Abstract
The early secretory pathway (ESP) consisting of the endoplasmic reticulum (ER), pre-Golgi intermediates and the Golgi stack links protein synthesis to folding and vesicle trafficking to generate the membrane architecture of the eukaryotic cell. The fundamental principles that contribute to organization of the ESP remain largely unknown. We raise the possibility that assembly of the ESP is largely built on a foundation that is influenced by the kinetic and thermodynamic properties of the protein fold. Folding energetics may provide an adjustable platform for adaptor-dependent interactions with the transport machinery, suggesting the possibility that protein cargo energetics plays a central role in directing both trafficking patterns and global compartmental organization of the ESP. In this view, cargo energetics likely coordinates the composition and maturation of ER and Golgi compartments with the physiological state of the cell in different tissue and environmental settings.
Introduction
It is now appreciated that the early secretory pathway (ESP) is defined by a series of sequential and hierarchal protein interactions that direct membrane trafficking patterns from the endoplasmic reticulum (ER) to and through Golgi compartments. The ESP can be defined by its capacity to link ER-associated protein folding (ERAF) pathways [1••] to both degradative (ER-associated degradation, or ERAD [2]) and vesicle-based trafficking pathways involving coat (COPII and COPI) and tethering/fusion (SNARE) machineries (referred to as the membrome [3•]). These folding and trafficking pathways dictate the fate of nearly one-third of proteins encoded by the eukaryotic genome, and are used to build the strikingly diverse membrane architectures of eukaryotic cells.
Critical to understanding organization of the ESP is to define unifying molecular and biochemical features that could direct protein trafficking and thereby establish compartment function. The starting point for the ESP is the ER. Here, several lines of evidence support the view that the vast majority of export decisions are defined by interactions between newly synthesized protein cargo and adaptors that mediate coupling to a specialized scaffolding machine, the COPII vesicle coat system that directs ER export [4•] Adaptor interactions are dictated by the energetics of the protein fold [1••,5] and the biology and chemistry of the local folding environment [6••]. We use the phrase ‘folding energetics’ as a catch-all to mean the combination of folding kinetics and thermodynamics, that is the kinetic and thermodynamic properties of the protein fold, as a measure of how fast a protein folds (kinetics) and how stable it is once folded (thermodynamics). These are described as folding energetics because they are both dictated by energies: the kinetics by the energy of the transition state relative to the folded and unfolded states (which determines the rate of folding) and thermodynamics by the energy of the folded state relative to the unfolded state (which determines the population of the unfolded and folded states). The link between the cargo fold and adaptor function raises the possibility that the combination of folding energetics and the local environment particular to each cell type may manifest together a variable, rather than a fixed, energetic profile that defines the unique structures and composition of the pre-Golgi and Golgi compartments in different cell types.
Herein, we highlight three potential integrating features of membrane trafficking through the ESP. These include (1) protein folding energetics, (2) the local folding environment and (3) adaptor recognition. Together they help explain the considerable variability found in the operation of ER to Golgi trafficking systems for both maintaining and evolving eukaryotic cell function.
Protein cargo—a foundation for global cellular architecture
The ESP encompassing the ER, pre-Golgi intermediates and Golgi compartments has complex morphologies that differ considerably between cell types (Box 1). The underlying difference reflects in large part the type of protein cargo generated by the ribosome mediated co-translational insertion of protein into the ER - including both lumenal and transmembrane anchored cargo - in response to developmental signaling pathways. Two types of cargo dominate the ESP. One group (class I) is the ‘resident’ machinery that includes, for example, protein folding chaperones found in the ER or glycan processing enzymes found in the Golgi in nearly all cell types (Box 2). A second group (class II) encompasses the ‘itinerant’ cargo, the variable, transiting cargo load that provides unique temporal identity to both ESP compartments and defines the final composition and function of downstream exocytic and endocytic compartments and the cell surface for each cell type. Imposed on this cargo load are trafficking machineries (Box 3) comprising the membrome [3•], the latter including the COPII [4•] and COPI [7] coats, and tethering [8] and fusion (SNARE) components that facilitate protein cargo distribution [9]. Strikingly, the level and composition of membrome components varies considerably between each cell type [3•]. As the activity of these trafficking components reflect the local protein cargo load, it is apparent that a deeper understanding of the contribution of the folding energetics of class I and II cargo in a given cell type may be an important and underappreciated factor for globally assessing the operation and organization of the ESP.
Box 1 Morphology of the ESP.
The ESP is defined morphologically as sequential compartments comprising the cisternal/tubular ER, pre-Golgi intermediates (also referred to as vesicular tubular clusters (VTCs) or ER-Golgi intermediates (ERGIC) [55•]) and the Golgi stack. ER exit sites (ERES) are thought to generate transit vesicles and pleiomorphic tubular carriers through the activity of the COPII coat machinery (Box 3) to yield pre-Golgi intermediates [29]. Pre-Golgi intermediates are defined by the recruitment of the COPI coat machinery (Box 3) and have been suggested to be formed by either homotypic fusion of COPII vesicles [4•,30••,31] or to serve as stationary elements for receiving COPII generated vesicles [55•]. Pre-Golgi intermediates have been shown to move en bloc to the Golgi stack [32,33], or have been proposed to be involved in the generation of tubular transport intermediates that traffic to the Golgi [6••,34], both processes being microtubule dependent. The Golgi is a stack of polarized tubular/saccular compartments with a defined cis to trans content reflecting the presence of specialized processing enzymes that extensively modify newly synthesized proteins [35••,36,37]. The overall morphology of the ER-Golgi system can vary remarkably between cell types-being composed of completely separated and randomly distributed stacks (budding yeast), fragmented mini-stacks juxtaposed to ERES (fission yeast, plants), or elaborate inter-connected tubules and cisternal saccules that function as a central, peri-nuclear structure where cargo delivered to cis face exits at the trans-most face (higher eukaryotes) (Figure 4). Compartments comprising the ESP are highly dynamic structures whose physical organization and modus operandi largely remains elusive, but are currently thought to function through maturation models [38••,39••].
Box 2 The chaperome and UPR machineries.
The ER chaperome [6••,13] facilitates the folding of structurally unique ER cargo to prevent their premature degradation by ERAD and facilitate folding for export [2]. Canonical chaperones such as lumenal BiP (Hsp70-like) and GRP94 (Hsp90-like), and cytosolic Hsp90 and Hsp40/70 utilize ATP-dependent pathways to sequester polypeptides via hydrophobic patches preventing aggregation [14]. While these chaperones are promiscuous in their function, other chaperones and co-chaperones provide specificity to folding [14]. Moreover, specialized lumenal chaperones are required for the folding of protein cargo modified by N-linked glycans—the most well-characterized of these pathways being the lectin-based calnexin/calreticulin monitoring pathway [5,40]. This pathway is thought to monitor protein conformation through modification of N-linked glycans covalently attached to Asn residues through the activity of glycosyl transferases, glucosidases and chaperones. Folding enzymes such as PDI and ERP57 interact with polypeptides displaying non-native disulfide bonds, effectively reorganizing these covalent cross-links to their native pattern [17]. Through processive interactions with chaperone networks, the ER environment prevents misfolding events that can lead to disruption of folding homeostasis. Importantly, in order to maintain the proper balance between the chaperome and protein cargo load, three sensors of the unfolded protein response (UPR) (IRE1, ATF6, and PERK) actively monitor the state of misfolded proteins in the lumen [24]. These sensors are activated in response to the accumulation of misfolded proteins resulting in a PERK-mediated translational attenuation reducing cargo flux into the ER and a re-organization of the chaperome and ERAD pathways by transcriptional upregulation mediated by IRE1 and ATF6. Thus, the UPR continuously matches protein cargo load to the folding capacity of the ER in response to both endogenous and exogenous stimuli.
Box 3 Membrome trafficking machinery components.
Trafficking through the ESP is directed by components belonging to the membrome [3•]. Export from the ER can occur at exit sites (ERES) (Box 1) whose formation involves the Sec16 component [29,41] and where vesicle formation is directed by the COPII coat machinery. COPII consists of the cytosolic Sar1 GTPase that is activated by the ER-associated Sec12 guanine nucleotide exchange factor (GEF) to recruit the cytosolic Sec23-24 adaptor complex that binds protein cargo containing various ‘exit’ sorting codes [4•,26••]. These adaptor-cargo ternary-complexes are collected and concentrated into vesicles [42] or larger pleiomorphic elements [32,43]in a PI-4-dependent fashion [44•] by the self-assembling properties of the Sec13-31 cage complex [25••] that undergo fission from the ER in a Sar1-dependent fashion [45•,46•]. Following disassembly of the COPII coat, potentially by the activity of the Sec23 and Sec31 GTPase activating proteins (GAPs) [4•], newly formed pre-Golgi intermediates (Box 1) recruit the COPI complex, consisting of the F-‘adaptor’ subcomplex containing β, γ, δ, and ζ subunits, and the B-‘cage’ subcomplex containing the α, β’, ε subunits [4•,7]. COPI interacts with a subset of cargo in an ARF GTPase-dependent reaction [7] to promote retrieval and retrograde transport to the ER in a fashion that is sensitive to membrane curvature [47,48•]. The Golgi stack (Box 1) consists of a complex collection of components and cargo [35••] that appear to utilize different ARF-dependent reactions to recruit COPI and maintain the organization the stack by directing either anterograde and/or retrograde transport between stacks [7,35••]. Exit from the trans face of the stack to post-Golgi compartments occurs through clathrin coat-dependent and independent mechanisms using cargo selective adaptors. Tethering components including golgins [49,50••] and COGs [51], that dictate membrane recognition, in conjunction with Rab GTPases [3•] and their effectors [30•• ,52] and SNARE machinery components [51], mediate vesicle docking and fusion [8•] through Hsp90-dependent chaperoning mechanisms [56•]. It is thought that linked anterograde/retrograde recycling pathways (Box 1) insure a steady-state balance of the essential lipid components and membrane embedded and lumenal proteins necessary to maintain functionality of the ESP.
Protein energetics in the ESP
The contribution of protein cargo to membrane trafficking is strongly influenced by a protein’s ability to fold (Figure 1a) [10•]. The folding environment is defined by the composition of both the organic phase (the lipid bilayer) and the soluble phase (e.g., the lumenal content of each cellular compartment and the surrounding cytosol). Together, they provide an energetically favorable ‘solvent’ to support protein-folding pathways dictated by the primary sequence of the polypeptide chain [10•]. Since membrane trafficking in the ESP is closely linked to the conformation-based interactions between folding polypeptides and the membrome trafficking machinery [3•], trafficking decisions are strongly influenced by state of the fold [10•].
Figure 1.
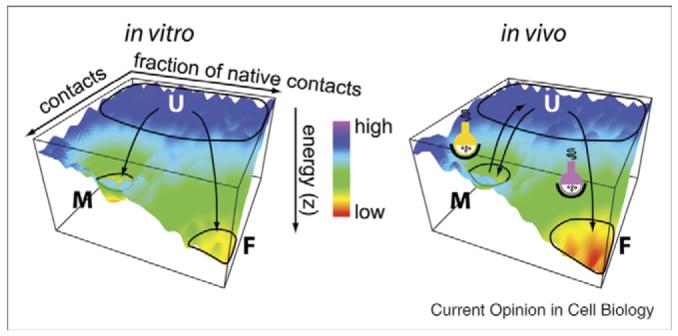
Energy landscapes for protein folding in the ESP. (left panel) The energy landscape for a protein folding in vitro [10•]. The x and y axes specify protein conformations, while the z-axis represents the energy of each conformation, with red being energetically more stable. The areas circled on the plot indicate conformations corresponding to the unfolded (U), folded (F) and misfolded (M) protein populations. (right panel) An energy landscape for the same protein as described in left panel, but in vivo. The axes are as in the left panel but the in vivo folding environment is such that folding and misfolding is managed (more) efficiently by the chaperome and local metabolites (indicated by the colored reaction flasks), particularly for multi-domain and membrane-spanning proteins [10•].
The energetics of the protein fold are defined by thermodynamic stability of the folded state and the kinetics of protein folding pathway(s) (that are defined by the stability of the transition state or states) that allow a specific polypeptide sequence to achieve its ‘native’ folded state. In such an energetic view of ESP function, a critical question becomes- what could be the kinetic and thermodynamic basis for cargo selection by COPII membrome components that contributes to the formation and function of downstream exocytic and endocytic compartments? Conversely, what are the energetic and structural parameters that dictate cargo delivery to the ERAD pathway? While the answers to these questions are still unknown, export or degradation likely depend on the populations of the various folding conformations (Figure 1a) [10•]. Of course, a problem arises in defining these populations as biologists typically only measure endpoints of trafficking decisions (e.g., the appearance of protein cargo in downstream compartments based on imaging techniques, biochemical processing by Golgi-specific enzymes or delivery to the cell surface), not the initiating events (e.g., the defining events directing folding and export from the ER by COPII (Box 3)). Thus, a possible alternative approach for understanding the first step of the exocytic pathway is to simply define the relationship between folding, degradation and export efficiency in terms of the protein folding energetics that are necessary to gain access to the COPII pathway [1••,10•].
To address the role of energetics in organizing the function of the exocytic pathway, a combined biochemical, biophysical and cell biological experimental approach was recently applied to study the export of the homotetrameric protein transthyretin (TTR) and numerous TTR variants that are known to predispose individuals to amyloid disease, that is, extracellular aggregation in response to a destabilized folded state [1••]. Notably, the COPII trafficking pathway was found to equally secrete TTR molecules with a surprisingly broad range of thermodynamic stability (ΔG from 5 to 35 kcal/mol), thus allowing the export of even highly destabilized protein variants to the extracellular space (Figure 2). Moreover, different cell types had different export efficiencies. This is so because consideration of the combined thermodynamic and kinetic parameters allowed a high proportion of even the most destabilized TTR molecules to be recognized by the COPII machinery for trafficking [1••,4•]. The reader is recommended to see reference [1••] for a more in-depth description of TTR trafficking energetics.
Figure 2.
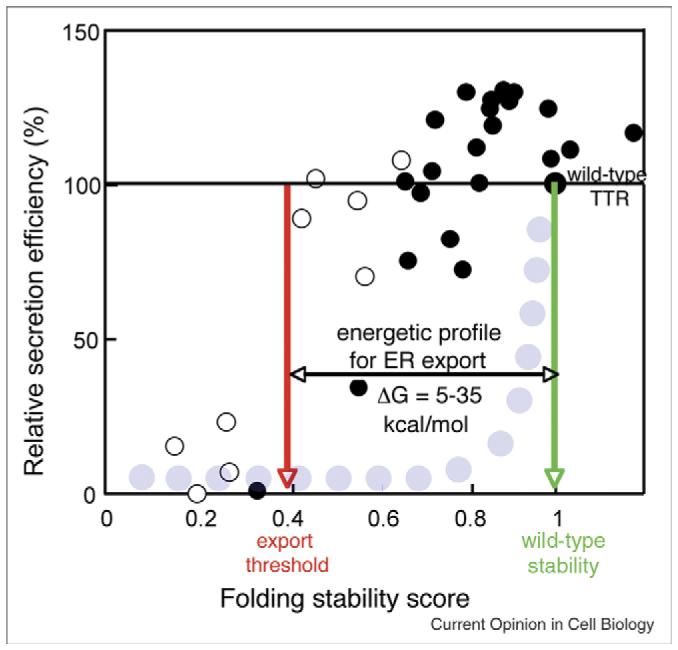
Plot of the export efficiency of variant TTR. A plot of secretion efficiency (y axis) for different TTR variants (open circles (monomer) and closed circles (homotetramer) [1••]) with differing folding stability scores, a value that includes both the thermodynamic and kinetic stability of the protein fold [1••]. The broad energetic permissiveness of TTR export (an energetic profile ranging from 5 to 35 kcal/mole indicated by the red and green arrows) demonstrates that the COPII export machinery is relatively tolerant to destabilized protein products. This differs from more traditional quality control (QC) views of ER export (gray circles) whereby protein-folding defects compromising wild type energetics would lead to ERAD [1••].
This study of TTR export energetics from the ER revealed what might be considered the first feature of membrane trafficking in the ESP - here, protein populations are selected for trafficking based on a fundamental, but flexible standard defined by protein folding energetics. While the mechanisms linking energetics and trafficking remain to be fully defined, the variable range of energetics that contribute to TTR export is likely applicable to trafficking decisions directing the exit of all cargo from the ER. Thus, we speculate that protein folding energetics, which is heavily influenced by the sequence of the polypeptide chain, may provide a primary framework for initiating membrane trafficking patterns in the ESP [1••,10•]. Indeed, several studies support this point indirectly by demonstrating that cargo largely controls vesicle budding from the ER [1••,4•,11,12,26••].
The environment counts—the metabolome and chaperome defining the ESP
While it is simpler to think of protein folding conformations in purely biophysical terms (Figure 1a), polypeptides associated with the ER must fold in rich biological and chemical environments consisting of protein chaperones (the chaperome [5,13,14]) defining the biological environment, and small, soluble and hydrophobic metabolites (the chemical or metabolome environment) that together generate a general solvent that defines the local lipid and cytosolic or lumenal environments of each compartment [10•]. Moreover, unlike in vitro folding of purified protein in a buffer solution, in vivo folding in the lumen of the ER or the cytosol occurs in a highly crowded environment that favors misfolding and subsequent misassembly leading to aggregation. Through both ATP-dependent and independent processes, it is now thought that chaperones enable protein folding pathways by interacting with hydrophobic patches exposed in unfolded and misfolded protein conformations, preventing their misassembly and aggregation [14]. In addition, some folding enzymes are known to, for instance, catalyze slow steps including peptidyl-prolyl amide bond isomerization or to promote disulfide bond formation within the lumen of the ER [15-17]. Thus, chaperones and folding enzymes provide an opportunity for unfolded/misfolded/misassembled protein to remain in the productive folding pathway for repeated opportunities to gain access to the ESP, thus mediating the overall energetic signature of the export pathway.
By reducing the probability of misfolding and aggregation, chaperones have a major impact on membrane trafficking by altering the distribution of the folded states (Figure 1b). How variable are these chaperome networks? Protein folding by the ER involves both lumenal - and cytosol - oriented chaperones [15] (Box 2), the latter required to manage the folding of the cytosolic domains of transmembrane spanning proteins [10•]. Microarray analyses (http://symatlas.gnf.org/SymAtlas/) reveal that the content of these protein solvent networks varies significantly between cell types, likely reflecting both a baseline level of folding activity required for the more universal class of resident (class I) proteins, and a need to sustain efficient folding of highly abundant and frequently highly specialized itinerant (class II) cargo species specific to a particular cell type [6••]. The establishment of these tissue-specific chaperome networks not only occurs in response to developmental regulation, but can be modified by a robust inducible folding pathway—the unfolded protein response (UPR) (Box 2).
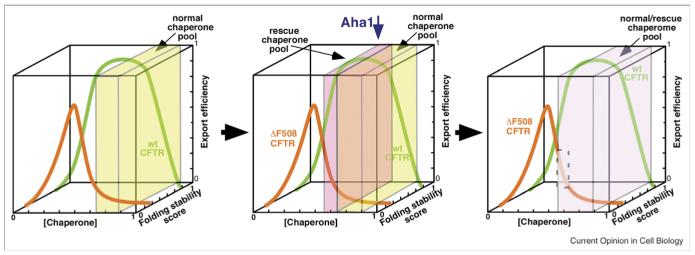
The UPR is now recognized as a prominent ER-based signaling pathway that continuously monitors the chaperome and membrome content of the ER and downstream trafficking pathways in the ESP, coupling cargo load to ERAF and ERAD pathways in order to maintain normal function in response to both intracellular and extracellular environmental challenge. For example, increased expression of chaperones has been shown to increase the trafficking of specific substrates from the ER by increasing the population in folded state (Figure 1b) (e.g., [16]) and, conversely, ERAD components likely direct a potentially stable population of cargo for degradation through interactions with the unfolded and misfolded states (Figure 1b) (e.g., [18]). Thus, the composition of downstream compartments may reflect the molecular and biochemical properties of the local folding environment(s). Consistent with this possibility, it has recently been shown that alteration of the chaperome composition can significantly attenuate misfolding disease by changing the apparent ‘buffering’ capacity of the Hsp90-dependent chaperone cycle associated with the folding of the cytoplasmic domains of cystic fibrosis transmembrane conductance regulator (CFTR) [6••](Figure 3).
Figure 3.
Augmenting the buffering capacity of the cellular chaperome for export of CFTR. (left panel) Depicted is the concentration range (indicated by the x axis) of the normal cellular chaperome pool (defined by the yellow box) that supports the folding and ER export (z axis) of the energetically stable CFTR wild-type protein (green curve). The folding stability score of wild-type CFTR is indicated by its location along the y-axis. This chaperome pool cannot support export of the more unstable ΔF508 mutant of CFTR (orange curve) as folding does not occur within the concentration range of the normal chaperome environment (yellow box). (middle panel) Modification of the chaperome pool by siRNA reduction of the Hsp90 cochaperone Aha1 generates a rescue pool (pink box) that adds folding capacity to the normal chaperome environment (yellow box) and results in increased export from the ER (orange line). (Right panel) The new chaperome pool created by reducing the level of Aha1 creates an altered cellular folding environment (green box) that can now support stabilization and export of mutant CFTR (gray highlighted region) without altering the function of the wild-type protein, thereby contributing to an increase of CFTR activity in the cell expressing mutant CFTR [6••]. See Wang et al. [6••] for experimental details.
Not surprisingly, the chemical metabolome, that is, the lipid and soluble metabolite content of compartments or the cytosol, can dramatically affect traffic through the ESP. For example, previous studies have demonstrated that even non-specific chemical chaperones (e.g., osymolytes) can increase the stability of proteins in the ER reflecting the rather remarkable influence of high concentrations of osmolytes on protein folding pathways and thermodynamic stability [19]. Moreover, modification of the bilayer composition, or the changes in the oxidizing or Ca2+ environments of the ER can also significantly affect protein export by affecting the protein fold [20,21••]. This raises the possibility that alterations in the local chemistry of the folding environment can shift the distribution of protein conformations on a more global scale to favor or disfavor export and trafficking by altering the general protein populations occupying the various folded states [10•](Figure 1b). Similarly, protein cargo-specific metabolites found in the metabolome of each cell type may influence membrane trafficking by stabilizing or destabilizing-specific protein cargo conformations in the ER. Here, examples are provided by the level and composition of microsomal triglycerides required for folding of ApoB100 in hepatocytes for export from the ER [4•], the capacity of lipid-bilayer sterol concentrations to regulate HMG-CoA ERAD and SREBP trafficking to the Golgi [22••], and small molecule metabolites such as thyroxine that increase the export efficiency of destabilized proteins TTR variants from the cell by altering the energetic stability of the protein fold [1••]. Interestingly, the metabolic and regulatory pathways defined by changes in either specific (e.g., sterols) or global (e.g., Ca2+) metabolites are often found to be linked to the UPR and biological folding pathways (e.g., chaperome [6••] composition), thus coupling the metabolome to protein folding conformation states and hence, folding energetics (Figure 1b) [23,24].
In general, the view derived from our quantitative energetic analysis of TTR trafficking [1••] suggests that the local metabolome/chaperome networks provide an adjustable environment that can both facilitate and/or alter the folding energetics of the protein cargo population. Thus, a second defining feature of the ESP is that trafficking in different cell types is not a fixed parameter, rather uses a flexible standard reflecting the local cargo folding energetics in response to the local environment [6••]. This is also likely responsive to diverse stimuli, either cell autonomous or cell non-autonomous [10•]. The mechanism(s) by which protein folding energetics is quantitatively manipulated by biology for function remains a formidable barrier to understanding the ESP.
Energetic coupling of cargo load to membrome machineries
While we have highlighted the importance of polypeptide chain folding energetics and the ESP folding environment [1••,10•], by necessity, a third layer of interactions encompasses the biochemical machines that respond to cargo load to direct movement and composition of downstream compartments. Two coat systems, COPII and COPI, directing anterograde and retrograde trafficking, respectively [4•,25••], and specific tethering and fusion machineries (Box 3) maintain the organization of the ER, pre-Golgi and Golgi compartments at steady-state (Box 1) [4•,6••]. While the coat systems direct cargo selection and vesicle budding, the tethering and fusion machineries direct targeting and delivery of cargo carriers. Given the different roles of COPII and COPI in trafficking in the ESP (Box 3), they must clearly differ in their ability to respond to the energetics of the cargo fold, although the mechanism(s) are only beginning to be elucidated. One aspect that is currently a strong focus of attention is the dependence of trafficking in the ESP on ‘adaptor protein complexes’ or APCs that recognize protein cargo and thereby link cargo to the coat components that generate budding vesicles (Box 3).
How do APCs sort cargo to different pathways? The conventional view focuses on the importance of the primary sequence embedded in the polypeptide chain. For example, COPII-mediated export requires a specific APC that recognizes ER ‘exit codes’ found in the cytosolic domain of transmembrane cargo, or in the case of lumenal cargo, cargo receptors [4•] (Box 3). COPI found in the Golgi, on the other hand, uses a different set of APCs along with accessory proteins to selectively filter misfolded/misassembled protein conformations displaying a ‘retrieval code’ for return to the ER (Box 3). Of course, interaction with APCs will be affected by the conformational accessibility or stability of export and retrieval codes—a feature that is strongly influenced by protein folding energetics [1••,5]. Thus, APCs define a third functional feature of the ESP, the role of which is to integrate folding energetics with cargo content to direct compartment organization. Here, one might speculate that APCs utilize an energetic framework to selectively direct forward or retrograde movement (Figure 4). For example, cargo competing for exit from the ER via COPII likely populates conformations with different degrees of stability pending the composition of the local chaperone and metabolome environments [1••,10•]. Those that lack the appropriate recognition machinery (e.g., export codes), of course, remain in the ER [1••,26••]. Those that are sufficiently stable (Figure 4, red boxes) move efficiently from the ER across the cis, medial and trans Golgi to post-Golgi compartments and the cell surface. By contrast, less stable folds (Figure 4, orange and yellow boxes) could reach earlier Golgi compartments, but be subject to retrieval if sufficiently unstable- movement being dictated by the biochemical properties of the COPII and COPI APCs and their ability to assess folding energetics associated with presentation of transit codes (Box 3).
Figure 4.
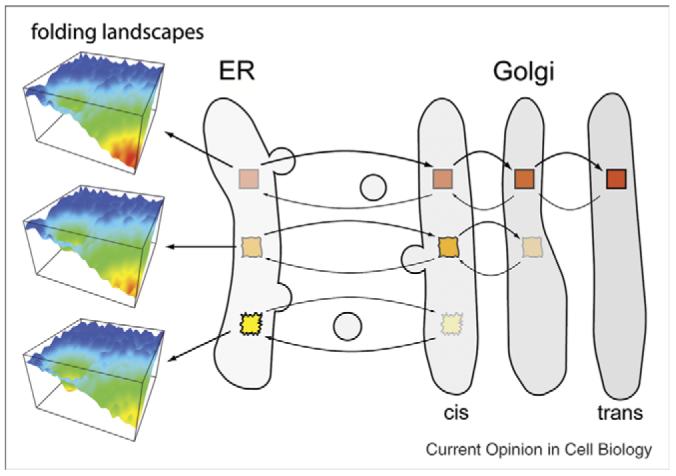
Folding energetics in trafficking through the ESP. Cargo exiting the ER populates ‘folded’ conformations with different degrees of stability. Those that are stable (red boxes) move efficiently to the trans Golgi, whereas less stable folds (orange and yellow boxes) occupy earlier Golgi compartments, or are quickly retrieved to the ER from pre-Golgi compartments if sufficiently unstable. The protein load in the various compartments may be actively maintained by a variety of different adaptor components that link to COPII and COPI cage assemblies in response to protein folding energetics (Box 3). This model suggests that it is the folding energetics of the cargo load in a particular compartment that determines the structure and composition of maturation events that dictate the dynamic features of the ESP.
Given that multi-domain or oligomeric (multi-subunit) proteins may contain both folded and unstructured domains, trafficking by membrome components in the ESP through exit codes may reflect the folding energetics of either a single domain or the integration of folding energetics of these domains or subunits [4•,26••]. Moreover, there are now many examples to suggest that folding energetics in the ESP are likely affected by cargo-specific accessory factors that augment their interaction with adaptors [4•] and/or with the tethering and fusion machineries (Box 3). These could provide critical regulatory constraints on the folding energetics facilitating membrane traffic [3•,8•,27]. For example, pro-sequences found on numerous cargo may provide additional energetic signals to direct protein stability (or instability) that is altered by further trafficking and processing (e.g., proteolytic removal) in downstream compartments. Even class I resident cargo such as Golgi processing enzymes would be expected to be continuously monitored through either COPI or other adaptor mechanisms that detect the energetic status of the fold on the basis of their location in early or late compartments. Perhaps the continuously changing chemical metabolome content of the lumenal environment or changes in the polarized distribution of lipids such as cholesterol along the cis to trans axis of the Golgi [53] leads to differences in the chemical potential of the bilayer [54] that could significantly contribute to the stability (energetics) of the protein fold (Figure 4).
Thus, a more energetics-based view of the ESP suggests that kinetics and thermodynamics of the cargo fold in a particular compartment defines the structure and capacity of the compartment to function and mature. In this view, we raise the possibility that the entire ESP, not just the ER, represents a flexible folding environment that allows for continuous energetic maturation of a protein to not only direct its trafficking but also to generate exocytic and, potentially, endocytic compartments.
Conclusion
One of the fundamental features of an energetics influenced view of membrane trafficking through the ESP [1••] is that transport of protein cargo within each compartment can be permissive or restrictive depending on the local environment that is, in turn, dependent on its local chemical and biological composition (Figure 4). The permissiveness and variability of export and trafficking through the ESP in different cell types observed with wild-type and variant TTR [28], and likely other cargo, is difficult to envision with the more traditional quality control (QC) views of ER export. Here protein folding defects compromising wild type energetics through the activity of glycan folding monitors are suggested to lead to ERAD [5,40]. Instead, we suggest that decisions to mobilize a protein through the various ESP compartments are flexible and dependent on the energetics of various folding populations [1••] (Figure 1)—states that are dynamically influenced by the composition of the metabolome, chaperome and membrome of a particular cell type (Figure 1b), thereby providing a variable standard in different cell types to define the role of cargo load in the directing the organization and function of the ER, pre-Golgi and Golgi compartments comprising the ESP (Figure 4). We suggest that ESP compartments may have co-evolved with increasing cargo folding complexity to segregate and optimize energetically sensitive steps in protein cargo folding with further processing by glycosyl transferases, proteases and the like to achieve functionality in evolving environments. Such environmental optimization on the basis of a protein folding energetics platform through compartmentalization would considerably enhance the versatility of the protein cargo fold for function by the eukaryotic cell in order to respond to complex tissue and host-specific needs
Acknowledgement
We apologize to those authors whose important work we have not cited but are clearly reference in the cited work given the emphasis of this review format on publications within the past few years. This work is supported by GM42336, HL67202 and GM33301, and the Cystic Fibrosis Foundation to WEB, and DK46335, AG18917, and DK75295 to JWK.
References and recommended reading
Papers of particular interest, published within the annual period of the review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1••.Sekijima Y, Wiseman RL, Matteson J, Hammarstrom P, Miller SR, Sawkar AR, Balch WE, Kelly JW. The biological and chemical basis for tissue-selective amyloid disease. Cell. 2005;121:73–85. doi: 10.1016/j.cell.2005.01.018. A paper that defines quantitatively the contribution of thermodynamics and kinetics as an energetic basis for understanding export from the ER.
- 2.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 3•.Gurkan C, Lapp H, Alory C, Su AI, Hogenesch JB, Balch WE. Large-scale profiling of Rab GTPase trafficking networks: the membrome. Mol Biol Cell. 2005;16:3847–3864. doi: 10.1091/mbc.E05-01-0062. A systems biology description of trafficking components directing Rab and SNARE dependent trafficking in the exocytic and endocytic pathways.
- 4•.Gurkan C, Stagg SM, Lapointe P, Balch WE. The COPII cage: unifying principles of vesicle coat assembly. Nat Rev Mol Cell Biol. 2006;7:727–738. doi: 10.1038/nrm2025. A contemporary review of the parameters governing assembly of COPII vesicles mediating export of cargo from the ER.
- 5.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 6••.Wang X, Venable J, LaPointe P, Hutt DM, Koulov AV, Coppinger J, Gurkan C, Kellner W, Matteson J, Plutner H, et al. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127:803–815. doi: 10.1016/j.cell.2006.09.043. A paper that describes the role of the chaperome environment in directing the export of wild-type and mutant CFTR proteins from the ER.
- 7.Bethune J, Wieland F, Moelleken J. COPI-mediated transport. J Membr Biol. 2006;211:65–79. doi: 10.1007/s00232-006-0859-7. [DOI] [PubMed] [Google Scholar]
- 8•.Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell. 2007;12:671–682. doi: 10.1016/j.devcel.2007.04.005. A very contemporary review of the interactions between coat, tether, Rab and SNAREs to generate networks of trafficking complexes that facilitate membrane traffic.
- 9.Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2005;38:1–47. doi: 10.1017/S0033583505004051. [DOI] [PubMed] [Google Scholar]
- 10•.Kelly JW, Balch WE. The integration of cell and chemical biology in protein folding. Nat Chem Biol. 2006;2:224–227. doi: 10.1038/nchembio0506-224. A chemical biology perspective of folding and trafficking through the exocytic and endocytic pathways.
- 11.Sato K, Nakano A. Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol. 2005;12:167–174. doi: 10.1038/nsmb893. [DOI] [PubMed] [Google Scholar]
- 12.Forster R, Weiss M, Zimmermann T, Reynaud EG, Verissimo F, Stephens DJ, Pepperkok R. Secretory cargo regulates the turnover of COPII subunits at single ER exit sites. Curr Biol. 2006;16:173–179. doi: 10.1016/j.cub.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y, Hendershot LM. Organization of the functions and components of the endoplasmic reticulum. Adv Exp Med Biol. 2007;594:37–46. doi: 10.1007/978-0-387-39975-1_4. [DOI] [PubMed] [Google Scholar]
- 14.Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. doi: 10.1016/j.cell.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 15.van Anken E, Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Crit Rev Biochem Mol Biol. 2005;40:191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- 16.Inan M, Aryasomayajula D, Sinha J, Meagher MM. Enhancement of protein secretion in Pichia pastoris by overexpression of protein disulfide isomerase. Biotechnol Bioeng. 2006;93:771–778. doi: 10.1002/bit.20762. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta Proteins Proteom. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Olivari S, Molinari M. Glycoprotein folding and the role of EDEM1, EDEM2 and EDEM3 in degradation of folding-defective glycoproteins. FEBS Lett. doi: 10.1016/j.febslet.2007.04.070. in press. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa T, Ejima D, Kita Y, Tsumoto K. Small molecule pharmacological chaperones: from thermodynamic stabilization to pharmaceutical drugs. Biochim Biophys Acta. 2006;1764:1677–1687. doi: 10.1016/j.bbapap.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Norez C, Antigny F, Becq F, Vandebrouck C. Maintaining low Ca2+ level in the endoplasmic reticulum restores abnormal endogenous F508del-CFTR trafficking in airway epithelial cells. Traffic. 2006;7:562–573. doi: 10.1111/j.1600-0854.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 21••.Runz H, Miura K, Weiss M, Pepperkok R. Sterols regulate ER-export dynamics of secretory cargo protein ts O45-G. EMBO J. 2006;25:2953–2965. doi: 10.1038/sj.emboj.7601205. An important report describing the general role of cholesterol lipids in modulating the efficiency of ER export.
- 22••.Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols black transpsort by binding to Insig. Proc Natl Acad Sci U S A. 2007;104:6511–6518. doi: 10.1073/pnas.0700899104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sun LP, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Pro Natl Acad Sci U S A. 2007;104:6519–6526. doi: 10.1073/pnas.0700907104. Linked papers illustrating the latest in an important series of papers that define the role of cholesterol and exit codes in mediating ER to Golgi trafficking of cargo.
- 23.Harding HP, Zhang Y, Khersonsky S, Marciniak S, Scheuner D, Kaufman RJ, Javitt N, Chang YT, Ron D. Bioactive small molecules reveal antagonism between the integrated stress response and sterol-regulated gene expression. Cell Metab. 2005;2:361–371. doi: 10.1016/j.cmet.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 25••.Stagg SM, Gurkan C, Fowler DM, LaPointe P, Foss TR, Potter CS, Carragher B, Balch WE. Structure of the Sec13/31 COPII coat cage. Nature. 2006;439:234–238. doi: 10.1038/nature04339. Using cryo-EM and single particle analysis the paper describes for the first time the unprecedented structure of the cuboctahedral COPII cage mediating cargo collection and budding of vesicles from the ER.
- 26••.Kincaid MM, Cooper AA. Misfolded proteins traffic from the endoplasmic reticulum (ER) due to ER export signals. Mol Biol Cell. 2007;18:455–463. doi: 10.1091/mbc.E06-08-0696. An important paper that provides insights into the key role of export signals in directing trafficking from the ER through the ESP.
- 27.Puthenveedu MA, Linstedt AD. Subcompartmentalizing the Golgi apparatus. Curr Opin Cell Biol. 2005;17:369–375. doi: 10.1016/j.ceb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Sekijima Y, Dendle MA, Kelly JW. Orally administered diflunisal stabilizes transthyretin against dissociation required for amyloidogenesis. Amyloid. 2006;13:236–249. doi: 10.1080/13506120600960882. [DOI] [PubMed] [Google Scholar]
- 29.Connerly PL, Esaki M, Montegna EA, Strongin DE, Levi S, Soderholm J, Glick BS. Sec16 is a determinant of transitional ER organization. Curr Biol. 2005;15:1439–1447. doi: 10.1016/j.cub.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 30••.Cai H, Yu S, Menon S, Cai Y, Lazarova D, Fu C, Reinisch K, Hay JC, Ferro-Novick S. TRAPPI tethers COPII vesicles by binding the coat subunit Sec23. Nature. 2007;445:941–944. doi: 10.1038/nature05527. A paper that significantly advances our understanding of the link between the COPII vesicle budding machinery and targeting machinery components.
- 31.Yu S, Satoh A, Pypaert M, Mullen K, Hay JC, Ferro-Novick S. mBet3p is required for homotypic COPII vesicle tethering in mammalian cells. J Cell Biol. 2006;174:359–368. doi: 10.1083/jcb.200603044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marra P, Salvatore L, Mironov A, Jr, Di Campli A, Di Tullio G, Trucco A, Beznoussenko G, Mironov A, De Matteis MA. The biogenesis of the Golgi ribbon: the roles of membrane input from the ER and of GM130. Mol Biol Cell. 2007 doi: 10.1091/mbc.E06-10-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson JC, Nilsson T, Pepperkok R. Biogenesis of tubular ER-to-Golgi transport intermediates. Mol Biol Cell. 2006;17:723–737. doi: 10.1091/mbc.E05-06-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Tekaya H, Miura K, Pepperkok R, Hauri HP. Live imaging of bidirectional traffic from the ERGIC. J Cell Sci. 2005;118:357–367. doi: 10.1242/jcs.01615. [DOI] [PubMed] [Google Scholar]
- 35••.Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, et al. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–1281. doi: 10.1016/j.cell.2006.10.036. A landmark proteomic description of the composition of Golgi compartments and COPI Golgi derived vesicles that sheds insight into Golgi organization and trafficking.
- 36.Cosson P, Ravazzola M, Varlamov O, Sollner TH, Di Liberto M, Volchuk A, Rothman JE, Orci L. Dynamic transport of SNARE proteins in the Golgi apparatus. Proc Natl Acad Sci U S A. 2005;102:14647–14652. doi: 10.1073/pnas.0507394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang CW, Hamamoto S, Orci L, Schekman R. Exomer: A coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J Cell Biol. 2006;174:973–983. doi: 10.1083/jcb.200605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. A striking live-cell imaging description of cargo trafficking through the Golgi, emphasizing the maturation model of Golgi function.
- 39••.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441:1007–1010. doi: 10.1038/nature04737. A striking live-cell imaging description of cargo trafficking through the Golgi, emphasizing the maturation model of Golgi function.
- 40.Ruddock LW, Molinari M. N-glycan processing in ER quality control. J Cell Sci. 2006;119:4373–4380. doi: 10.1242/jcs.03225. [DOI] [PubMed] [Google Scholar]
- 41.Watson P, Townley AK, Koka P, Palmer KJ, Stephens DJ. Sec16 defines endoplasmic reticulum exit sites and is required for secretory cargo export in mammalian cells. Traffic. 2006;7:1678–1687. doi: 10.1111/j.1600-0854.2006.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeuschner D, Geerts WJ, van Donselaar E, Humbel BM, Slot JW, Koster AJ, Klumperman J. Immuno-electron tomography of ER exit sites reveals the existence of free COPII-coated transport carriers. Nat Cell Biol. 2006;8:377–383. doi: 10.1038/ncb1371. [DOI] [PubMed] [Google Scholar]
- 43.Luini A, Ragnini-Wilson A, Polishchuck RS, De Matteis MA. Large pleiomorphic traffic intermediates in the secretory pathway. Curr Opin Cell Biol. 2005;17:353–361. doi: 10.1016/j.ceb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 44•.Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M. Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell. 2006;11:671–682. doi: 10.1016/j.devcel.2006.09.001. A critical set of observations describing the role of PI-4-P metabolite in directing COPII vesicle formation.
- 45•.Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, Schekman R. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 2005;122:605–617. doi: 10.1016/j.cell.2005.07.025. An important paper that identifies the Sar1 GTPase as a potentially important component of fission machinery directing release of COPII vesicles from the ER in yeast.
- 46•.Bielli A, Haney CJ, Gabreski G, Watkins SC, Bannykh SI, Aridor M. Regulation of Sar1 NH2 terminus by GTP binding and hydrolysis promotes membrane deformation to control COPII vesicle fission. J Cell Biol. 2005;171:919–924. doi: 10.1083/jcb.200509095. An important paper that identifies the Sar1 GTPase as a potentially important component of fission machinery directing release of COPII vesicles from the ER in mammalian cells.
- 47.Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 48•.Antonny B, Bigay J, Casella JF, Drin G, Mesmin B, Gounon P. Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation. Biochem Soc Trans. 2005;33:619–622. doi: 10.1042/BST0330619. A critical analysis of the role of membrane curvature in controlling COPI vesicle formation.
- 49.Sztul E, Lupashin V. Role of tethering factors in secretory membrane traffic. Am J Physiol Cell Physiol. 2006;290:C11–C26. doi: 10.1152/ajpcell.00293.2005. [DOI] [PubMed] [Google Scholar]
- 50••.Malsam J, Satoh A, Pelletier L, Warren G. Golgin tethers define subpopulations of COPI vesicles. Science. 2005;307:1095–1098. doi: 10.1126/science.1108061. A insightful paper describing the differential role of tethers in mediating COPI vesicle traffic.
- 51.Ungar D, Oka T, Krieger M, Hughson FM. Retrograde transport on the COG railway. Trends Cell Biol. 2006;16:113–120. doi: 10.1016/j.tcb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Kim YG, Raunser S, Munger C, Wagner J, Song YL, Cygler M, Walz T, Oh BH, Sacher M. The architecture of the multisubunit TRAPP I complex suggests a model for vesicle tethering. Cell. 2006;127:817–830. doi: 10.1016/j.cell.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 53.Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- 54.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 55•.Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. A contemporary discussion of the function of potential transport intermediates in the ESP.
- 56•.Chen CY, Balch WE. The Hsp90 chaperone complex regulates GDI-dependent Rab recycling. Mol Biol Cell. 2006;17:3494–3507. doi: 10.1091/mbc.E05-12-1096. A report describing the likely universal role of Hsp90 chaperome systems in vesicle trafficking through regulation of assembly of Rabs, tethers and SNAREs complexes.