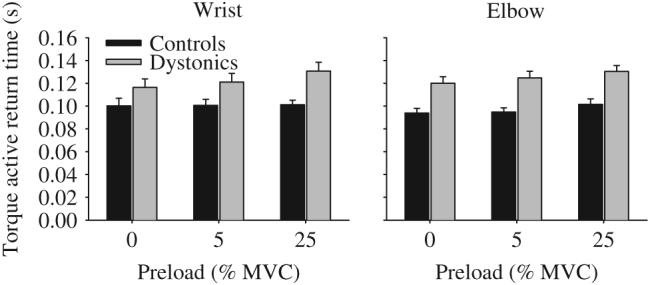
Effect of no preload, a 5% preload, and a 25% preload on active torque return times in healthy control subjects (black bars) and dystonic subjects (gray bars) at the wrist (left panel) and elbow (right panel). Data are the mean and standard error.
Table 5.
Statistical results from the two-way ANOVA of the effect of preload on torque active return time
| 0% preload |
5% preload |
25% preload |
||||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Group | 6.75 | 0.015* | 10.73 | 0.003* | 16.99 | <0.001* |
| Joint | 0.13 | 0.716 | 0.11 | 0.742 | 0.01 | 0.942 |
| Joint × Group | 2.50 | 0.125 | 2.24 | 0.145 | 0.00 | 0.989 |
Degrees of freedom are 1,28.
p<0.05.
4. Discussion
There are three main findings from this study. The first is that the ability to rapidly turn on force was impaired in the patients with FHD and that this deficit extended to include force output in a proximal joint that was not manifesting dystonic symptoms. This was despite the fact that dystonic subjects produced less force than controls, and therefore had a lower target force [4]. Furthermore, pre-activating force output did not improve the ability to rapidly produce force in dystonia. Second, the ability to voluntarily relax force output was impaired in the patients with dystonia at both a clinically involved and a clinically uninvolved joint within the dystonic limb. Third, the ability to actively return force from a steady-state flexion contraction to zero was longer in the dystonic subjects compared to controls.
Previous work has identified that dystonic patients are slow when performing voluntary movements [5-10]. We have extended this finding to include slowness in isometric force production at the wrist (a clinically involved joint) and the elbow (a joint that did not present with overt clinical symptoms of dystonia). The fact that we saw comparable deficits at the elbow joint suggests a more widespread dysfunction of movement control in FHD than just the hand. This finding is consistent with our previous study showing that maximal force output was decreased at both these joints [4]. In that study, decreased force output could not be explained by excessive co-contraction. Similarly, co-contraction did not account for movement slowing during ballistic movements of the wrist [5]. For this reason, co-contraction of antagonist muscles was unlikely to contribute to the reduced torque rise times we observed.
Pre-activating force output did not improve the ability of dystonic patients to rapidly reach the target force. Therefore, it is unlikely that reduced excitability of spinal or descending motor pathways was the mechanism of dysfunction in the dystonic group. Instead, we suggest that the movement slowness seen in patients with dystonia is mediated by dysfunction of cortical drive to the motorneuron pool. Oga et al. [17] have shown using fMRI that there is underactivation of the primary sensorimotor cortex and SMA during an isometric wrist contraction task in dystonic patients compared to controls. They interpreted this underactivation as reflective of dysfunction in the motor circuitry connecting the basal ganglia to cortical areas. Abnormal firing patterns have previously been found in the internal segment of the globus pallidus of the basal ganglia of patients with dystonia [20,21], and it is interference from this abnormal firing pattern which could disrupt cortical activation and lead to slowness in rapid force production. The behavioral deficits seen at the elbow joint in our patient group suggests a more widespread disorder of motor control than just control of the body part displaying dystonic symptoms (i.e. the hand). This is consistent with what is seen in movement disorders of basal ganglia origin such as PD, which show dysfunction across multiple limb segments. Alternatively, deficits in the production and control of force could arise from dysfunction of brainstem nuclei that control motor output and have been implicated in the pathophysiology of DYT1 primary dystonia [22].
Our findings are also consistent with previous studies showing that the control of voluntary muscle relaxation is impaired in patients with dystonia. Buccolieri et al. [18] showed that the time to relax a muscle was impaired in patients with dystonia, although the time to relax as used by that group included the reaction time from an auditory signal to the cessation of muscle activity. It was not clear from their work whether the deficit in dystonia was attributable to the reaction time or to the time it actually took to turn off the muscle. In the present study we have shown that the time to actually relax force output is impaired in dystonia. Consistent with these difficulties in force relaxation in dystonia are findings of abnormal cortical mechanisms associated with voluntary muscle relaxation. This has been shown using a variety of methods including movement-related potentials [16], transcranial magnetic stimulation applied to the motor cortex [23], and event-related fMRI [17]. Our results are similar to those found in patients with PD, another disorder associated with basal ganglia dysfunction [11,19,24].
Slowness in dystonia also extended to slowness in reversing force output from a steady-state contraction. This finding is consistent with the work by Agostino et al. [7] who have previously shown longer movement times when switching from one movement to another as part of a movement sequence in patients with dystonia. It is of interest however that there was less of a difference between dystonics and controls in rapidly reversing force output than the difference between them in rapidly producing force from rest (Fig. 5 compared to Fig. 2). It is not clear what underlies this difference, but several possibilities might be considered. First, it is possible that a different goal in the two tasks was responsible for the disparity of findings. Torque rise time was to a target, but during the active torque return condition, torque overshoots were encouraged. It is possible that the difficulty in rapidly generating force from rest in the dystonic group was related to the presence of a target, which interfered with the motor plan in some way. In contrast, actively returning force to zero from a steady-state contraction did not require moving to a target. When force is rising to a target, sensory feedback must be used and compared to the visual signal. There is a significant body of work to suggest a role of dysfunctional sensorimotor integration in the pathogenesis of dystonia (for reviews see Abbruzzese and Berardelli [25] and Tinazzi et al. [26]). If sensory feedback is abnormally processed in dystonia, then the patient will either miss the target, or must slow down to acquire a target.
Another possibility to explain why the difference between groups was more pronounced for rapid torque rise time compared to active return time may lie in a dysfunction of reciprocal inhibition in the dystonic group. It has previously been shown that the second phase of reciprocal inhibition reflecting presynaptic inhibition is reduced in patients with dystonia [27]. This has been attributed to reduced reciprocal spinal inhibition possibly from defective descending control of the excitability of the presynaptic inhibitory pathway [27-30]. Paradoxically, abnormal reciprocal inhibition in dystonia may make it easier to switch recruitment from an agonist to an antagonist muscle to reverse the direction of force output.
In conclusion, patients with primary FHD show deficits in both the rate of production and relaxation of force output. These findings are consistent with deficits observed in other neurological diseases affecting the basal ganglia. These deficits extend to include force production at joints that do not yet show clinical evidence of dystonic symptoms suggesting a global dysfunction in dystonia. Further study of regions not directly related to the clinically involved limb (e.g. the opposite hand or foot) is required to investigate how widespread the deficits are.
Acknowledgments
We would like to thank the staff at the Section for Movement Disorders in the Department of Neurological Sciences at Rush University Medical Center and Dr. Tanya Simuni in the Department of Neurology at Northwestern Memorial Hospital for their assistance in patient recruitment. Also, we thank Dr. Annette Weis-McNulty and Ms. Emily Gilley for their assistance with some aspects of data collection. This study was supported in part by the National Institutes of Health Grants AR33189, NS21827, and NS40902.
References
- [1].Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, editors. Movement disorders 2. Butterworth; London: 1987. pp. 332–58. [Google Scholar]
- [2].Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121(Pt 7):1195–212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- [3].Vitek JL. Pathophysiology of dystonia: a neuronal model. Movement Disord. 2002;17(Suppl 3):S49–62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]
- [4].Prodoehl J, Mackinnon CD, Comella CL, Corcos DM. Strength deficits in primary focal hand dystonia. Movement Disord. 2006;21(1):18–27. doi: 10.1002/mds.20623. [DOI] [PubMed] [Google Scholar]
- [5].MacKinnon CD, Velickovic M, Drafta C, Hesquijarosa A, Brin MF. Corticospinal excitability accompanying ballistic wrist movements in primary dystonia. Movement Disord. 2004;19(3):273–84. doi: 10.1002/mds.20017. [DOI] [PubMed] [Google Scholar]
- [6].van der Kamp W, Berardelli A, Rothwell JC, Thompson PD, Day BL, Marsden CD. Rapid elbow movements in patients with torsion dystonia. J Neurol Neurosurg Psychiatr. 1989;52:1043–9. doi: 10.1136/jnnp.52.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M. Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain. 1992;115:1481–95. doi: 10.1093/brain/115.5.1481. [DOI] [PubMed] [Google Scholar]
- [8].Inzelberg R, Flash T, Schechtman E, Korczyn AD. Kinematic properties of upper limb trajectories in idiopathic torsion dystonia. J Neurol Neurosurg Psychiatr. 1995;58(3):312–9. doi: 10.1136/jnnp.58.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Curra A, Berardelli A, Agostino R, Giovannelli M, Koch G, Manfredi M. Movement cueing and motor execution in patients with dystonia: a kinematic study. Movement Disord. 2000;15(1):103–12. doi: 10.1002/1531-8257(200001)15:1<103::aid-mds1016>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [10].Inzelberg R, Flash T, Korczyn AD. Kinematic properties of upper-limb trajectories in Parkinson’s disease and idiopathic torsion dystonia. Adv Neurol. 1990;53:183–9. [PubMed] [Google Scholar]
- [11].Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson’s disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39(1):79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- [12].Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson’s disease. Movement Disord. 2001;16(6):1048–65. doi: 10.1002/mds.1220. [DOI] [PubMed] [Google Scholar]
- [13].Berardelli A, Rothwell JC, Day BL, Marsden CD. Movements not involved in posture are abnormal in Parkinson’s disease. Neurosci Lett. 1984;47(1):47–50. doi: 10.1016/0304-3940(84)90384-7. [DOI] [PubMed] [Google Scholar]
- [14].Ceballos-Baumann AO, Passingham RE, Warner T, Playford ED, Marsden CD, Brooks DJ. Overactive prefrontal and underactive motor cortical areas in idiopathic dystonia. Ann Neurol. 1995;37(3):363–72. doi: 10.1002/ana.410370313. [DOI] [PubMed] [Google Scholar]
- [15].Ceballos-Baumann AO, Sheean G, Passingham RE, Marsden CD, Brooks DJ. Botulinum toxin does not reverse the cortical dysfunction associated with writer’s cramp—a PET study. Brain. 1997;120(Part 4):571–82. doi: 10.1093/brain/120.4.571. [DOI] [PubMed] [Google Scholar]
- [16].Yazawa S, Ikeda A, Kaji R, Terada K, Nagamine T, Toma K, Kubori T, Kimura J, Shibasaki H. Abnormal cortical processing of voluntary muscle relaxation in patients with focal hand dystonia studied by movement-related potentials. Brain. 1999;122(Part 7):1357–66. doi: 10.1093/brain/122.7.1357. [DOI] [PubMed] [Google Scholar]
- [17].Oga T, Honda M, Toma K, Murase N, Okada T, Hanakawa T, Sawamoto N, Nagamine T, Konishi J, Fukuyama H, Kaji R, Shibasaki H. Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer’s cramp: an fMRI study. Brain. 2002;125(Part 4):895–903. doi: 10.1093/brain/awf083. [DOI] [PubMed] [Google Scholar]
- [18].Buccolieri A, Avanzino L, Marinelli L, Trompetto C, Marchese R, Abbruzzese G. Muscle relaxation is impaired in dystonia: a reaction time study. Movement Disord. 2004;19(6):681–7. doi: 10.1002/mds.10711. [DOI] [PubMed] [Google Scholar]
- [19].Robichaud JA, Pfann K, Vaillancourt DE, Comella C, Corcos DM. Force control and disease severity in Parkinson’s disease. Movement Disord. 2005;20(4):441–50. doi: 10.1002/mds.20350. [DOI] [PubMed] [Google Scholar]
- [20].Hutchinson WD, Lang AE, Dostrovsky JO, Lozano AM. Pallidal neuronal activity: implications for models of dystonia. Ann Neurol. 2003;53:480–8. doi: 10.1002/ana.10474. [DOI] [PubMed] [Google Scholar]
- [21].Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, Triche S, Mewes K, Hashimoto T, Bakay RA. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46(1):22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [22].McNaught KS, Kapustin A, Jackson T, Jengelley TA, Jnobaptiste R, Shashidharan P, Perl DP, Pasik P, Olanow CW. Brainstem pathology in DYT1 primary torsion dystonia. Ann Neurol. 2004;56(4):540–7. doi: 10.1002/ana.20225. [DOI] [PubMed] [Google Scholar]
- [23].Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatr. 1995;59(5):493–8. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jordan N, Sagar HJ, Coper JA. A component analysis of the generation and release of isometric force in Parkinson’s disease. J Neurol Neurosurg Psychiatr. 1992;55:572–6. doi: 10.1136/jnnp.55.7.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Movement Disord. 2003;18(3):231–40. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- [26].Tinazzi M, Rosso T, Fiaschi A. Role of the somatosensory system in primary dystonia. Movement Disord. 2003;18(6):605–22. doi: 10.1002/mds.10398. [DOI] [PubMed] [Google Scholar]
- [27].Nakashima K, Rothwell JC, Day BL, Shannon K, Thompson PD, Marsden CD. Reciprocal inhibition between forearm muscles in patients with writer’s cramp and other occupational dystonias and patients with hemiparesis due to stroke. Brain. 1989;112:681–97. doi: 10.1093/brain/112.3.681. [DOI] [PubMed] [Google Scholar]
- [28].Chen RS, Tsai CH, Lu CS. Reciprocal inhibition in writer’s cramp. Movement Disord. 1995;10(5):556–61. doi: 10.1002/mds.870100505. [DOI] [PubMed] [Google Scholar]
- [29].Valls-Sole J, Hallett M. Modulation of electromyographic activity of wrist flexor and extensor muscles in patients with writer’s cramp. Movement Disord. 1995;10(6):741–8. doi: 10.1002/mds.870100607. [DOI] [PubMed] [Google Scholar]
- [30].Panizza ME, Hallett M, Nilsson J. Reciprocal inhibition in patients with hand cramps. Neurology. 1989;39(1):85–9. doi: 10.1212/wnl.39.1.85. [DOI] [PubMed] [Google Scholar]
- [31].Sheehy MP, Marsden CD. Writers’ cramp—a focal dystonia. Brain. 1982;105(Part 3):461–80. doi: 10.1093/brain/105.3.461. [DOI] [PubMed] [Google Scholar]
- [32].Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35(1):73–7. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]


