Abstract
Recent studies suggest that a bronchial-derived relaxing factor (BrDRF) decreases the contractility of newborn, but not fetal, rat pulmonary arteries (PAs) by a nitric oxide (NO)-mediated mechanism. We studied the effect of an adjacent bronchus on PA contractility to norepinephrine (NE) in late-gestation fetal (n = 7), neonatal (1 day old, n = 9), ventilated neonatal (24-h ventilation from birth with 100% oxygen, n = 9), and adult sheep (n = 6) in the presence and absence of the NO synthase inhibitor Nω-nitro-L-arginine (L-NNA). The sheep were anesthetized and killed, and fifth-generation PA rings with and without an attached adjacent bronchus (PA+Br) were contracted in standard tissue baths with NE (10−8–10−6 M). NE contractions were expressed as fraction of KCl (118 mM) contraction and as grams of contraction force. NE contractions were significantly diminished by the presence of an attached bronchus in the neonatal and ventilated neonatal and adult, but not fetal, lambs. Hyperoxic ventilation markedly increased NE contractions in PA and PA+Br. L-NNA significantly enhanced NE contractions in PA+Br in postnatal but not in fetal lambs. Pretreatment with L-NNA abolished the difference between NE contractions in PA and PA+Br in neonatal but not in hyperoxic ventilated neonatal lambs. We conclude that there is a BrDRF that is developmentally regulated and has vascular activity postnatally but not during fetal life. The effect of BrDRF is predominantly mediated by NO in air-breathing neonatal lambs but may involve a second non-NO mediator following hyperoxic ventilation. We speculate that BrDRF may have an important role in postnatal changes in pulmonary arterial reactivity.
Keywords: nitric oxide, pulmonary vascular resistance, developmental changes
Pulmonary vascular resistance is high during fetal life, and only 8–10% of combined ventricular output enters the pulmonary circulation (10). Successful transition at birth results in a dramatic reduction in pulmonary vascular resistance, with an 8- to 10-fold increase in pulmonary blood flow. Inhibition of nitric oxide (NO) synthase (NOS) impairs this transition, suggesting an important role for NO in the pulmonary circulatory changes at birth (1, 7, 11, 12).
Belik et al. recently reported the presence of a bronchial-derived relaxing factor (BrDRF) that reduced pulmonary artery (PA) force generation in newborn rats (4) and that this factor is either absent or not active in the fetus (3). This factor mediates its effect by stimulating NO production, as inhibition of NOS blocks the effect of this factor on the adjacent PA (4). Similar effects of airway epithelium influencing adjacent arterial tone have been reported by others (5, 6). Our laboratory has recently reported that a similar factor exists in juvenile lambs (8). The ontogeny of the bronchial effect on adjacent PA contractility has not been studied in larger mammals such as lambs, whose pulmonary developmental physiology and weight correspond more closely to those of humans.
We hypothesized that, similar to rodents, BrDRF is either absent or inactive in fetal ovine pulmonary circulation, contributes to high pulmonary vascular resistance during this period, and appears or is activated in the postnatal period. We further hypothesized that ventilation under hyperoxic conditions would activate this factor. We, therefore, determined the developmental changes in the effect of an adjacent bronchus on PA contractility in fetal, neonatal, and adult sheep. We also studied the effect of hyperoxic ventilation on BrDRF in neonatal sheep. Because this factor appears to mediate its effect on adjacent PA in a NO-dependent mechanism (4), we studied the effect of NOS inhibitor on norepinephrine (NE)-induced contractile responses in PAs, with and without an adjacent bronchus.
METHODS
The State University of New York at Buffalo Laboratory Animal Care Committee approved this study. Thirty-one sheep in the following age groups were studied: 1) near-term fetal lambs (135–136 days; term being 146–150 days) (n = 7); 2) neonatal lambs delivered at the farm (24–48 h old) (neonatal group; n = 9); 3) neonatal lambs delivered by cesarean section and ventilated with 100% oxygen for 24 h (neonatal ventilated group, n = 9); and 4) adult sheep (n = 6): four postpartum ewes (7–11 days following delivery or abortion) and two adult rams.
Near-term gestation pregnant ewes were anesthetized with pentothal sodium (750 mg iv) and halothane (2% inhalation), and the fetus was delivered by cesarean section. The fetus was killed before the first breath by rapid exsanguination through a cardiac puncture under anesthesia. The postpartum ewes and adult rams were killed with an intravenous injection of Fatal Plus (Vortech Pharmaceuticals, Dearborn, MI; pentobarbital 390 mg/ml; dose: 1 ml/10 lb. body wt). Neonatal lambs were anesthetized with pentothal sodium and exsanguinated as described above.
Near-term lambs in the “neonatal ventilated” group (135–139 days gestation) were exteriorized by cesarean section. A small incision was made in the neck, and systemic arterial and venous access was established through the carotid artery and jugular vein, respectively. Lambs were intubated and then delivered and ventilated. Lambs were sedated with fentanyl (2 mg · kg−1 · dose−1 every 2 h as needed) and received an initial dose of pancuronium bromide (0.1 mg · kg−1 · dose−1) at birth, which was repeated only if necessary for vigorous spontaneous movement, despite adequate sedation. They were placed under servo-controlled radiant warmers, and rectal temperature was maintained between 37.9 and 39°C (normal temperature for lambs). Intravenous fluids (dextrose 10% solution with 25 meq of sodium chloride, 20 meq of potassium chloride, and 10 meq of sodium bicarbonate per liter) were administered continuously at 100 ml · kg−1 · day−1. Fluid composition and rate were adjusted based on serum electrolyte values. The lambs were ventilated with Servo 300 ventilators (Seimens, Mississauga, ON, Canada) with the following settings: positive end-expiratory pressure, 4 cm H2O; rate, 60 breaths/min; peak inspiratory pressure: ~25 cm H2O (adjusted to deliver 10 ml/kg tidal volume using a BiCore CP-100 Monitor, BiCore Monitoring Systems, Irvine, CA); and 100% oxygen. Arterial blood gases were monitored frequently (every 5–15 min) during initial stabilization. Ventilator settings (peak inspiratory pressure and rate) were adjusted to maintain arterial PCO2 between 35 and 50 Torr. A neonatologist, neonatal fellow, neonatal nurse practitioner, or neonatal nurse from the Women and Children’s Hospital of Buffalo provided continuous care for the lambs. After 24 h of ventilation, lambs were anesthetized with pentothal sodium and killed by rapid exsanguination through a direct cardiac puncture.
Organ bath studies
The heart and lungs were removed en bloc, and fifth-generation PAs (inner diameter of ~500 μm in fetal and neonatal lambs and 1–2 mm in adult sheep) were dissected, isolated, and cut into rings as previously described (15). Some rings were carefully dissected with an attached bronchus (PA+Br). Rings were suspended in water-jacketed chambers filled with aerated (94% O2–6% CO2) modified Krebs-Ringer solution (in mM: 118 sodium chloride, 4.7 potassium chloride, 2.5 calcium chloride, 1.2 magnesium sulfate, 1.2 potassium biphosphate, 25.5 sodium bicarbonate, and 5.6 glucose). A continuous recording of isometric force generation was obtained by tying each vessel ring to a force displacement transducer (model UC2, Statham Instruments, Hato Rey, PR) that was connected to a recorder (Gould Instrument Systems, Valley View, OH). After the arterial rings were mounted, they were allowed to equilibrate for 20 min in the bathing solution. A micrometer was used to stretch the tissues repeatedly in small increments over the following 45 min until resting tone remained stable at a passive tension of 0.8 g. Preliminary experiments determined that this procedure provided optimal length for generation of active tone to exogenous NE.
Materials
The following pharmacological agents were used: DL-propranolol, NE hydrochloride, and NOS antagonist Nω-nitro-L-arginine (L-NNA). All drugs were obtained from Sigma-Aldrich (St. Louis, MO). L-NNA was dissolved in warmed Kreb’s solution. All other drugs were dissolved in distilled water. Experiments were performed in a dark room, as L-NNA is sensitive to light.
Protocols
Isolated PAs were pretreated with propranolol (10−6 M) to block β-adrenergic receptors. Some arteries were pretreated with L-NNA (10−3 M). In PA with attached bronchus, both PA and the bronchus were pretreated with L-NNA. Following these pretreatments, the vessels were contracted with increasing doses of NE (10−8 to 10−6 M). The arteries were then washed and, when their tone returned to baseline, contracted with 118 mM of potassium chloride. Contraction responses to NE were recorded as grams of force and were normalized as a fraction of the response to 118 mM of potassium chloride and to PA tissue weight. PA rings were blotted dry on tissue paper and weighed. In PA rings with attached bronchi, the bronchus was dissected and removed, and the PA rings were weighed.
Statistical analysis
All data are expressed as means ± SE, with n representing the number of animals studied. Two to four vessels were studied in each protocol from an animal and then averaged. Statistical comparisons of the curves were performed with factorial or repeated-measures ANOVA, as appropriate. Fisher’s protected least significant difference post hoc testing was used as needed to compare multiple groups. All statistical analysis was performed with StatView software (Abacus Concepts, Berkley, CA). Significance was accepted at P < 0.05.
RESULTS
We studied NE-induced contraction in fetal PA, with and without attached bronchi. NE contracted fetal PA in a concentration-dependent manner (Fig. 1). The presence of an adjacent bronchus did not alter NE-induced contraction. Pretreatment with L-NNA did not enhance the contraction to NE in PA with attached bronchus. L-NNA enhanced contractions to NE in PA alone at 10−8 and 10−6 M concentrations.
Fig. 1.
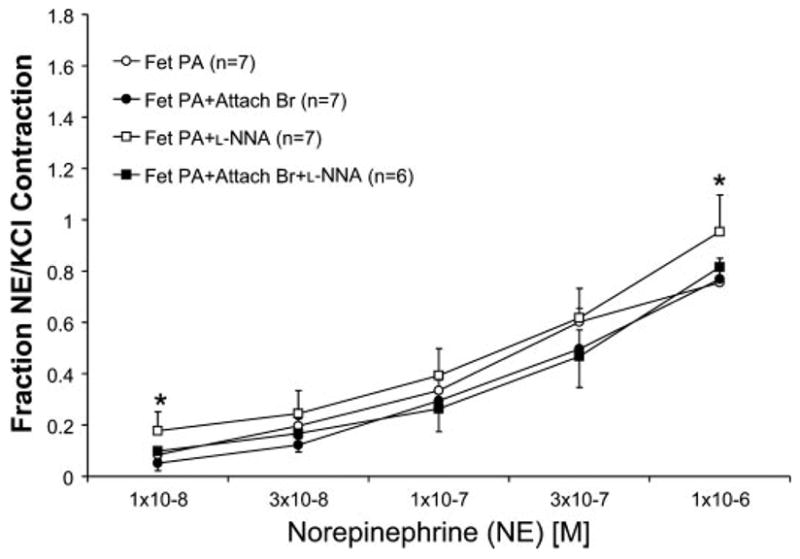
Concentration-response curves of fetal (Fet) ovine pulmonary arteries (PAs) with and without an attached bronchus (PA+Br) to norepinephrine (NE, 10−8 to 10−6 M). Some tissues were pretreated with a nitric oxide synthase (NOS) inhibitor Nω-nitro-L-arginine (L-NNA). Data are represented as means ± SE of a fraction of NE contraction of the PA to contraction obtained with 118 mM of KCl. All tissues were pretreated with propranolol (10−6 M). n, No. of lambs. *P < 0.05 compared with PA alone by Fisher’s protected least significant difference (PLSD) for that concentration of NE only.
Next, we evaluated PA with and without bronchi isolated from 24- to 48-h-old air-breathing neonatal lambs following spontaneous delivery at the farm. In contrast with fetal PA, the presence of an adjacent bronchus impaired contractions to NE in neonatal PA. Also, in contrast to fetal PA, pretreatment with L-NNA significantly enhanced contractions to NE in both PA and PA with attached bronchus (Fig. 2). The contractions to NE following L-NNA pretreatment were not significantly different between PA with and without an attached bronchus.
Fig. 2.

Concentration-response curves of 24- to 48-h-old neonatal ovine PAs, with and without an attached bronchus (PA+Br) to NE (10−8 to 10−6 M). Some tissues were pretreated with L-NNA, a NOS inhibitor. Data are presented as means ± SE of a fraction of NE contraction of the PA to contraction obtained with 118 mM of KCl. All tissues were pretreated with propranolol (10−6 M). n, No. of lambs. **P < 0.001 by ANOVA repeated measures compared with corresponding vessel without L-NNA. †P < 0.05 compared with PA without attached bronchus.
Following ventilation with 100% oxygen for 24 h (neonatal ventilated group), contraction in PA was greater than those in PA with attached bronchus (Fig. 3). In fact, the contraction response of PA to 10−6 M of NE was greater than that to 118 mM of KCl. Pretreatment with L-NNA resulted in increased contraction in PA with attached bronchus (Fig. 3). L-NNA did not significantly enhance contractions in PA alone. In sharp contrast to air-breathing neonatal lambs, the NE contractions following L-NNA pretreatment in PA were significantly increased compared with PA with attached bronchus at 10−7 and to 10−6 M of NE concentrations.
Fig. 3.

Concentration-response curves of PAs with and without an attached bronchus (PA+Br) to NE (10−8 to 10−6 M) isolated from 1-day-old neonatal lambs ventilated with 100% oxygen since birth (Vent). Some tissues were pretreated with a NOS inhibitor, L-NNA. Data are presented as means ± SE of a fraction of NE contraction of the PA to contraction obtained with 118 mM of KCl. All tissues were pretreated with propranolol (10−6 M). n, No. of lambs. *P < 0.05 by ANOVA repeated measures compared with corresponding vessel without L-NNA. †P < 0.05 compared with PA without attached bronchus. ‡P < 0.05 by ANOVA with Fisher’s PLSD post hoc test compared with PA+Attach Br+L-NNA for that concentration of NE only.
In adult sheep, the presence of an attached bronchus decreased contractions to NE (Fig. 4). Pretreatment with L-NNA did not enhance contractions to NE in adult PA with and without attached bronchus.
Fig. 4.

Concentration-response curves of PAs with and without an attached bronchus (+Br) to NE (10−8 to 10−6 M) isolated from adult sheep. Some tissues were pretreated with L-NNA, a NOS inhibitor. Data are represented as means ± SE of the fraction of NE contraction of the PA to contraction obtained with 118 mM of KCl. All tissues were pretreated with propranolol (10−6 M). n, No. of sheep. †P < 0.05 by Fisher’s PLSD for 10−6 M concentration only.
Comparative data from all of the age groups studied are presented in Fig. 5. In all postnatal age groups, the presence of an attached bronchus significantly decreased contraction responses to NE (10−6 M), whether expressed as a fraction of response to potassium chloride (Fig. 5A) or as grams of force contraction per gram of PA weight (Fig. 5B). Ventilation with 100% oxygen significantly enhanced contraction response to NE in PA without attached bronchus (Fig. 5) compared with neonatal PA. Oxygen ventilation significantly increased the contraction response in PA with attached bronchus when expressed as a fraction of KCl contraction (Fig. 5A) but not when expressed as grams per gram force (Fig. 5B).
Fig. 5.
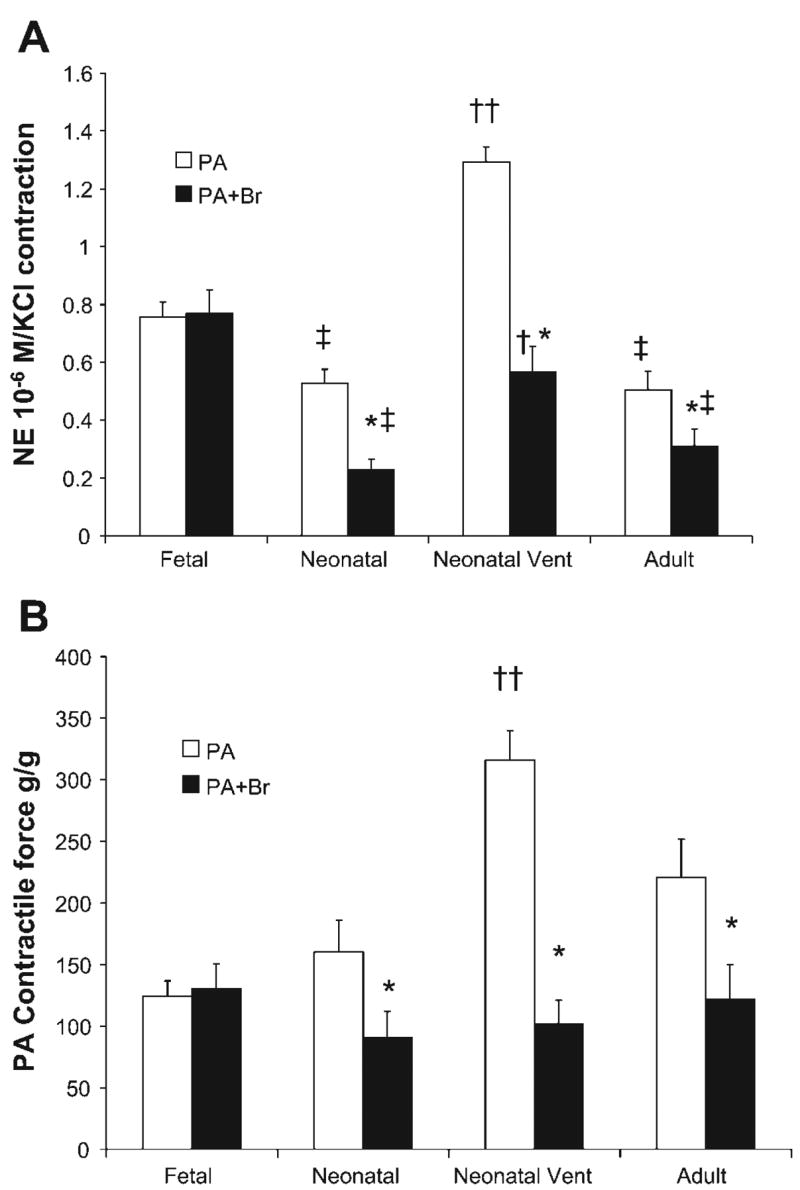
Developmental changes in PA contractility to NE in fetal, 24- to 48-h-old newborn (Neonatal), 24-h-old neonatal lambs ventilated with 100% oxygen from birth (Neonatal Vent), and adult sheep (Adult). Data are presented as contractions to 10−6 M of NE as a fraction of contraction to 118 mM of KCl (A) or as grams of force/gram PA tissue weight (B). In case of PA+Br, the PA was dissected out and weighed for the g/g force calculation. *P < 0.05 compared with PA alone from the same age group. †P < 0.05 and ††P < 0.01 compared with neonatal group. ‡P < 0.05 compared with corresponding vessel in the fetal group.
The contractile responses to NE (10−6 M) in neonatal lambs breathing room air and following hyperoxic ventilation in the presence and absence of an attached bronchus, with and without pretreatment with L-NNA, is presented in Fig. 6. Ventilation with 100% oxygen induces a 2.5-fold enhancement in NE contraction in both PA and PA with attached bronchus. Hyperoxic ventilation does not enhance contraction in PA with attached bronchus pretreated with L-NNA.
Fig. 6.

Effect of hyperoxic ventilation on PA contractility to NE in the presence and absence of an attached bronchus (PA+Br) and with and without pretreatment with L-NNA (10−3 M). Open bars indicate neonatal lambs delivered at the farm and spontaneously breathing room air (Neonatal). Solid bars indicate lambs delivered by cesarean section and ventilated with 100% oxygen for 24 h (Neonatal Vent). †††P < 0.0001, ††P < 0.005, and †P < 0.05 compared with corresponding vessel in the neonatal group. ***P < 0.0001 and *P < 0.05 compared with corresponding vessel without L-NNA pretreatment. ‡ ‡ ‡P < 0.0001, ‡ ‡P < 0.001, and ‡P < 0.05 compared with corresponding PA without bronchus.
DISCUSSION
We found developmental differences in the effect of an adjacent bronchus on PA contractility to NE in the fetus, neonate, and adult sheep. This BrDRF reduces PA contractility in the postnatal period but not in the fetus, suggesting that it may play an important role in modulation of pulmonary vascular reactivity in the postnatal period.
We chose to present contraction responses to NE as a fraction of contraction response to 118 mM of potassium chloride. This form of normalization was chosen because of subtle differences in the PA weight and surface area between PA alone and PA isolated from PA+Br tissues. We also analyzed contraction data normalized to PA weight (in case of PA with an attached bronchus, the bronchus was removed and PA segment alone was weighed; Fig. 5B) and also to PA surface area (data not shown). Data were similar with all forms of normalization in postnatal lambs and adult sheep.
In fetal lambs, the presence of an adjacent bronchus did not decrease the PA contractile response to NE. These data indicate that the BrDRF either is not produced or is ineffective, in an age group with high pulmonary vascular resistance. Pretreatment with L-NNA significantly enhanced the contractile response in PA alone, but not in PA with attached bronchus, providing further support for lack of this factor during fetal life (Fig. 1).
In contrast, in neonatal lambs, an attached bronchus significantly diminished contractile responses to NE in PA (Fig. 2). Pretreatment with L-NNA enhanced contractions in PA with attached bronchus. These results indicate that BrDRF is produced and has vascular activity in the immediate postnatal period. L-NNA also markedly enhanced responses in PA, with or without an attached bronchus, and there was no significant difference in NE-induced contractions between L-NNA-treated PA and PA with attached bronchus. We speculate that decreased contraction noted in PA with attached bronchus is secondary to NO-dependent BrDRF, and this difference is abolished by pretreatment with L-NNA. This provides indirect evidence that NO is the predominant mediator of BrDRF in neonatal lambs breathing air.
We recently reported that ventilation with 100% oxygen for 24 h significantly increases PA contractility in neonatal lambs (9). Interestingly, the presence of an adjacent bronchus markedly decreased contractions to NE in these PA, and pretreatment with L-NNA partly prevented this decrease (Fig. 3). These results indicate that high oxygen exposure does not alter release of BrDRF. The reverse phenomenon, suppression of BrDRF with postnatal hypoxia, has been reported recently (2). Despite pretreatment with L-NNA, the difference between contraction in PA and PA with attached bronchus persists following hyperoxic ventilation (Figs. 3 and 6). This finding is in stark contrast to that observed in room air-breathing neonatal lambs. This suggests that, although BrDRF is functional following hyperoxic ventilation, its effect is no longer predominantly mediated by NO. A second non-NO mediator appears to play a role in mediating the effects of BrDRF following hyperoxic ventilation.
The effect of pretreatment with L-NNA on NE-induced PA contraction in the fetal, neonatal, and neonatal lambs exposed to hyperoxia merits further attention. Pretreatment with L-NNA minimally enhanced the contractile response to NE in fetal PA (Fig. 1), suggesting low levels of NOS activity. Following spontaneous breathing for 24–48 h, we observed a significant enhancement of PA contraction following L-NNA pretreatment (Fig. 2), indicating activation of NOS. In contrast, following ventilation with 100% oxygen for 24 h, pretreatment with L-NNA failed to increase NE-induced contraction significantly in PA (Fig. 3), indicating decreased NOS activity compared with spontaneously room air-breathing lambs. We recently reported that endothelial NOS (eNOS) protein levels in fifth-generation PA are low in fetal lambs but increase significantly 24–48 h after spontaneous delivery. In sharp contrast, following ventilation with 100% oxygen for 24 h, eNOS protein levels in PA remained low, similar to fetal lambs (13). Thus hyperoxic ventilation appears to selectively inhibit eNOS in neonatal PA without impairing production or activity of BrDRF. However, pretreatment with L-NNA abolishes the effect of an attached bronchus in air-breathing neonatal lambs but not in hyperoxic ventilated lambs. As mentioned earlier, this suggests that NO is no longer the only mediator of bronchial relaxing effect following hyperoxic ventilation.
We acknowledge the presence of several drawbacks in this study. The fetal studies were conducted in 135- to 136-day gestation near-term lambs delivered by cesarean section. The neonatal lambs delivered at the farm are usually at 145- to 150-day gestation and have gone through the process of labor and spontaneous delivery. This difference in maturation and the presence of labor may have contributed to the change in response to adjacent bronchus. However, the ventilation studies were done at the same gestation as the fetal studies and showed a dramatic change in the effect of an adjacent bronchus. Second, many laboratories (including ours) that study isolated vessels in conventional tissue baths use a 94% oxygen and 6% CO2 gas mixture (14, 16, 17). The PO2 of the buffer solution bathing the tissue is close to 500 Torr, and PCO2 is ~40 Torr. This approach is based on the assumption that the inner core of smooth muscle cells in the vessel may be relatively hypoxic because of lack of perfusion through the vasa vasorum in the isolated vessel bath. We acknowledge that the oxygen environment of our vessels may have influenced some of our results. Third, the presence of an attached bronchus can mechanically interfere with contraction response of the PA. We consider this possibility unlikely, because of the limited area (usually <20% of the circumference of PA ring) of attachment. Also, we used potassium chloride contraction generated by the PA and bronchus together to normalize contraction responses to NE. The size of the vessels studied varied in diameter between ~500 mm in fetuses to 1–2 mm in adult sheep. These vessels, especially in adult sheep, may not represent true resistance arteries. Finally, to study adult sheep, we included postpartum (7–11 days following delivery or abortion) ewes. The altered hormonal milieu of pregnancy and postpartum period may have influenced our results. However, results obtained from adult rams were similar to those of postpartum ewes.
In summary, we have shown that the bronchial modulation of PA reactivity is developmentally regulated, is effective in the postnatal period but not during fetal life, and remains effective after 24 h of hyperoxia-ventilation exposure, but may no longer be entirely NO dependent. We speculate that BrDRF may be involved in postnatal adaptation of pulmonary circulation in larger mammals, similar to that in rodents (3).
Acknowledgments
GRANTS
This study was funded in part by National Heart, Lung, and Blood Institute Grant HL-54705 (R. H. Steinhorn) and American Academy of Pediatrics/Neonatal Resuscitation Program Grant 1040244 (V. H Kumar).
References
- 1.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol Heart Circ Physiol. 1990;259:H1921–H1927. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 2.Belik J, Pan J, Jankov RP. Airway modulation of pulmonary arterial muscle tone in a newborn rat model of chronic hypoxia-mediated pulmonary hypertension (Abstract) PAS. 2005;57:84. [Google Scholar]
- 3.Belik J, Pan J, Jankov RP, Tanswell AK. Bronchial epithelium-associated pulmonary arterial muscle relaxation in the rat is absent in the fetus and suppressed by postnatal hypoxia. Am J Physiol Lung Cell Mol Physiol. 2005;288:L384–L389. doi: 10.1152/ajplung.00309.2004. [DOI] [PubMed] [Google Scholar]
- 4.Belik J, Pan J, Jankov RP, Tanswell AK. A bronchial epithelium-derived factor reduces pulmonary vascular tone in the newborn rat. J Appl Physiol. 2004;96:1399–1405. doi: 10.1152/japplphysiol.01004.2003. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes LB, Paterson JW, Goldie RG. Co-axial bioassay of a smooth muscle relaxant factor released from guinea-pig tracheal epithelium. Br J Pharmacol. 1989;96:117–124. doi: 10.1111/j.1476-5381.1989.tb11791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes LB, Preuss JM, Paterson JW, Goldie RG. Epithelium-derived inhibitory factor in human bronchus. Eur J Pharmacol. 1990;187:331–336. doi: 10.1016/0014-2999(90)90360-i. [DOI] [PubMed] [Google Scholar]
- 7.Fineman JR, Wong J, Morin FC, Wild LM, 3rd, Soifer SJ. Chronic nitric oxide inhibition in utero produces persistent pulmonary hypertension in newborn lambs. J Clin Invest. 1994;93:2675–2683. doi: 10.1172/JCI117281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshminrusimha S, Gugino SF, Russell JA, Mathew B, Kumar VH, Ryan RM, Morin FC., 3rd Pulmonary arterial vasoreactivity is decreased by the adjacent bronchus in juvenile lambs (Abstract) Proc Am Thorac Soc. 2005;2:A711. [Google Scholar]
- 9.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, Swartz DD, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2005;59:137–141. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol. 1999;26:601–619. [PubMed] [Google Scholar]
- 11.Rairigh RL, Parker TA, Ivy DD, Kinsella JP, Fan ID, Abman SH. Role of inducible nitric oxide synthase in the pulmonary vascular response to birth-related stimuli in the ovine fetus. Circ Res. 2001;88:721–726. doi: 10.1161/hh0701.088683. [DOI] [PubMed] [Google Scholar]
- 12.Rairigh RL, Storme L, Parker TA, Le Cras TD, Markham N, Jakkula M, Abman SH. Role of neuronal nitric oxide synthase in regulation of vascular and ductus arteriosus tone in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2000;278:L105–L110. doi: 10.1152/ajplung.2000.278.1.L105. [DOI] [PubMed] [Google Scholar]
- 13.Reda WJ, Wedgwood S, DeVol JM, Lakshminrusimha S, Russell JA, Gugino SF, Porta NF, Steinhorn RH. Inhaled nitric oxide restores eNOS protein levels in PPHN lambs (Abstract) PAS. 2005;57:2527. [Google Scholar]
- 14.Schindler MB, Hislop AA, Haworth SG. Postnatal changes in response to norepinephrine in the normal and pulmonary hypertensive lung. Am J Respir Crit Care Med. 2004;170:641–646. doi: 10.1164/rccm.200311-1551OC. [DOI] [PubMed] [Google Scholar]
- 15.Steinhorn RH, Morin FC, Gugino SF, 3rd, Giese EC, Russell JA. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol Heart Circ Physiol. 1993;264:H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- 16.Steinhorn RH, Morin FC, Gugino SF, Giese EC, Russell JA. Developmental differences in endothelium-dependent responses in isolated ovine pulmonary arteries and veins. Am J Physiol Heart Circ Physiol. 1993;264:H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- 17.Villamor E, Kessels CG, Fischer MA, Bast A, de Mey JG, Blanco CE. Role of superoxide anion on basal and stimulated nitric oxide activity in neonatal piglet pulmonary vessels. Pediatr Res. 2003;54:372–381. doi: 10.1203/01.PDR.0000077481.15081.C8. [DOI] [PubMed] [Google Scholar]


