Abstract
We report that physiological concentrations of both short- and long-chain ceramides, despite being lipids, form large stable pores in membranes. Some of these pores should be large enough to allow cytochrome c to permeate. Dihydroceramide differs from ceramide by the reduction of one double bond, and yet both its apoptogenic and channel-forming activities are greatly reduced. A structural model provides insight into how ceramides might form pores. According to a mathematical model, both the individual conductance of the channels and the overall membrane conductance are directly related to the overall concentration of ceramide in the membrane. Slight changes in concentration have dramatic effects on the size of the channels formed, providing an easy way for rapidly altering membrane permeability by changing the activity of local synthetic and catabolic enzymes. A possible role for these channels in apoptosis is discussed.
Ceramide, a sphingosine-based lipid second messenger, is known to be involved in the regulation of several cellular responses to extracellular stimuli, including differentiation, growth suppression, cell senescence, and apoptosis (1-3). The role of ceramide specifically in apoptosis has attracted great attention in recent years. Ceramide is generated within the cell via the hydrolysis of sphingomyelin or de novo synthesis. A net increase in ceramide levels within the cell is observed in response to several inducers of cellular stress (1-3). The contents of ceramide in the membranes of cells undergoing apoptosis are thought to reach 10 mol % of the total phospholipid (2). The wide range biological effects of ceramide, which depend on cell type, receptors involved, sub-cellular location, and concentration, suggest the existence of several downstream targets for distinct intracellular pathways.
Mitochondria have been shown to play a major regulatory role in cell death via apoptosis (4-8). A key feature is the release of mitochondrial proteins, including apoptosis-inducing factor, cytochrome c, procaspases, and heat shock proteins from the intermembrane space of mitochondria to the cytoplasm (4-7, 9). The release of intermembrane space proteins into the cytoplasm is crucial for the activation of specific caspases and DNases with apoptosis as a final result. Increased ceramide levels and exogenously added cell-permeable ceramide analogues have been reported to have an effect on various aspects of mitochondrial function. N-Acetyl-d-erythro-sphingosine (C2-ceramide) has been shown to induce cytochrome c release when added to whole cell cultures (10-14) and isolated mitochondria (15-17). This cytochrome c release can be prevented by preincubation with or overexpression of the anti-death protein, Bcl-2 (18), or transfection of cells with Bcl-xl (19, 20). In addition, solubilized long-chain C16-ceramide has been shown to induce a large release of cytochrome c when added to mitochondrial suspensions (16). Additional effects of ceramide analogues include enhanced generation of reactive oxygen species (10, 21, 22), alteration of mitochondrial calcium homeostasis (10, 21, 22), and ATP depletion followed by collapse in the inner mitochondrial membrane potential (Δψm) (10, 17). In addition, ceramide has been shown to destabilize membranes and cause leakage, fusion, and budding of vesicles (23-26). Ceramides have been reported to mix poorly with phospholipids in bilayers, form distinct ceramide-enriched microdomains, favor the stability of the gel over the lamellar phase, and facilitate the lamellar-hexagonal transition of phospholipids (26). Di Paola et al. (16) recently reported that C2-ceramide, when added to liposomes, causes a collapse in the membrane potential generated by reconstituted complex III of the mitochondrial electron transport chain. Their results indicate that C2-ceramide can somehow cause an increase in the permeability of membranes.
Although ceramides have been demonstrated to increase the permeability of membranes, the means by which they do so remains unclear. We therefore tested the ability of C2- and C16-ceramides to form channels in membranes lacking proteins, utilizing the planar membrane technique (27) as modified (28). Here we show that both short- and long-chain ceramides can form large stable channels in membranes and have provided both structural and mathematical models to provide insight as to how they might do so. As far as we are aware, this is the first report of stable pore formation by a lipid in a membrane. The implication for a role of these ceramide channels in apoptosis is discussed.
EXPERIMENTAL PROCEDURES
Drugs and Reagents
The following reagents were purchased from Avanti Polar Lipids: C2-ceramide, C2-dihydroceramide, C16-ceramide, C18-dihydroceramide, and asolectin (soybean phospholipids).
Electrophysiological Recordings
Planar membranes were formed by the monolayer method (27), as modified (28), across a 100-μm-diameter hole in a Saran partition using 1% (w/v) asolectin (soybean phospholipids), 0.2% (w/v) cholesterol in hexane solution. The aqueous solution contained 1 m KCl, 1 mm MgCl2, 5 mm MES (pH 6.0). The applied voltage was held constant at 9 mV. Me2SO was used as the vehicle for C2-ceramide and dihydroceramide experiments such that it was no more than 0.5% of the total volume of the aqueous solution. Equal amounts of the agent being tested were pipetted into the aqueous solution on both sides of the membrane while stirring to reach the total concentration indicated. Because of the harsh techniques required for solubilization of long-chain ceramides, the planar membrane experiments utilizing long-chain ceramides required that the planar membrane be made in their presence. The membrane consisted of 1% (w/v) asolectin, 0.2% (w/v) cholesterol, and 0.05% (w/v) either C16-ceramide or C18-dihydroceramide in hexane. All other conditions remained the same.
RESULTS
C2-Ceramide Increases Membrane Conductance by Forming Large Stable Channels
The effect of C2-ceramide on membrane conductance was monitored by utilizing the planar membrane technique (27) as modified (28). 5 μm C2-ceramide, a concentration less than or equal to those used in whole cell and isolated mitochondrial suspension experiments, when added to the aqueous phase (either one or both sides), resulted in stable pore formation in the planar phospholipid membrane. The discrete stepwise current increases are diagnostic of C2-ceramide pore formation in the membrane (Fig. 1, a–c). The channels had long lifetimes, and thus the overall conductance of the membrane increased in a stepwise manner as new channels formed. As the total membrane conductance increased and the experiment progressed, the conductance of the individual channels tended to increase, ranging from about 1 nS early to more than 200 nS late in the experiment (Fig. 2). Whereas the stability of the membrane was reduced by the addition of ceramide, the discrete conductance increments observed were not characteristic of simple membrane breakdown. Membranes lasted as long as 40 min after the formation of the first channel, achieving total conductance values as high as 2.3 × 103 nS.
Fig. 1. C2-ceramide forms channels in planar phospholipid membranes.
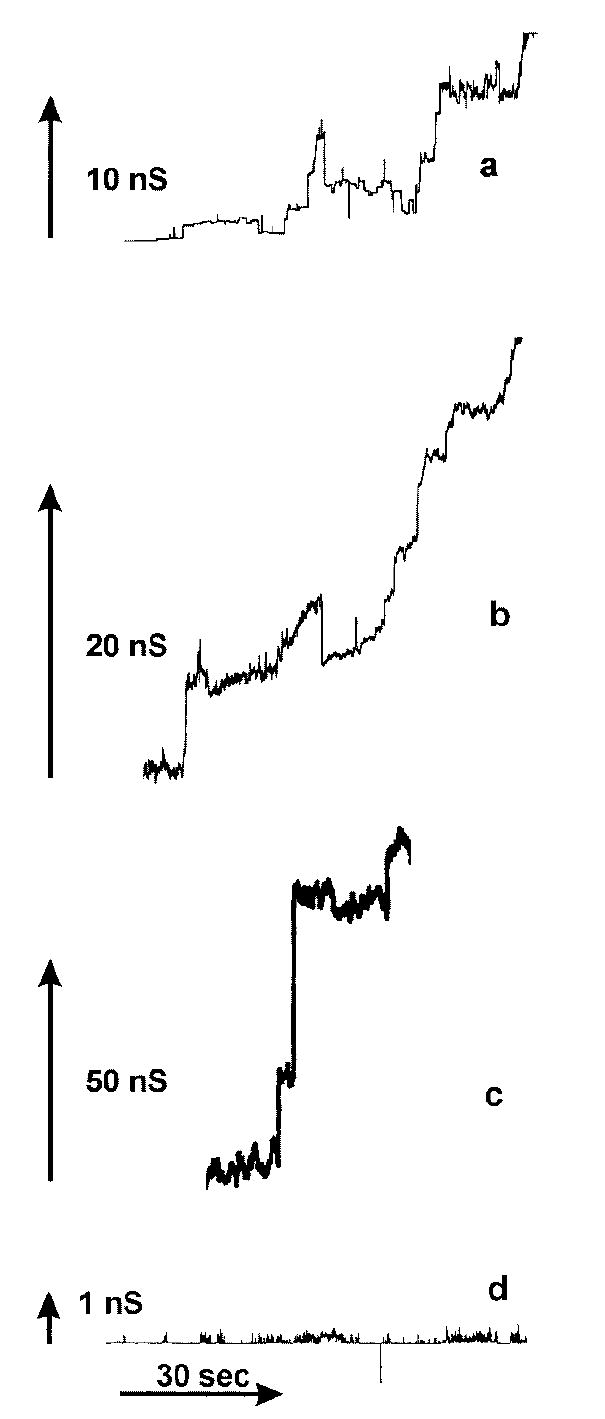
a–c, continuous current recordings of channel formation induced by the addition of 5 μm C2-ceramide to the aqueous solution, which consisted of 1 m KCl, 1 mm MgCl2, and 5 mm MES (pH 6). The applied voltage was clamped at 9 mV. d, current recordings in the presence of 120 μm C2-dihydroceramide. Conditions were as in a–c.
Fig. 2. Distribution of single channel conductances produced by 5 μm C2-ceramide.
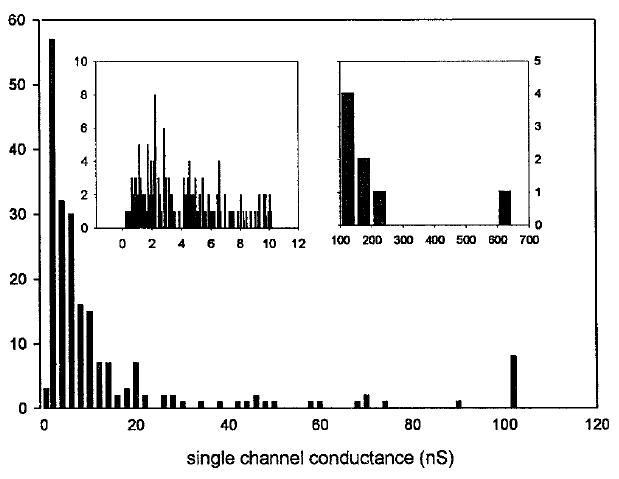
Data were compiled from four separate experiments using the conditions stated for Fig. 1, a–c. Only distinct vertical current increments were measured. The left and right insets are detailed distributions of channels with single channel conductances below 10 nS and greater than 100 nS, respectively.
The addition of C2-d-erythro-dihydroceramide to the aqueous phase, at concentrations up to 25 times greater than that of C2-ceramide, only resulted in a few, low conductive, transient pores (Fig. 1d). The rare stepwise current increases seen with dihydroceramide were up to 3 orders of magnitude smaller than those of C2-ceramide, and the current always returned to baseline.
A Structural Model For Ceramide Channel Formation
We propose that annuli of 5 or more ceramide molecules form a polar center, which can accommodate water and solutes (Fig. 3c). The annuli, stacked in register, should consist of 6 or 7 layers to form a pore that spans the 3- to 3.5-nm-thick hydrophobic portion of the membrane. The stacking of the annuli would be stabilized by intermolecular hydrogen bonds between amide nitrogens and carbonyl groups located on opposite surfaces of each ceramide molecule (see Fig. 3a for the structure of C2-ceramide). These are the same types of hydrogen bonds that stabilize the α helices and β-pleated sheets of proteins and are consistent with the observations of Pascher (29). These interactions can be thought of as forming columns of ceramide residues that span the membrane (Fig. 3b). Multiple columns are held together by hydrogen bonding via the hydroxyl groups proposed to line the channel lumen. Thus, a hydroxyl hydrogen-bonded network, not unlike the structure of ice, would line the wall of the aqueous pore. The number of ceramide columns formed (or the number of ceramides in the annulus) would therefore determine the pore size.
Fig. 3. Structural model for ceramide pore formation.
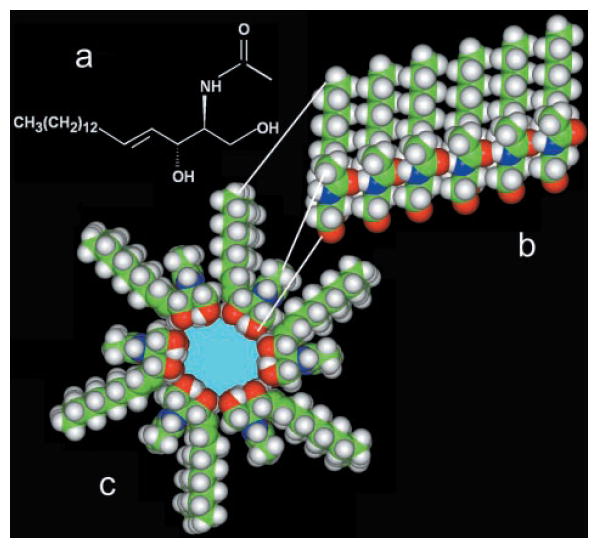
a, structure of a C2-ceramide molecule. b, a column of ceramide residues held together by intermolecular hydrogen bonds between amide and carbonyl groups. This column would span the hydrophobic portion of the membrane and, in association with other columns, would form pores of various sizes. c, cross-sectional view of a ceramide channel consisting of 6 columns of ceramide molecules. The columns are held together via intermolecular hydrogen bonds between hydroxyl groups along the channel lumen. The ceramide molecules form an annulus that encloses a polar inner space lined with hydroxyl groups. Note that the hydrocarbon chain of the sphingoid base backbone has been shortened to increase the image size of the polar region.
The nonapoptogenic precursor, C2-d-erythro-dihydroceramide, lacks the 4,5-trans double bond present in ceramide. Rotation about the 4,5 single bond in dihydroceramide could inhibit the formation of the large stable pore structures either by making these structures unstable or preventing their formation. Because of restricted rotation about the 4,5-trans double bond, the C3 hydroxyl group would be held in better position for participating in the hydrogen-bonding network proposed to line the channel lumen. Note that the secondary alcohol group on C3 of the sphingoid base backbone is an allyl alcohol and thus a particularly effective hydrogen-bond donor. This may also be the functional advantage of the double bond in ceramide and may explain the ineffectiveness of dihydroceramide.
A Mathematical Model Relates Overall Ceramide Concentration in the Membrane to Individual Channel Conductance and Overall Membrane Conductance
The proposed structure allows for a variable pore size depending on the number of ceramide molecules forming each channel. Therefore, the likelihood of forming channels of a particular size would strongly depend on the ceramide concentration in the membrane. Likewise, the overall conductance of the membrane, reflecting the number of channels that have formed, would also depend on the ceramide concentration in the membrane. As was observed, the conductance of newly formed channels would depend on the overall conductance of the membrane at that time. A simple theory puts these observations on a quantitative footing and yields interesting insights. The concentration of pores, made up of an i number of ceramide molecules ([Ci]), is related to the concentration of ceramide molecules in the membrane ([C]) as shown in Equation 1,
| (Eq. 1) |
where the constants(k1) and are for the formation of the first channel and the enlargement of the channel, respectively. The single channel conductance (gi) of a pore made up of an i number of ceramide molecules is represented in Equation 2.
| (Eq. 2) |
The total conductance of the membrane (GT) is the summation of the individual conductances of all the pores in the membrane,
| (Eq. 3) |
where n is the number of ceramides in the smallest channel, m is those in the largest, and A is the area of the membrane. Theoretical values for average single channel conductances for a range of total membrane conductances were generated using Equation 4,
| (Eq. 4) |
and were compared with the experimental observations (Fig. 4a). For both the theory and the data, as the total membrane conductance increases, the single channel conductance increases. In addition, the total concentration of ceramide in the membrane can be related to the number of monomers making up the channel and the total membrane conductance (Fig. 4b). Slight changes in concentration have dramatic effects on the size(s) of channels represented. For a given total membrane conductance range, there are distinct single channel conductances that are represented. At low membrane conductances and low concentrations of ceramide in the membrane, channels of smaller size are represented. Likewise, at higher total membrane conductances, there are higher concentrations of ceramide in the membrane, and larger channels are observed. This mathematical model is consistent with both the experimental observations and the structural model presented above.
Fig. 4. The average size of ceramide channels increased with increasing ceramide content in the membrane.
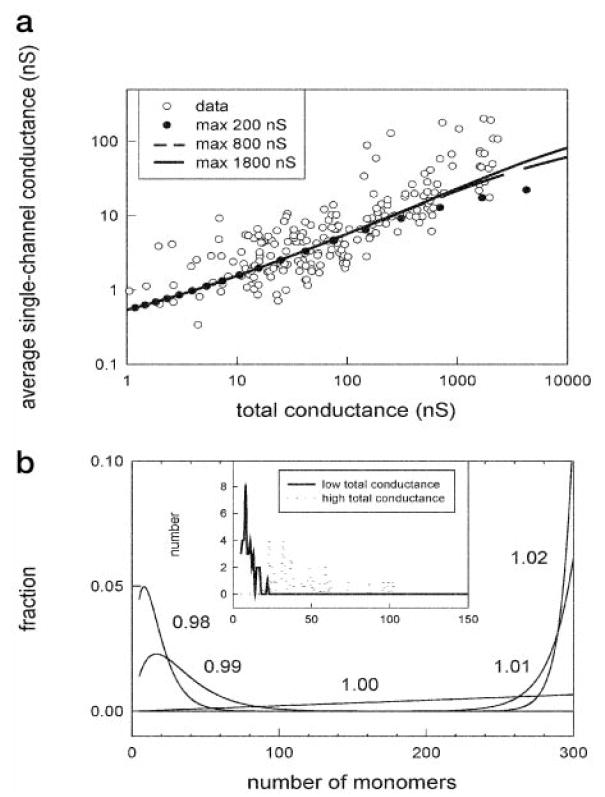
a, the average single channel conductance increased as the total conductance increases. The dots are individual experimental observations, and Equation 4 was used to generate the fitted lines. The size of the largest possible channel was chosen to be 200, 800, or 1800 nS. b, the main figure is a theoretical distribution of single-channel sizes as a function of ceramide monomer concentration in the membrane (numbers next to curves). The inset is a plot of the number of channels observed that would correspond to channels with the indicated number of monomers. The solid line represents the channels formed at low membrane conductance (bottom fifth), and the dotted line represents the channels formed at high conductance (top fifth).
C16-Ceramide, like C2-Ceramide, Forms Stable Channels in Planar Membranes
To determine the physiological significance of C2-ceramide channel formation, the naturally occurring long-chain C16-ceramide was tested for its effects on membrane conductance. When the membrane was made in the presence of 5 mol % (0.05% w/v) C16-ceramide, stable pore formation in the membrane was observed (Fig. 5a). C18-Dihydroceramide (lacking the 4,5-trans double bond) did not have any effects on membrane conductance (Fig. 5b). The known nonideal mixing of ceramides with phospholipids and the formation of ceramide-enriched microdomains may select for membranes with fewer channels and lower total membrane conductances, and therefore the histogram in Fig. 5c is probably biased toward lower channel conductances.
Fig. 5. C16-Ceramide forms channels in planar phospholipid membranes.
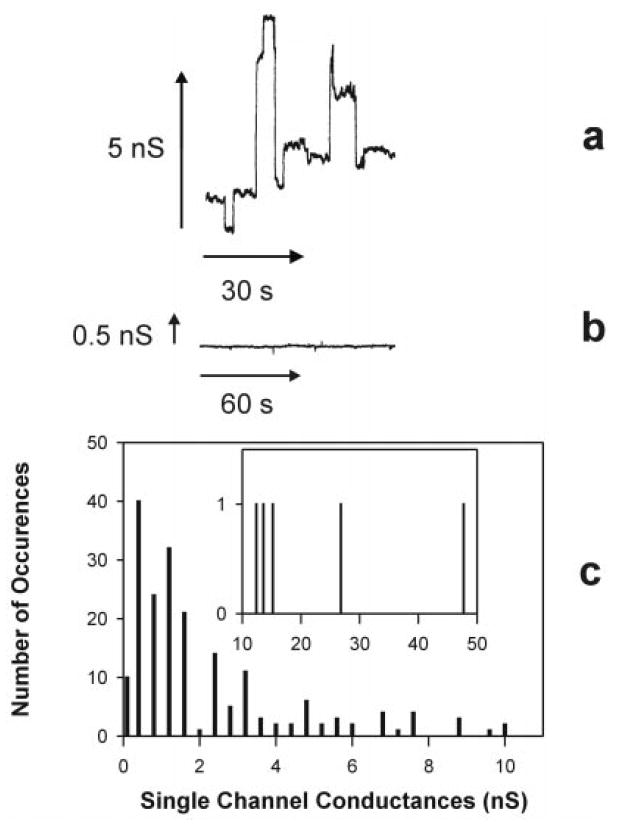
a, continuous current recording of channel formation induced by membranes made in the presence of 5 mol % C16-ceramide. The aqueous solution was identical to that used in C2-ceramide experiments. b, typical current recording from a membrane formed in the presence of 5 mol % C18-dihydroceramide. c, distribution of single channel conductances produced by membranes made in the presence of 5 mol % C16-ceramide.
DISCUSSION
The results of this paper provide evidence that both C2- and C16-ceramide form large stable pores in membranes, whereas the biologically inactive C2- and C18-dihydroceramides do not. The inability of other amphiphiles, including detergents, phospholipids, and products of phospholipase activity, to form stable channels may reside in their inability to form an extensive hydrogen-bonded network. In our model, hydrogen bonding stabilizes not only columns of ceramide molecules but also the polar ring that forms the wall of the aqueous pore.
Channel formation by C2- and C16-ceramides explains their ability to increase the permeability of membranes. The pores formed by both short- and long-chain ceramides could be directly or indirectly related to their apoptotic activity. According to the bulk properties of water, the pores with conductances ranging from 1 to 200 nS would have estimated diameters ranging from 0.8 to 11 nm, respectively. The voltage-dependent anion channel, located in the outer mitochondrial membrane, is considered to be a relatively large pore. In the high conductance state, it only has a single channel conductance of 4 nS (in the equivalent solution) and cannot allow the passage of cytochrome c (30). The larger ceramide pores, if formed in the outer mitochondrial membrane, would provide a pathway large enough for cytochrome c to pass. Alternatively, the ceramide pores could indirectly cause cytochrome c release through alterations in ion homeostasis (increased calcium levels alone are known to induce cytochrome c release) or through interactions with Bcl-2 family members. In addition, if ceramide pores formed in the inner mitochondrial membrane, they would dissipate Δψm and could accelerate electron transport, resulting in enhanced generation of reactive oxygen species. Results using C2- and C16-ceramides applied to mitochondrial suspensions indicated that only C2-ceramide was able to dissipate the inner mitochondrial membrane potential (16). It has been reported that the relative proportion of ceramide and phospholipids plays a large role in the formation of ceramide-enriched microdomains and the way in which ceramides perturb the structure of bilayers (26, 31). In addition, the 14 extra CH2 groups of C16-ceramide would make it much more difficult for the long-chain ceramides to move between the outer and inner mitochondrial membranes through the intervening aqueous phase. Using an estimated 3 kJ/mol/CH2 residue, the free energy difference between the hydrocarbon phase and water would increase by 42 kJ/mol, which translates to a change in partitioning of 3 × 107. Therefore, natural ceramides generated via de novo synthesis would exert their effects on local membrane fractions depending on the location of generation resulting in effects that would differ from those elicited by the more soluble ceramides.
Ceramide channels consist of hundreds of ceramide molecules, hence their formation and size would be exceedingly sensitive to the local ceramide concentration. García-Ruiz et al. (21) reported that mitochondria isolated from cells treated with tumor necrosis factor showed a 2–3-fold increase in mitochondrial ceramide concentrations as compared with control cells (21). They showed that this ceramide is not locally produced within mitochondria by action of sphingomyelinases acting on sphingomyelin, as incubation of isolated mitochondria with sphingomyelinases did not result in an increase in mitochondrial ceramide levels (21). Enzymes capable of both de novo synthesis (ceramide synthase) and hydrolysis (ceramidase) have been found in mitochondria (32, 33). Thus the dynamic channel formation and breakdown seen in vitro should also occur in vivo. These could increase or decrease local levels of ceramide, and even small changes could alter mitochondrial membrane permeability by channel formation or dissolution. Overall ceramide levels are known to increase during apoptosis, and thus regulation of the enzymes in the ceramide pathway is somehow involved in the apoptotic process. Further characterization of the pores formed by ceramide is underway and will hopefully provide answers to some of these questions.
Acknowledgments
We thank Dr. Jason Kahn for computer assistance with the structural models and Dr. Stephen Wolniak for reading of the manuscript.
Abbreviations
- C2-ceramide
N-acetyl-d-erythro-sphingosine
- C16-ceramide
N-hexadecyl-d-erythro-sphingosine
- C18-ceramide
N-octadecyl-d-erythro-sphingosine
- C2-dihydroceramide
N-acetyl-d-erythro-sphinganine
- C18-dihydroceramide
N-octadecyl-d-erythro-sphinganine
- MES
4-morpholineethanesulfonic acid
- nS
nanosiemens
References
- 1.Kolesnick RN, Krönke M. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 3.Ariga T, Jarvis WD, Yu RK. J Lipid Res. 1998;39:1–16. [PubMed] [Google Scholar]
- 4.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 5.Crompton M. Biochem J. 1999;342:233–249. [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G, Dallaporta B, Resche-Rigon M. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 7.Susin SA, Zamzami N, Kroemer G. Biochim Biophys Acta. 1998;1366:151–165. doi: 10.1016/s0005-2728(98)00110-8. [DOI] [PubMed] [Google Scholar]
- 8.Green DR, Reed JC. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 9.Narula J, Pandey P, Arbustini E, Haider N, Narula N, Kolodgie FD, Dal Bello B, Semigran MJ, Bielsa-Masdeu A, Dec GW, Israels S, Ballester M, Virmani R, Saxna S, Kharbanda S. Proc Natl Acad Sci U S A. 1999;96:8144–8149. doi: 10.1073/pnas.96.14.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsh T, Susin SA, Petit PX, Mignotte B, Kroemer G. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castedo M, Hirsch T, Susin SA, Zamzami N, Marchetti P, Macho A, Kroemer G. J Immunol. 1996;157:512–521. [PubMed] [Google Scholar]
- 12.Susin SA, Zamizami N, Castedo M, Daugas E, Wang EG, Geley S, Fassy F, Reed JC, Kroemer G. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Susin SA, Zamzami N, Larochette N, Dallaporta B, Marzo I, Brenner C, Hirsch T, Petit PX, Geuskens M, Kroemer G. Exp Cell Res. 1997;236:397–403. doi: 10.1006/excr.1997.3733. [DOI] [PubMed] [Google Scholar]
- 14.DeMaria R, Lenti L, Malisan F, d’Agostino F, Tomasini B, Zeuner A, Rippo MR, Testi R. Science. 1997;277:1652–1655. doi: 10.1126/science.277.5332.1652. [DOI] [PubMed] [Google Scholar]
- 15.Arora AS, Jones BJ, Patel TC, Bronk SF, Gores GJ. Hepatology. 1997;25:958–963. doi: 10.1002/hep.510250428. [DOI] [PubMed] [Google Scholar]
- 16.Di Paola M, Cocco T, Lorusso M. Biochemistry. 2000;39:6620–6628. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 17.Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. J Biol Chem. 1999;274:6080–6084. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Proc Natl Acad Sci U S A. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottschalk A, Boise L, Thompson C, Quintáns J. Proc Natl Acad Sci U S A. 1994;91:7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiesner DA, Kilkus JP, Gottschalk AR, Quintáns J, Dawson G. J Biol Chem. 1997;272:9868–9876. doi: 10.1074/jbc.272.15.9868. [DOI] [PubMed] [Google Scholar]
- 21.García-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 22.Quillet-Mary A, Jaffrézou J, Mansat V, Bordier C, Naval J, Laurent G. J Biol Chem. 1997;272:21388–21395. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 23.Begona Ruiz-Arguello M, Basanez G, Goni FM, Alonoso A. J Biol Chem. 1996;271:26616–26621. doi: 10.1074/jbc.271.43.26616. [DOI] [PubMed] [Google Scholar]
- 24.Begona Ruiz-Arguello M, Goni FM, Alonoso A. J Biol Chem. 1998;273:22977–22982. doi: 10.1074/jbc.273.36.22977. [DOI] [PubMed] [Google Scholar]
- 25.Holopainen JM, Angelova MI, Kinnunen PKJ. Biophys J. 2000;78:830–838. doi: 10.1016/S0006-3495(00)76640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veiga MP, Arrondo JLR, Goni FM, Alonso A. Biophys J. 1999;76:342–350. doi: 10.1016/S0006-3495(99)77201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montal M, Mueller P. Proc Natl Acad Sci U S A. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombini M. J Membr Biol. 1989;111:103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- 29.Pascher I. Biochim Biophys Acta. 1976;455:433–451. doi: 10.1016/0005-2736(76)90316-3. [DOI] [PubMed] [Google Scholar]
- 30.Colombini M. Methods Enzymol. 1987;148:465–475. doi: 10.1016/0076-6879(87)48045-2. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, Goldberg EM, Zidovetzki R. Eur Biophys J. 1998;27:361–366. doi: 10.1007/s002490050143. [DOI] [PubMed] [Google Scholar]
- 32.Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T. Lipids. 1998;33:601–605. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- 33.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. J Biol Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]


