Abstract
Rationale
Acute doses of buprenorphine can precipitate withdrawal in opioid dependent persons. The likelihood of this withdrawal increases as a function of the level of physical dependence.
Objectives
To test the acute effects of sublingual buprenorphine/naloxone tablets in volunteers with a higher level of physical dependence. The goal was to identify a dose that would precipitate withdrawal (Phase 1), then determine if withdrawal could be attenuated by splitting this dose (Phase 2).
Methods
Residential laboratory study; subjects (N=16) maintained on 100 mg per day of methadone. Phase 1: Randomized, double blind, triple dummy, within subject study. Conditions were sublingual buprenorphine/naloxone (4/1, 8/2, 16/4, 32/8 mg), intramuscular naloxone (0.2 mg), oral methadone (100 mg), or placebo. Medication conditions were randomized, but buprenorphine/naloxone doses were ascending within the randomization. Phase 2: Conditions were methadone, placebo, naloxone, 100% of the buprenorphine/naloxone dose that precipitated withdrawal in Phase 1 (full dose), and 50% of this dose administered twice in a session (split dose). Analyses covaried by trough methadone serum levels.
Results
Six subjects did not complete the study. Of the ten who completed, three tolerated up to 32/8 mg of buprenorphine/naloxone without evidence of precipitated withdrawal. For the seven completing both phases, split doses generally produced less precipitated withdrawal compared to full doses.
Conclusions
There is considerable between subject variability in sensitivity to buprenorphine's antagonist effects. Low, repeated doses of buprenorphine/naloxone (e.g., 2/0.5 mg) may be an effective mechanism for safely dosing this medication in persons with higher levels of physical dependence.
1.0 Introduction
Buprenorphine is available in many countries for use as a sublingual medication that is effective in the treatment of opioid dependence (Ling and Wesson, 2003; Mattick et al., 2004; Strain, 2002). It acts as a partial agonist at the mu opioid receptor and as a kappa opioid antagonist; preclinical and clinical studies have shown that it exhibits a bell-shaped dose response curve and produces less of a maximal effect than full agonist opioids (Doxey et al., 1982; Liguori et al., 1996; Strain, 2006; Walsh et al., 1994). When administered to opioid dependent persons, it can precipitate withdrawal under certain experimental and clinical conditions (Clark et al., 2002; Strain et al., 1995; Walsh et al., 1995). Buprenorphine-related precipitated withdrawal is thought to occur due to its partial mu agonist effects (Strain et al., 1992; Walsh and Eissenberg, 2003).
The risk of buprenorphine-precipitated withdrawal is increased as a function of three parameters: higher doses of buprenorphine, a shorter time interval between the exposure to the full agonist and buprenorphine administration (which may vary as a function of the half life of the full agonist), and higher levels of physical dependence. With respect to the last of these, clinical guidelines have recommended patients with levels of physical dependence greater than 30 mg of daily methadone should not be given acute doses of buprenorphine because of the risk of precipitated withdrawal (McNicholas, 2004). It is quite common for patients to have levels of physical dependence that are higher than that produced by 30 mg per day of methadone, so this recommendation makes it problematic to consider transferring such patients to sublingual buprenorphine. While one strategy to address this problem is to increase the time since the last dose of opioid agonist, this can be difficult in practice. Patients may be unwilling or unable to wait for dosing, and may begin to experience spontaneous opioid withdrawal and use illicit opioids before the first dose of buprenorphine can be administered. These experiences may decrease patient acceptance of initiating buprenorphine therapy.
The risk of precipitated withdrawal from a partial agonist also increases as the dose of the partial agonist increases. However, acute smaller doses of partial agonists do not precipitate withdrawal (Strain et al., 1995). This suggests that it may be possible to administer small, repeated doses of buprenorphine in a person with a higher level of physical dependence and avoid precipitated withdrawal. While smaller doses of buprenorphine may produce minimal or no detectable precipitated withdrawal, the patient may experience sufficient cumulative opioid agonist effect to produce the desired therapeutic outcome with repeated dosing. Such an observation would suggest that partial agonist-induced precipitated withdrawal is related to the immediate delivery of total dose rather than a cumulative effect of dose exposure over time. Conversely, if the same degree of partial agonist-induced precipitated withdrawal were observed after both a single acute larger dose and repeated smaller doses, this would suggest that a specific amount of drug produces precipitated withdrawal without respect to the rate of delivery.
The purpose of this study was to examine the relationship between buprenorphine delivery and occurrence of buprenorphine-induced precipitated withdrawal. In this study, the buprenorphine/naloxone combination product (Suboxone®) was employed rather than the single buprenorphine formulation (Subutex®), because the former is the more commonly prescribed medication in the United States. It is important to note that the naloxone in sublingual buprenorphine/naloxone does not increase the risk of precipitated withdrawal compared to buprenorphine alone, because sublingual naloxone has poor bioavailability and bioactivity (Ciraulo et al., 2006; Harris et al., 2000; Harris et al., 2004; Preston et al., 1990; Strain et al., 2004). This study first identified a dose of sublingual buprenorphine that produced precipitated withdrawal in individuals with higher levels of opioid physical dependence, and then examined whether this same dose of buprenorphine could be administered as split doses separated by a relatively short time interval without producing the same severity of precipitated withdrawal.
2.0 Methods
2.1 Subjects
A total of sixteen subjects were enrolled in the study. Participants were adult male and female volunteers eligible for methadone maintenance treatment. Pregnancy, significant medical, and severe non-substance use psychiatric illnesses (e.g., schizophrenia) were exclusionary. Prior to study enrollment, applicants to the study were screened by medical staff not directly involved in the study as investigators; assessments included a medical history and physical examination, psychiatric history, electrocardiogram, and basic chemistry, hematology and urinalysis testing. There were five White and eleven African-American participants, with ten of the sixteen being males. Subjects had an average age of 37.4 years (range 20-51 years), average duration of lifetime illicit opioid use of 6.5 years, and an average of 28.2 out of 30 days of illicit opioid use prior to study entry. The study was approved by a local Institutional Review Board, and all volunteers gave written informed consent and were provided a modest daily payment for their study participation.
2.2 Study setting
Participants were initially stabilized as outpatients on methadone (100 mg/day) for an average of 25 days prior to starting the residential portion of the study. Subjects ingested methadone under medical supervision at a treatment/research clinic each day. After this outpatient stabilization period, subjects were admitted to a closed, 14-bed clinical pharmacology residential research unit for the active period of the study. Urine samples were collected daily from subjects residing on the unit, and tested for the presence of illicit drugs on a random schedule. There was no evidence of unauthorized drug use during the residential period.
2.3 General study procedures
The duration of the residential study period was up to eight weeks for each volunteer, and consisted of two phases. Subjects and staff were unaware that the study consisted of different phases; daily study procedures were indistinguishable between phases and are described in more detail below. During the residential period, participants underwent experimental challenge sessions scheduled a maximum of twice per week with at least two days between sessions. Methadone was administered at 0900 on each non-session day; this standing methadone dose was not provided on session days. After completion of the inpatient study, subjects were discharged to an outpatient treatment/research clinic where they could be withdrawn off opioids and transferred to a drug-free treatment program or receive assistance in transferring to another methadone maintenance treatment program.
2.4 Laboratory sessions
Experimental sessions followed procedures used in previous, similar studies (Correia et al., 2006; Strain et al., 2000; Strain et al., 1997). They occurred twice weekly with at least 48 hours between them. Sessions began at 0830 on experimental days and lasted 4.5 hours. They were run by a research technician blind to the drug, dose administered, and study phase, who was present throughout each session. During the first 30 minutes of each session, baseline physiological data were obtained, all subject and observer questionnaires were completed, and pupil photos were taken.
There were two drug administrations during each session timed to occur at 0900 and 1100. At the time of each administration, participants received an oral liquid medication, an intramuscular injection in the upper extremity, and sublingual tablets.
2.5 Study phases
The purpose of the first study phase was to identify a dose of sublingual buprenorphine/naloxone that reliably produced precipitated withdrawal. In the second study phase, that same dose of buprenorphine/naloxone was given, but as two divided doses, separated by a 2-hour interval to determine if less precipitated withdrawal would be produced by smaller, repeated doses. There could be a variable number of sessions during the first study phase (depending upon the range of buprenorphine/naloxone doses tested before identifying one that produced precipitated withdrawal). In order to maintain the study blind, there were two drug administrations during the first phase, but only the first (0900) was active drug; all administrations at 1100 were placebo. The second study phase had the potential to include active dosing at 1100.
2.6 Study conditions
During the first phase of the study, all participants received three control conditions in separate experimental sessions: placebo, methadone (100 mg oral), or naloxone (0.2 mg intramuscular). In addition, subjects received ascending doses of sublingual buprenorphine/naloxone during this phase (4/1, 8/2, 16/4, and 32/8 mg). These seven conditions were partially randomized; buprenorphine/naloxone was administered in ascending doses within the randomization. In each session, only one of the administered drugs was active. Once a buprenorphine/naloxone dose was identified that precipitated withdrawal, it was repeated in Phase 1 for confirmation purposes. Once confirmed (and the three control conditions were completed), the subject entered Phase 2.
There were five experimental test conditions in Phase 2, and drug administration was randomized. The conditions consisted of the same three control conditions (placebo, methadone 100 mg oral, naloxone 0.2 mg intramuscular); the sublingual dose of buprenorphine/naloxone that precipitated withdrawal (as a single dose) in Phase 1, and that same buprenorphine/naloxone dose split evenly into two doses and administered with a 2-hour interval between doses.
2.7 Session drugs
Methadone (Mallinckrodt, St. Louis, MO) was administered as a 40 ml oral solution mixed with cherry syrup and containing 9 mcg of denatonium benzoate (Spectrum Pharmacy Products, New Brunswick, NJ) as a flavor mask. Placebo methadone doses for session days consisted of the same volume but contained only the cherry syrup vehicle and 12 mcg of the denatonium benzoate flavor mask. A commercial form of naloxone hydrochloride (0.4 mg/ml; Endo Pharmaceuticals, Inc., Chadds Ford, PA) was diluted with bacteriostatic water and used for the antagonist control condition. Bacteriostatic water was used for placebo injections.
Buprenorphine/naloxone was supplied by the National Institute on Drug Abuse, Research Technology Branch (Rockville, MD) from a supply provided by Reckitt Benckiser Pharmaceuticals, Inc. (Hull, England). Each tablet weighed 100 mg and contained 2 mg of buprenorphine combined with 0.5 mg of naloxone. Placebo tablets, matched by weight, color, taste, and shape, were also provided. For session days on which an active buprenorphine/naloxone dose was scheduled, subjects received a combination of active and placebo tablets (except for the highest dose condition of 32/8 mg, in which case all tablets were active). A total of sixteen tablets were administered at one time in each session.
2.8 Methadone serum levels
There can be considerable variability in methadone blood levels for patients maintained on the same dose of methadone (Eap et al., 1998; Eap et al., 2002; Horns et al., 1975; Kreek, 1973). In order to assess this potential variability, each participant had blood samples collected on a non-session day 23.5 hours after a daily methadone dose (i.e., a trough level). Samples were collected on up to three occasions while residing on the residential unit and were tested at a commercial laboratory (Quest Diagnostics, Pittsburgh, PA) for methadone serum levels.
2.9 Physiological measures
Heart rate, blood pressure, skin temperature, respiratory rate, and oxygen saturation were monitored throughout the session following procedures used in similar studies (Correia et al., 2006; Strain et al., 2000; Strain et al., 1997). Data for each measure were collected and stored using a Macintosh computer (Apple Computer, Inc., Cupertino, CA), and averaged across time intervals: baseline (the 15-minute interval from 15 minutes to 1 minute before drug administration), and then 15-minute intervals following the first drug administration (1-15, 16-30, 31-45, …). Pupil diameter was determined from photographs taken in standardized ambient room lighting using a Polaroid camera with 2X magnification. Pupil photographs were taken three times at 15 minutes before drug administration, and at 15-minute intervals (15, 30, 45, 60, …) after the first drug administration. There was no pupil photo taken at 120 minutes after the first drug administration (the time of the second drug administration), but pupil photos then continued at 15-minute intervals for the remainder of the session. The mid-value of the three pre-first drug administration pupil photos was used as the baseline measure.
2.10 Subject and observer measures
Subjective effect reports and observer rating questionnaires were completed 15 minutes before and at 15-minute intervals following the first drug administration (15, 30, 45, …) for 210 minutes. However, no collection occurred at 120 minutes (the time of the second drug administration). Subjects were instructed to respond describing how they felt at the time the questionnaire was answered.
Subjects completed visual analog scales, an adjective rating questionnaire, and a pharmacological class questionnaire, following procedures similar to those used in previous studies (Correia et al., 2006; Strain et al., 2000; Strain et al., 1997). There were six visual analog scales: High, Drug Effects, Good Effects, Bad Effects, Liking, and Sick. The adjective rating questionnaire consisted of 37 items that the participant rated on a five-point scale from 0 (not at all) to 4 (extremely); the items constituted two scales: a 16-item opioid Agonist scale (adjectives associated with morphine-like effects), and a 21-item Withdrawal scale (adjectives associated with opioid withdrawal-like effects). Adjective items were selected based upon earlier related work (Fraser et al., 1961; Jasinski, 1977), and this instrument has been used in previous opioid pharmacology studies (Strain et al., 1993, 1995). For the pharmacological class questionnaire, the subject selected one of ten drug classes to which the administered drug was most similar: Placebo, Opioids, Opioid Antagonists, Phenothiazines, Barbiturates and Sleeping Medications, Antidepressants, Hallucinogens, Benzodiazepines, Stimulants, and Other. Examples for each drug class were listed on the questionnaire.
Observer ratings, done at the same times as the subject ratings, were performed by research assistants trained to assess signs and symptoms of opioid agonist and withdrawal effects. Observer ratings included the same adjective rating scale scored in the same manner. In addition, an observer-rated assessment of seven signs of opioid withdrawal (lacrimation, rhinorrhea, perspiration, piloerection, yawning, restlessness, and bowel sounds) was performed. Each opioid withdrawal item was scored using standardized criteria, as 0, 1 or 2 (with higher scores corresponding to greater severity), and scores for all items were summed to produce a total observer Withdrawal Signs Score (WSS).
2.11 Psychomotor/cognitive performance measures
Subjects completed three psychomotor/cognitive performance tasks during the session: a computerized form of the Digit Symbol Substitution Task (DSST) (McLeod et al., 1982), a recall (memory) task in which the participant was assessed for ability to recall an eight digit number, and a computerized form of the Trail-Making Test (Strain et al., 2000). This latter test was a Macintosh-based version of the Trail-Making Test (Reitan, 1958). Each of the three tasks was completed during the baseline period (15 minutes before drug administration), and at the same time periods as subject ratings.
2.12 Determination of precipitated withdrawal
During the first study phase, a dose of buprenorphine/naloxone that produced precipitated withdrawal was identified. Results from each session were reviewed by a non-blind investigator prior to the onset of the next scheduled session, and evidence of precipitated withdrawal was based upon the subject achieving at least two of three criteria: 1) a peak visual analog scale Bad Effects rating of 30 or greater, 2) a pupil diameter dilation (change from baseline) of at least 0.4 mm, and 3) an increase from baseline in the WSS of at least 5 points. These criteria were based upon previous studies in which opioid dependent volunteers had received naloxone challenges and demonstrated clinically significant evidence of opioid withdrawal (Stoller et al., 2001; Strain et al., 1992, 1993).
2.13 Data analysis
Primary analyses compared five study conditions: the Phase 1 results for the three control conditions (placebo, methadone, naloxone), the first buprenorphine/naloxone dose that produced reliable precipitated withdrawal, and that same dose given as two divided doses in Phase 2. Peak values for each session were determined for each measure. This was an increased effect for most measures. However, because some measures may decrease in response to acute opioid agonist effects (e.g., pupil diameter, certain psychomotor tasks), the absolute nadir effect for these measures was examined. A conservative one-step procedure, Tukey's honestly significant difference (HSD), was used for pairwise comparisons of peak values for each condition. The mean square error term needed to perform these tests was calculated using a repeated-measures analysis of covariance. In order to allow for individual variability in methadone levels for subjects, analyses were covaried by each subject's average methadone serum level results. Comparisons for which the Tukey q-value corresponded to p<0.05 are reported.
Participants received the same dose of buprenorphine/naloxone that precipitated withdrawal twice during Phase 1 and once during Phase 2. Results for this dose of buprenorphine/naloxone on the three different occasions were compared with a repeated measures analysis of variance to determine if subjects had changes over time upon repeated exposure to the same dose of buprenorphine/naloxone.
3.0 Results
Six of the sixteen subjects left without completing both study phases. Three other subjects received all doses of buprenorphine/naloxone (i.e., up to 32/8 mg) without reliably showing evidence of precipitated withdrawal. While one of the three subjects had the lowest trough methadone serum level (0.11 mg/L), the other two participants had levels (0.48 and 0.41 mg/L) that were close or identical to the mean for all subjects (0.41 mg/L). The remaining seven subjects completed both phases of the study. Four of these seven had precipitated withdrawal with the 4/1 mg dose, two with the 8/2 mg dose, and one with the 16/4 mg dose. The following sections describe responses for the seven subjects who completed both study phases.
3.1 Peak effects
3.1.1 Subjective effects
Figure 1 shows results for four of the visual analog scale ratings as representative measures of self-reported effects. Compared to placebo, methadone produced significantly higher ratings of drug effects, while the full dose of buprenorphine/naloxone and the naloxone control condition produced elevated ratings that were not significantly different from placebo. There were no significant differences between the conditions for ratings of good effects and liking, although methadone produced the highest peak ratings for these measures (top right panel of Figure 1, Table 1).
Figure 1.
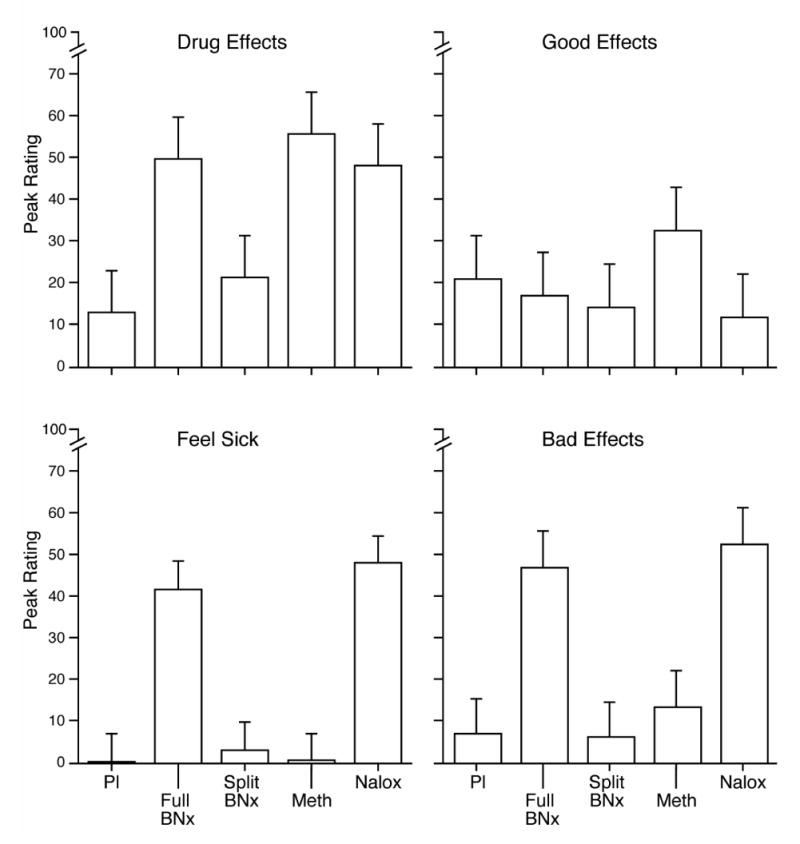
Subject-reported peak visual analog scale ratings. Each bar represents the mean value (SEM) for the seven subjects who entered the second phase of the study. Pl = placebo, BNx = buprenorphine/naloxone, Meth = methadone, and Nalox = naloxone.
Table 1.
Summary of Peak Change from Baseline Resultsa
| Methadone | Naloxone | Buprenorphine/naloxone | |||||
|---|---|---|---|---|---|---|---|
| F (df=4, 24)b | Placebo | (100 mg po) | (0.2 mg im) | Full | Split | Post hoc test results | |
| Subjective measures | |||||||
| Visual Analog Scales | |||||||
| High | 2.10 | 6.4 (3.0) | 35.4 (11.8) | 10.4 (6.7) | 12.3 (7.9) | 17.0 (6.5) | |
| Drug effects | 3.77 | 12.7 (4.9) | 55.7 (13.0) | 48.0 (13.2) | 49.7 (10.4) | 21.1 (6.9) | M>P |
| Good effects | 1.31 | 21.1 (12.5) | 32.6 (10.8) | 11.9 (9.5) | 17.0 (10.7) | 14.0 (5.0) | |
| Bad effects | 6.51 | 6.9 (6.5) | 13.4 (9.0) | 52.6 (12.2) | 47.0 (11.3) | 6.0 (3.9) | F>P,S; N>P,M,S |
| Liking | 2.38 | 18.7 (10.8) | 22.0 (9.8) | 3.0 (1.9) | 9.9 (6.5) | 4.1 (2.7) | |
| Sick | 10.73 | 0.1 (0.1) | 0.4 (0.3) | 48.0 (13.0) | 41.7 (12.3) | 3.1 (3.1) | F>P,M,S; N>P,M,S |
| Adjective rating scales | |||||||
| Agonist | 0.82 | 1.6 (0.8) | 4.4 (1.5) | 2.0 (1.1) | 1.4 (1.7) | 2.3 (1.6) | |
| Withdrawal | 4.05 | 1.4 (0.6) | 1.9 (0.8) | 13.1 (5.0) | 11.1 (2.4) | 4.3 (1.6) | N>P; F>M |
| Observer-rated measures | |||||||
| Adjective rating scales | |||||||
| Agonist | 3.43 | 2.3 (0.9) | 5.4 (1.0) | 1.9 (1.0) | 1.4 (0.7) | 2.9 (1.2) | M>F,N |
| Withdrawal | 1.65 | 1.6 (0.3) | 2.4 (0.8) | 11.7 (6.6) | 7.6 (2.0) | 5.4 (1.8) | |
| Withdrawal Signs Score | 6.03 | 1.3 (0.4) | 1.4 (0.5) | 6.3 (1.2) | 4.2 (1.5) | 4.7 (1.1) | N>P,M |
| Physiologic measures | |||||||
| Diastolic blood pressure | 4.38 | 3.1 (0.9) | 7.9 (1.9) | 9.1 (1.6) | 13.7 (2.6) | 11.4 (2.9) | F>P; S>P |
| Systolic blood pressure | 6.38 | 3.7 (2.9) | 5.3 (1.3) | 14.8 (2.2) | 17.3 (2.2) | 12.9 (4.5) | F>P,M; N>P |
| Heart rate | 5.55 | −2.2 (1.2) | −1.3 (1.4) | 6.1 (2.2) | 2.9 (2.1) | 0.8 (1.6) | N>P,M |
| Temperature | 3.15 | 1.5 (0.6) | 1.8 (0.4) | −0.3 (0.6) | 0.7 (0.5) | 1.8 (0.5) | M>N |
| Pupil diameter (max | 5.46 | 0.8 (0.2) | 0.6 (0.2) | 1.8 (0.2) | 1.8 (0.3) | 1.2 (0.3) | N>P,M; F>M |
| Pupil diameter (min | 3.95 | −1.1 (0.3) | −1.1 (0.3) | −0.3 (0.3) | −0.1 (0.2) | −0.5 (0.3) | |
| Oxygen saturation (%) | 5.57 | 0.9 (0.2) | 0.7 (0.3) | 1.2 (0.2) | 1.5 (0.2) | 1.3 (0.2) | F>P,M |
Results shown are for n=7 subjects except for oxygen saturation (n=6 due to missing data). Values are means (SEM). “Full” refers to the phase one dose of buprenorphine/naloxone that produced precipitated withdrawal, and “Split” is that same dose administered in two one-half increments separated by two hours. F=full, M=methadone, N=naloxone P=placebo, S=split.
Bold values indicate significant effect (p<0.05).
Elevations on visual analog scale ratings of sick and bad effects are consistent with precipitated opioid withdrawal. Both the full dose of buprenorphine/naloxone and the naloxone control condition produced significantly higher ratings on these two measures compared to placebo (bottom two panels of Figure 1, Table 1). The magnitude of effect produced by the acute dose of buprenorphine/naloxone was similar to that seen with naloxone. These effects also significantly differed from those seen with the split dose of buprenorphine/naloxone (which was virtually identical to the ratings associated with placebo), and ratings of sick and bad effects associated with methadone.
Naloxone and the full dose of buprenorphine/naloxone also produced the highest ratings on the subject withdrawal adjective rating scale (Table 1), while the split dose of buprenorphine/naloxone produced more modest ratings on this measure. In contrast, the agonist adjective rating scale produced modest peak effects for all conditions and none were significantly different from each other.
The pharmacologic class identification assessment provides a global characterization of the subjective effects produced by a study condition (Table 2). The placebo condition was most commonly identified as a placebo, methadone was most commonly identified as an opioid agonist, and naloxone was primarily identified as placebo and an opioid antagonist. The full dose of buprenorphine/naloxone was most commonly identified as an opioid antagonist, although one-third of identifications were as placebo. The split dose of buprenorphine/naloxone was most commonly identified as a placebo, and the second most common identification was as an opioid agonist.
Table 2.
Pharmacologic Class Identifications*
| Identifications | ||||
|---|---|---|---|---|
| Condition: | Placebo | Opioid Agonist | Opioid Antag | Other |
| Placebo # | 58 | 21 | 6 | 6 |
| % | 63.74 | 23.08 | 6.59 | 6.59 |
| Methadone # | 25 | 63 | 1 | 2 |
| % | 27.47 | 69.23 | 1.10 | 2.20 |
| Naloxone # | 52 | 8 | 27 | 4 |
| % | 57.14 | 8.79 | 29.67 | 4.40 |
| Full dose # | 34 | 12 | 41 | 1 |
| % | 38.64 | 13.64 | 46.59 | 1.14 |
| Split dose # | 54 | 21 | 15 | 0 |
| % | 60.00 | 23.33 | 16.67 | 0.00 |
Total number (#) of identifications was 91 for each condition (13 identifications for each of seven subjects); three identifications were missing for the full dose condition, and one for the split dose condition. Full dose refers to that dose of buprenorphine/naloxone which produced precipitated withdrawal; split dose refers to the corresponding dose of buprenorphine/naloxone that was given in two, divided doses.
3.1.2 Observer-rated effects
While subject ratings generally produced a pattern of effects for the split dose condition that was similar to that seen for placebo, observer ratings for the split dose condition were more similar to those seen for the full dose condition rather than placebo. For example, peak ratings for the WSS were 4.71 and 4.21 for the split and full dose conditions, respectively. Adjective withdrawal scores for the split condition also were more similar to the full dose condition than the placebo condition (Table 1). However, the highest observer ratings for withdrawal were associated with the naloxone control condition; both buprenorphine/naloxone conditions produced peak effects that were midway between the placebo and naloxone conditions. Similarly, the highest observer ratings of opioid agonist effects were seen for the methadone control condition.
3.1.3 Physiologic effects
The absolute greatest pupillary dilation, which is associated with opioid withdrawal, was seen with naloxone (Table 1), but the full dose of buprenorphine/naloxone produced a similar response. The split dose of buprenorphine/naloxone produced peak pupillary dilation that was greater than placebo but less than the full dose of buprenorphine/naloxone. As expected for a full agonist opioid, methadone produced peak pupillary dilation that was similar to placebo. Pupillary constriction, an indication of opioid agonist effects, was greatest for the methadone condition, while the naloxone and full buprenorphine/naloxone conditions produced minimal pupillary constriction (Table 1).
Other physiologic measures showed the buprenorphine/naloxone conditions had variable effects compared to the naloxone and methadone control conditions. For example, peak systolic blood pressure changes for the buprenorphine/naloxone conditions tended to be similar to that seen for the naloxone condition. In contrast, while naloxone produced a decrease in skin temperature, both buprenorphine/naloxone conditions produced increases in skin temperature, as did methadone (with the split dose producing an increase in temperature very similar to that seen with methadone; Table 1). Heart rate increased after naloxone and mildly decreased with methadone. The full dose of buprenorphine/naloxone produced a modest increase in heart rate (consistent with precipitated withdrawal), while the split dose of buprenorphine/naloxone produced essentially no change in heart rate (Table 1).
3.1.4 Psychomotor effects1
In general, there were no significant differences between conditions for peak changes on these tasks. Naloxone was associated with mild impairments on tasks consistent with precipitated withdrawal. For example, relative to placebo, total line length on the Trails B task decreased for naloxone; likewise, the total length decreased for the full dose buprenorphine/naloxone condition. However, total line length for the split buprenorphine/naloxone dose condition was very similar to that seen with placebo. Other measures showed minimal differences between conditions (e.g., errors on the Digit Recall).
3.2 Repeated administration of buprenorphine/naloxone over study phases
Subjects received the same dose of buprenorphine/naloxone on three separate occasions (twice in Phase 1 and once in Phase 2). There was a tendency for peak effects on several self-report measures related to opioid withdrawal to decrease across successive exposures (e.g., top and middle panels of Figure 2); this was also the case for pupillary dilation (bottom panel of Figure 2). However, this pattern was not consistently seen for all measures (e.g., observer ratings of withdrawal). While maximal ratings were often seen with the first exposure to a dose of buprenorphine/naloxone, for most measures there were not significant pairwise differences between exposures for the same dose of buprenorphine/naloxone2.
Figure 2.
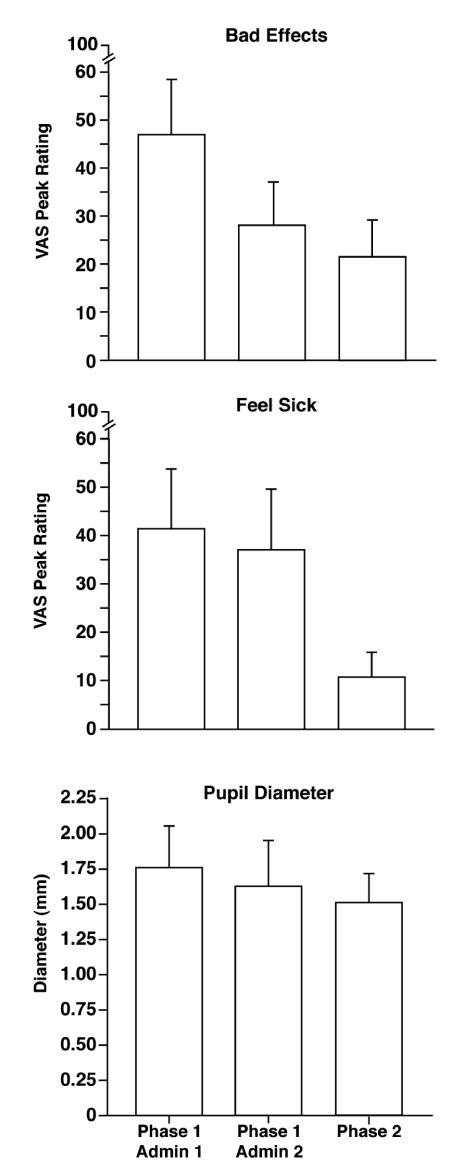
Results for doses of buprenorphine/naloxone that produced precipitated withdrawal. The dose that first produced precipitated withdrawal in the first phase (Phase 1 Admin 1) was repeated for confirmation of withdrawal effects (Phase 1 Admin 2). This dose was administered a third time in Phase 2 of the study. Each bar represents the mean peak value (SEM) for the seven subjects who entered the second phase of the study. VAS = visual analog scale.
4.0 Discussion
This study tested the acute effects of sublingual buprenorphine/naloxone in subjects maintained on 100 mg of daily methadone. This dose of methadone was selected to represent a relatively high level of physical dependence and is a dose commonly used in clinical practice. Given evidence that buprenorphine can precipitate withdrawal in subjects maintained on daily doses of 30 and 60 mg of methadone (Strain et al., 1995; Walsh et al., 1995), the selection of 100 mg per day for this study was thought to have a high likelihood of demonstrating buprenorphine-related precipitated withdrawal.
Three subjects in the study did not demonstrate buprenorphine-related precipitated withdrawal, despite the administration of acute buprenorphine/naloxone doses as high as 32/8 mg. The bioavailability and elimination rate of methadone can vary considerably between persons maintained on the same dose of methadone; thus, one possible explanation for the lack of withdrawal in these three subjects could have been relatively poor absorption of methadone or rapid elimination with subsequent low levels of physical dependence. However, an examination of their trough blood level results does not support such an explanation.
For participants who entered the second phase of the study, splitting the dose of buprenorphine/naloxone and administering it as two halves over a 2-hour interval resulted in minimal subject-reported precipitated withdrawal. The duration of buprenorphine's effects is long; it dissociates very slowly from the mu opioid receptor, and its terminal half-life may be as long as 32 hours (Chiang and Hawks, 2003; Marquet, 2002). This suggests that differences between split and full doses found in this study were not likely due to decreased buprenorphine availability from the first one-half dose at the time of the second half dosing (i.e., resulting in an overall lower exposure to buprenorphine). Alternatively, the observed precipitated withdrawal could be attributable to sublingual absorption of naloxone rather than buprenorphine, whereby splitting the dose diminished the impact of naloxone given its short half-life (approximately 60 minutes). However, studies have shown that sublingual naloxone has poor bioavailability (Harris et al., 2000; Preston et al., 1990; Strain et al., 2004), suggesting that the effects observed in the present study were not due to naloxone absorption.
The finding of diminished withdrawal with split dosing would thus seem most consistent with a process in which low cumulative levels exert minimally detectable effects that are tolerated and not significantly different from placebo. While significant precipitated withdrawal in response to the split dose was not seen on subject-rated measures (Figure 1, Table 1), observer ratings and physiologic measures did show a profile of withdrawal-related effects that was more similar to the full dose of buprenorphine/naloxone and the naloxone control condition. This disconnection between subjective versus observer and physiologic measures is somewhat surprising; participants might be expected to report any detection of withdrawal-like effects. However, pharmacologic class identifications (Table 2), which provide a global assessment of the type of drug effect detected (if any), showed that the split dose was rarely identified as an opioid antagonist, consistent with the other subject-rated measures. This disconnection between subjective and objective measures was not seen for the prototypic opioid antagonist naloxone nor the full dose of buprenorphine/naloxone (Table 1), suggesting these findings are specifically related to the conditions of the split dosing procedure.
Participants had three exposures to the same dose of buprenorphine/naloxone during the study. There was a tendency for subjects to report decreased effects across successive administrations (Figure 2)2, although these differences were not statistically significant, and this pattern was not clearly seen for observer ratings of withdrawal. It is unlikely participants developed marked tolerance to buprenorphine/naloxone effects, given the interval between exposures and their baseline level of physical dependence. However, some mild acclimation to the features of precipitated withdrawal may have occurred, especially for subjective effects, and this may have contributed to the observed decrease in withdrawal ratings associated with the split dose condition.
There are several limitations to this study that should be noted. For example, while variability in methadone blood levels was assessed, variability in buprenorphine absorption was not examined. Variations in buprenorphine blood levels might contribute to differences seen in the first phase of the study. There was also a limited sample size for the phase one study results, suggesting these findings be interpreted with caution. The phase two results, however, represent a within subject study that are of primary interest. Finally, this study addresses acute response to buprenorphine, but did not examine a chronic dosing regimen more consistent with clinical practice.
These findings have implications for the use of buprenorphine/naloxone. Specifically, they suggest that patients with high levels of physical dependence, including those maintained on daily doses of methadone of up to 100 mg, may receive repeated small doses of buprenorphine/naloxone and not experience significant precipitated withdrawal. Furthermore, as three participants in the present study had no evidence of buprenorphine-related precipitated withdrawal even with high acute doses of buprenorphine, this suggests there may be some patients with higher levels of physical dependence who find it particularly easy to tolerate the transition to buprenorphine. While the study used a model based upon methadone maintenance, the findings may be extended to patients with higher levels of physical dependence on other mu agonist opioids. The findings also suggest that partial agonist-related precipitated withdrawal is not an inevitable outcome for persons with high levels of physical dependence, and that the bolus of drug administration is an important variable in its production. The administration of repeated, small doses of buprenorphine/naloxone may be the optimal mechanism for transitioning patients with higher levels of opioid physical dependence onto sublingual buprenorphine/naloxone.
Supplementary Material
Acknowledgments
Supported by U.S. Public Health Service Scientist Development Award K02 DA00332 and R01 DA08045 from the National Institute on Drug Abuse. Dr. Bigelow has received, or anticipates receiving, research support from Purdue Pharma L.P., Biotek, Inc. and Titan Pharmaceuticals, Inc. for studies of other buprenorphine formulations. Drs. Strain and Walsh have served as speakers for Reckitt Benckiser, and Dr. Strain has provided consulting services to Titan Pharmaceuticals. Some of these data were presented at the annual meeting of the College on Problems of Drug Dependence, San Juan, Puerto Rico, June of 2004.
Footnotes
A table showing peak change from baseline results for all three full dose administrations of buprenorphine/naloxone can be found as supplementary material by accessing the online version of this paper at http://dx.doi.org by entering doi:xxxxxxxxx
A summary of analyses for psychomotor tasks can be found in the online supplementary table by accessing the online version of this paper at http://dx.doi.org by entering doi:xxxxxxxxx.
Data for all three full dose administrations, along with the split dose administration and all Phase 1 control conditions, are available in the online supplementary table.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chiang CN, Hawks RL. Pharmacokinetics of the combination tablet of buprenorphine and naloxone. Drug Alcohol Depend. 2003;70:S39–47. doi: 10.1016/s0376-8716(03)00058-9. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Hitzemann RJ, Somoza E, Knapp CM, Rotrosen J, Sarid-Segal O, Ciraulo AM, Greenblatt DJ, Chiang CN. Pharmacokinetics and pharmacodynamics of multiple sublingual buprenorphine tablets in dose-escalation trials. J Clin Pharmacol. 2006;46:179–92. doi: 10.1177/0091270005284192. [DOI] [PubMed] [Google Scholar]
- Clark NC, Lintzeris N, Muhleisen PJ. Severe opiate withdrawal in a heroin user precipitated by a massive buprenorphine dose. Med J Aust. 2002;176:166–7. [PubMed] [Google Scholar]
- Correia CJ, Walsh SL, Bigelow GE, Strain EC. Effects associated with double-blind omission of buprenorphine/naloxone over a 98-h period. Psychopharmacology (Berl) 2006;189:297–306. doi: 10.1007/s00213-006-0571-4. [DOI] [PubMed] [Google Scholar]
- Doxey JC, Everitt JE, Frank LW, MacKenzie JE. A comparison of the effects of buprenorphine and morphine on the blood gases of conscious rats. Br J Pharmacol. 1982;75:118P. [Google Scholar]
- Eap CB, Bertschy G, Baumann P, Finkbeiner T, Gastpar M, Scherbaum N. High interindividual variability of methadone enantiomer blood levels to dose ratios. Arch Gen Psychiatry. 1998;55:89–90. doi: 10.1001/archpsyc.55.1.89. [DOI] [PubMed] [Google Scholar]
- Eap CB, Buclin T, Baumann P. Interindividual Variability of the Clinical Pharmacokinetics of Methadone: Implications for the Treatment of Opioid Dependence. Clin Pharmacokinet. 2002;41:1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- Fraser HF, Van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (A) “Attitude” of opiate addicts toward opiate-like drugs, (B) A short-term “direct” addiction test. J Pharmacol Exp Ther. 1961;133:371–87. [PubMed] [Google Scholar]
- Harris DS, Jones RT, Welm S, Upton RA, Lin E, Mendelson J. Buprenorphine and naloxone co-administration in opiate-dependent patients stabilized on sublingual buprenorphine. Drug Alcohol Depend. 2000;61:85–94. doi: 10.1016/s0376-8716(00)00126-5. [DOI] [PubMed] [Google Scholar]
- Harris DS, Mendelson JE, Lin ET, Upton RA, Jones RT. Pharmacokinetics and subjective effects of sublingual buprenorphine, alone or in combination with naloxone: lack of dose proportionality. Clin Pharmacokinet. 2004;43:329–40. doi: 10.2165/00003088-200443050-00005. [DOI] [PubMed] [Google Scholar]
- Horns WH, Rado M, Goldstein A. Plasma levels and symptom complaints in patients maintained on daily dosage of methadone hydrochloride. Clin Pharmacol Ther. 1975;17:636–49. doi: 10.1002/cpt1975176636. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. Assessment of the abuse potentiality of morphine-like drugs (methods used in man) In: Martin WR, editor. Drug Addiction I. Springer-Verlag; New York: 1977. pp. 197–258. [Google Scholar]
- Kreek MJ. Plasma and urine levels of methadone. Comparison following four medication forms used in chronic maintenance treatment. N Y State J Med. 1973;73:2773–7. [PubMed] [Google Scholar]
- Liguori A, Morse WH, Bergman J. Respiratory effects of opioid full and partial agonists in rhesus monkeys. J Pharmacol Exp Ther. 1996;277:462–72. [PubMed] [Google Scholar]
- Ling W, Wesson DR. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend. 2003;70:S49–57. doi: 10.1016/s0376-8716(03)00059-0. [DOI] [PubMed] [Google Scholar]
- Marquet P. Pharmacology of high-dose buprenorphine. In: Kintz P, Marquet P, editors. Buprenorphine therapy of opiate addiction. Humana Press; Totowa, New Jersey: 2002. pp. 1–11. [Google Scholar]
- Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2004:CD002207. doi: 10.1002/14651858.CD002207.pub2. [DOI] [PubMed] [Google Scholar]
- McLeod D, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods Instrum. 1982;14:463–6. [Google Scholar]
- McNicholas L. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. U.S. Department of Health and Human Services; 2004. [Google Scholar]
- Preston KL, Bigelow GE, Liebson IA. Effects of sublingually given naloxone in opioid-dependent human volunteers. Drug Alcohol Depend. 1990;25:27–34. doi: 10.1016/0376-8716(90)90136-3. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Perceptual Motor Skills. 1958;8:271–6. [Google Scholar]
- Stoller KB, Bigelow GE, Walsh SL, Strain EC. Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl) 2001;154:230–42. doi: 10.1007/s002130000637. [DOI] [PubMed] [Google Scholar]
- Strain EC. High dose buprenorphine for the treatment of opioid dependence. In: Kintz P, Marquet P, editors. Buprenorphine Therapy of Opiate Addiction. Humana Press; Totowa, New Jersey: 2002. pp. 29–49. [Google Scholar]
- Strain EC. Pharmacology of buprenorphine. In: Strain EC, Stitzer ML, editors. The Treatment of Opioid Dependence. The Johns Hopkins University Press; Baltimore, Maryland: 2006. pp. 213–229. [Google Scholar]
- Strain EC, Moody DE, Stoller KB, Walsh SL, Bigelow GE. Relative bioavailability of different buprenorphine formulations under chronic dosing conditions. Drug Alcohol Depend. 2004;74:37–43. doi: 10.1016/j.drugalcdep.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Strain EC, Preston KL, Liebson IA, Bigelow GE. Acute effects of buprenorphine, hydromorphone and naloxone in methadone- maintained volunteers. J Pharmacol Exp Ther. 1992;261:985–93. [PubMed] [Google Scholar]
- Strain EC, Preston KL, Liebson IA, Bigelow GE. Precipitated withdrawal by pentazocine in methadone-maintained volunteers. J Pharmacol Exp Ther. 1993;267:624–34. [PubMed] [Google Scholar]
- Strain EC, Preston KL, Liebson IA, Bigelow GE. Buprenorphine effects in methadone-maintained volunteers: effects at two hours after methadone. J Pharmacol Exp Ther. 1995;272:628–38. [PubMed] [Google Scholar]
- Strain EC, Stoller K, Walsh SL, Bigelow GE. Effects of buprenorphine versus buprenorphine/naloxone tablets in non- dependent opioid abusers. Psychopharmacology (Berl) 2000;148:374–83. doi: 10.1007/s002130050066. [DOI] [PubMed] [Google Scholar]
- Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE. The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacology (Berl) 1997;129:329–38. doi: 10.1007/s002130050199. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70:S13–27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Walsh SL, June HL, Schuh KJ, Preston KL, Bigelow GE, Stitzer ML. Effects of buprenorphine and methadone in methadone-maintained subjects. Psychopharmacology (Berl) 1995;119:268–76. doi: 10.1007/BF02246290. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–80. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


