Abstract
Background
There are few data comparing risk factors for catheter-related (CR) versus non-CR bloodstream infection (BSI) or for BSI caused by gram-positive versus gram-negative organisms. The aims of this study were to compare risk factors for CR versus non-CR BSI and to compare risk factors for BSI associated with gram-negative versus gram-positive organisms among infants hospitalized in two neonatal intensive care units (NICUs).
Methods
Data were collected prospectively over a 2-year period to assess risk factors among 2,935 neonates from two NICUs.
Results
Among all neonates, in addition to low birth weight and presence of a central venous catheter, hospitalization in NICU 1 (relative risk [RR]: 1.60, 95% confidence intervals [CI]: 1.14, 2.24) was a significant predictor of BSI. In neonates with a central catheter total parenteral nutrition (TPN) was a significant risk factor for BSI (RR: 4.69, 95% CI: 2.22, 9.87). Ventilator use was a significant risk factor for CR versus non-CR BSI (RR: 3.74, 95% CI: 1.87, 7.48), and significantly more CR BSI were caused by gram-positive (77.1%) than by gram-negative organisms (61.4%), P = .03.
Conclusions
This study confirmed that central venous catheters and low birth weight were risk factors for neonates with late-onset healthcare-associated BSI and further elucidated the potential risks associated with TPN and ventilator use in subgroups of neonates with BSI. Additional studies are needed to examine the incremental risk of TPN among infants with central venous catheters and to understand the link between CR BSI and ventilator use. Preventive strategies for BSI in neonates in NICUs should continue to focus on limiting the use of invasive devices.
Health care-associated infections (HAI) among infants hospitalized in the neonatal intensive care unit (NICU) are a significant cause of morbidity and mortality and result in substantial health care costs.1-5 The most common type of neonatal HAI is bloodstream infections (BSI), often associated with the use of central venous catheters.6-8 The National Nosocomial Infections Surveillance (NNIS) System has reported rates of 9.1 umbilical and central line-associated BSI per 1000 device days for infants ≤1000 g, and 5.4 infections per 1000 catheter days for infants 1001 to 1500 g.9 Because advancing technology enables neonates to survive at lower birth weights, the number of preterm infants hospitalized in NICUs will continue to increase as will health care-associated BSI in this vulnerable population.
Although central venous catheters are a well-established risk factor for BSI,1,5 there are few data comparing risk factors for catheter-related (CR) BSI with risk factors for non-CR BSI or risk factors for BSI caused by gram-positive as compared with gram-negative pathogens. Hence, the aims of this study were to compare risk factors for bacterial CR versus non-CR BSI and to compare risk factors for BSI associated with gram-negative versus gram-positive organisms among infants hospitalized in 2 NICUs in different hospitals in the same health care system.
METHODS
Sample and setting
This study was 1 component of a larger prospective trial to assess the effect of staff hand hygiene on HAI in neonates performed in 2 NICUs from March 2001 through January 2003.10 During the study, either a traditional antiseptic soap containing 2% chlorhexidine gluconate or a waterless hand rinse containing 60% ethyl alcohol was used by all staff and visitors sequentially for half of the study period (11 months for each product) in a randomly assigned crossover design; no significant differences in infection rates were found in the parent study during the periods when either hand hygiene product was used. NICU 1 and NICU 2 are part of the New York-Presbyterian Hospital system in New York City and had 43 beds and 50 beds, respectively, during the study period. All neonates hospitalized for at least 24 hours were included in the analysis. The institutional review boards from both hospitals approved the study.
Despite the fact that the 2 study NICUs had the same intravascular catheter insertion and care policies, other characteristics of the 2 study NICUs varied. In NICU 1, more surgical procedures were performed, and more nasal cannula continuous positive airway pressure (NC-CPAP) was used. Furthermore, NICU 1 had approximately half the space per isolette as compared with NICU 2, and had more infants transferred from other hospitals and significantly more infants whose birth weight was < 1000 g compared with NICU 2 (12.8% and 8.7%, respectively, P < .001). Although the nurse-to-patient ratio in both units was the same (1:1 or 1:2), NICU 2 used more temporary staff than NICU 1. Nurses in NICU 2 were significantly younger and had worked fewer years on the unit than nurses in NICU 1.
Data collection and procedures
Data regarding BSI and risk factors were collected by an experienced infection control nurse hired for the study. Data were obtained from patient medical records, direct observation, and interviews with staff as well as laboratory and radiology reports. Birth weight; sex; length of stay; surgical procedures; and days of use of central venous catheters, ventilator, or NC-CPAP were recorded prospectively. For the purposes of this study, data for neonates with bacterial BSI were included from the day of admission to the day of first positive blood culture associated with the first episode of BSI, whereas data on neonates without BSI were included for the entire length of their hospital stay.
Definitions of variables
The outcome variable was the first occurrence of bacterial BSI. A BSI was defined according to the Centers for Disease Control and Prevention guidelines.11 The number of catheter days was calculated from the day of insertion to the day of removal minus 1 day. Cases of early-onset sepsis that occurred within the first 48 hours of life were excluded from this analysis. A health care-associated CR BSI (ie, late-onset sepsis) was defined as a BSI in the presence of a catheter with no other identifiable site of infection. A non-CR BSI was defined as a BSI that occurred without a central venous catheter present or when a catheter was present but another site of infection, such as a urinary tract infection or necrotizing enterocolitis with perforation, resulted in a secondary BSI.12 To avoid misclassification, the study nurse routinely reviewed equivocal cases with a physician coinvestigator in each NICU.
Data analysis
Descriptive statistics were calculated on variables including birth weight, sex, length of stay, study site, type of hand hygiene product used, and presence of and number of days of use of the following potential risk factors: ventilator, NC-CPAP, central venous catheter, total parenteral nutrition (TPN), and surgery. In addition, these risk factors were examined in different subgroups, including neonates with CR versus non-CR BSI and BSI caused by gram-positive versus gram-negative pathogens. For each aim, in bivariate analyses, the χ2 test was used for categorical variables, and either the Student t test or the Mann-Whitney test was used for continuous variables. Stepwise logistic regression models were then fit using predictors significant in the bivariate analyses (P < .05). For the logistic regression models comparing BSI causing gram-positive and gram-negative pathogens, 10 polymicrobial infections were excluded. Two-sided statistical tests were performed using SPSS software for Windows (version 13.0; SPSS, Inc., Chicago, IL), and a P value,.05 was considered statistically significant.
RESULTS
The characteristics of neonates with and without a health care-associated BSI are summarized in Table 1. In all, 2935 neonates were included in this study. Of these, 205 (7.0%) developed a bacterial BSI. The mean age of neonates at the time of the first health care-associated BSI was 21.4 days in NICU 1 and 35.7 days in NICU 2. Eighteen percent of neonates developed their first bacterial BSI after 30 days of life. Rates of BSI by unit were 18.5/1000 catheter days in NICU 1 and 13.0/1000 catheter days in NICU 2. Overall, 144 (70%) infections occurred in NICU 1 and 61 (30%) in NICU 2 (P < .001).
Table 1.
Characteristics of neonates with and without bacterial BSIs*
| Case (n = 205) |
Non-Case (n = 2730) |
||||
|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | P value† | |
| Sex | |||||
| Female | 96 (46.8) | 1199 (44.0) | .42 | ||
| Male | 109 (53.1) | 1531 (56.4) | |||
| Site | |||||
| NICU 1 | 144 (70.2) | 1550 (56.8) | <.001 | ||
| NICU 2 | 61 (29.8) | 1180 (43.2) | |||
| Length of stay (mean days) | 62.3 (42.9) | 14.3 (19.9) | <.001 | ||
| Birth weight (g) | |||||
| Mean birth weight | 1420 (947) | 2495 (997) | <.001 | ||
| <1000 | 104 (50.7) | 220 (8.1) | <.001 | ||
| 1000-1500 | 37 (18.0) | 296 (10.8) | |||
| 1501-2500 | 28 (13.7) | 841 (30.8) | |||
| >2500 | 36 (17.6) | 1373 (50.3) | |||
| Surgery | |||||
| Yes | 59 (28.8) | 437 (16.0) | <.001 | ||
| No | 146 (71.2) | 2293 (84.0) | |||
| Ventilator | |||||
| Yes | 138 (67.3) | 771 (28.2) | <.001 | ||
| No | 67 (32.7) | 1959 (71.8) | |||
| Ventilator days | 8.2 (12.0) | 2.2 (7.0) | <.001 | ||
| NC-CPAP | |||||
| Yes | 145 (70.7) | 1244 (45.6) | <.001 | ||
| No | 60 (29.3) | 1486 (54.4) | |||
| NC-CPAP days | 8.0 (9.5) | 3.5 (9.0) | <.001 | ||
| Central venous catheter‡ | |||||
| Yes | 182 (88.8) | 950 (34.8) | <.001 | ||
| No | 23 (11.2) | 1780 (65.2) | |||
| CVC days | 16.4 (14.2) | 4.4 (9.7) | <.001 | ||
| TPN | |||||
| Yes | 191 (93.2) | 1101 (40.3) | <.001 | ||
| No | 14 (6.8) | 1629 (59.7) | |||
| TPN days (mean) | 16.1 (13.8) | 4.7 (14.4) | <.001 | ||
| Hand hygiene product | |||||
| 2% Chlorhexidine | 95 (46.3) | 1421 (52.1) | .12 | ||
| Alcohol based | 110 (53.7) | 1309 (47.9) | |||
BSI, bloodstream infection; NC-CPAP, nasal cannula continuous positive airway pressure; TPN, total parenteral nutrition; CVC, central venous catether.
N = 2935.
All P values are for χ2 tests for categorical variables and t tests for continuous variables.
Includes all central venous catheters, ie, peripherally inserted central catheter (PICC), umbilical catheters, and Broviac catheters.
Risk factors for BSI
Significantly more neonates with BSI weighed < 1000 g as compared with neonates who did not develop BSI (50.7% and 8.1%, respectively, P < .001). Neonates with BSI were more likely to have had a central venous catheter (88.8% vs 34.8%, respectively, P < .001). In the bivariate analyses, neonates with BSI were more likely to have had surgery, have been on a ventilator, have been on NC-CPAP (all P < .001), and have had a longer mean length of stay (62.3 vs 14.3 days, respectively, P < .001) when compared with those without a BSI. The risk of BSI did not differ by the hand hygiene product used (P = .12).
In the final logistic regression model site, birth weight and presence of a central venous catheter were significant predictors of BSI (Table 2 section A). Neonates in NICU 2 had a longer average length of stay than those in NICU 1 (23.9 vs 17.0 days, respectively, P = .005) but a lower incidence of BSI; neonates from NICU 1 were 1.60 times more likely to develop a BSI than those from NICU 2 (95% confidence interval (CI): 1.14-2.24). Each 100 g decrease in birth weight conferred an additional 9.0% risk of developing a BSI (95% CI: 1.07-1.11). The presence of a central venous catheter was the greatest risk factor for BSI; infants with a catheter had a 9.3-fold increased risk of developing a BSI when compared with infants without a catheter (95% CI: 5.87-14.77).
Table 2.
Logistic regression models for predictors of BSI in different subgroups of NICU patients
| Subgroup | Significant variables | Relative risk | 95% Confidence interval |
|---|---|---|---|
| A. All neonates (N = 2935) | Site NICU 1 NICU 2 Birth weight* Central venous catheter† |
1.60 Referent 1.09 9.31 |
1.14-2.24 1.07-1.11 5.87-14.77 |
| B. Neonates with central venous catheter (n = 1132) | Birth weight* Total parenteral nutrition† |
1.09 4.69 |
1.07-1.12 2.22-9.87 |
| C. Neonates with CR BSI (compared with non-CR BSI) (n = 205) | Birth weight* Ventilator† |
1.05 3.74 |
1.02-1.09* 1.87-7.48 |
| D. Neonates with gram-positive pathogens (compared with gram-negative pathogens) (n = 195) | Site NICU 1 NICU 2 |
2.34 Referent |
1.16-4.73 |
Per 100 gram decrease.
Referent category is absence of variable or procedure.
Risk factors for BSI among neonates with a central venous catheter
This subgroup analysis assessed risk factors for BSI among the 1132 neonates who had at least 1 central venous catheter in place during their NICU stay, of whom 182 (16.1%) developed a BSI. The mean age at diagnosis of BSI in this subgroup analysis was 27.8 days of life (SD ± 36.4). In a logistic regression model of risk factors in this subgroup, birth weight and use of TPN remained significant predictors of BSI (Table 2 section B). Thus, among neonates with a central venous catheter, those who received TPN were 4.7 times more likely to have a BSI than those who did not receive TPN (95% CI: 2.22-9.87). Study site was not a significant predictor of BSI in this model.
Risk factors for catheter-related versus non-CR BSI
This subgroup analysis assessed risk factors in the 149 neonates with a CR BSI versus the 56 neonates with a non-CR BSI. Among the latter, 33 of 56 (58.9%) neonates had a central venous catheter during their hospitalization but were diagnosed with a non-CR BSI either because an alternative site of infection was identified associated with a secondary BSI or because the catheter had been removed before the BSI developed. Non-CR BSI were more likely to occur during the first 2 weeks of life (P < .001, Fig 1A). In a logistic regression model, birth weight and use of a ventilator remained significant risk factors for CR BSI compared with non-CR BSI. Neonates on mechanical ventilation were 3.7 times more likely to experience a CR BSI than a non-CR BSI (95% CI: 1.87-7.48, Table 2 section C).
Fig 1.
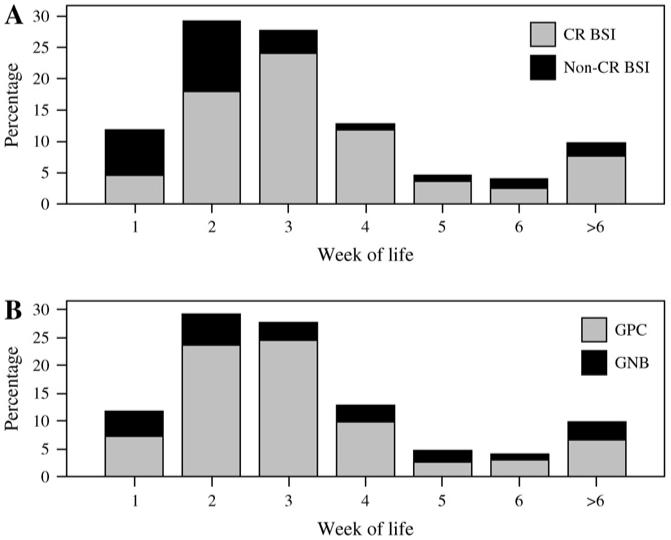
(A) Percentage of catheter-related versus non-CR BSI among neonates in the NICU by week of life. (B) Percentage of gram-positive (GPC) versus gram-negative (GNB) organisms associated with BSI among neonates in the NICU by week of life (excludes polymicrobial infections).
Risk factors for BSI caused by gram-positive versus gram-negative organisms
Overall, 205 episodes of BSI were caused by 179 gram-positive organisms and 57 gram-negative organisms (Table 3). The mean age of neonates with BSI caused by gram-positive organisms was similar to the mean age of neonates with BSI caused by gram-negative organisms (23.7 and 25.4 days, respectively, P = .79). The relative proportion of BSI caused by gram-positive compared with gram-negative organisms was not significantly different by week of life (P = .08,Fig 1B). Significantly more gram-positive BSI were catheter-related (77.1%, 138/179) compared with gram-negative BSI that were catheter-related (61.4%, P = .03). In bivariate analysis, birth weight, surgery, use of a central venous catheter, and hand hygiene product were not significantly different among neonates with a BSI caused by a gram-positive versus a gram-negative organism. Although NC-CPAP appeared to be a significant predictor of BSI caused by gram-positive organisms (data not shown), NC-CPAP was not included in the logistic regression analysis because usage was confounded by site, ie, NC-CPAP was used primarily in NICU 1. In the logistic regression, neonates in NICU 1 were 2.3 times more likely to have a BSI caused by gram-positive organisms than neonates in NICU 2 (95% CI: 1.16-4.73, Table 2 section D).
Table 3.
Species isolated from neonates with catheter- and non-catheter-related BSI*
| CR BSI (n = 173 isolates) | Non-CR BSI (n = 63 isolates) | N (%) | |
|---|---|---|---|
| Gram-positive organisms | |||
| Staphylococci | |||
| Staphylococcus aureus | 23 | 2 | 25 (13.9) |
| Staphylococcus epidermidis | 69 | 24 | 93 (51.7) |
| Staphylococcus warneri | 12 | 2 | 14 (7.8) |
| Other coagulase-negative staphylococci | 15 | 8 | 23 (12.8) |
| Enterococci | |||
| Enterococcus faecalis | 15 | 3 | 18 (10.0) |
| Enterococcus faecium | 1 | 0 | 1 (0.6) |
| Other gram-positive organisms | 3 | 2 | 5 (2.8) |
| Total gram-positive organisms | 138 (79.8) | 41 (65.1) | 179 (100) |
| Gram-negative organisms | |||
| Klebsiella pneumoniae | 9 | 11 | 20 (35.7) |
| Escherichia coli | 7 | 7 | 14 (25.0) |
| Enterobacter cloacae | 6 | 1 | 7 (12.5) |
| Pseudomonas aeruginosa | 5 | 0 | 5 (8.9) |
| Serratia marcescens | 4 | 1 | 5 (8.9) |
| Other gram-negative organisms | 4 | 2 | 6 (10.7) |
| Total gram-negative organisms | 35 (20.2) | 22 (34.9) | 57 (100) |
| Total number of isolates* | 173 (100) | 63 (100) | 236 |
n = 236 isolates in 205 neonates.
DISCUSSION
This is one of the largest studies to assess prospectively the risk factors for late-onset health care-associated BSI in the NICU population. Not surprisingly, we found that central venous catheters and lower birth weight placed infants at a higher risk of developing bacterial BSI, as has been shown in other studies.6-8,13,14 We also noted that study site was a significant predictor of BSI, even after controlling for differences in birth weight. Although neonates in NICU 2 had a longer average length of stay, they had a significantly lower risk of BSI. This discrepancy between the 2 NICUs could be explained in part by unmeasured host factors, underlying differences in patient care practices, or the physical environment. Notably, NICU 1 had significantly more neonates with low birth weight, performed more surgical procedures, used more NC-CPAP, was more crowded, and experienced outbreaks of Klebsiella pneumoniae and Staphylococcus epidermidis.15,16
This study was also large enough to allow us to examine risk factors for BSI (after controlling for birth weight) among subgroups of neonates, such as infants with central venous catheters, those with non-CR BSI, and those with BSI caused by gram-positive versus gram-negative organisms. Such subgroup analyses provide more precise estimates of risk, which can be used to guide clinical practice. Although both central venous catheters and TPN use have been associated with BSI in other studies,7,17-20 the independent contribution of TPN given the presence of a catheter has been less clear. Subgroup analyses of patients with catheters were potentially helpful in clarifying the independent contribution of TPN. In addition, study site was no longer predictive when only risk factors for CR-BSI were assessed, implying that the use of central catheters and study site were linked. This could be due to differences in the proportion of low-birth-weight infants between the NICUs or the increased frequency of surgery. Finally, the pathophysiology of the association between TPN and BSI has not been fully described, but studies performed in vitro and in vivo have suggested that TPN adversely affects phagocytosis in neutrophils and increases epithelial permeability.19,21
Among the subgroup of infants with a CR BSI as compared with those with a non-CR BSI, ventilator use was associated with an increased risk of the former, controlling for birth weight. An increased risk of sepsis associated with mechanical ventilation has previously been reported17,18,22 but not specifically in neonates. Furthermore, Raad et al23 noted an association between CR BSI, TPN, and mechanical ventilation among adult oncology patients. It is likely that more severely ill neonates required the use of both a ventilator and a catheter, but unmeasured factors such as preexisting colonization of the respiratory tract with potential pathogens may have increased the risk for CR BSI, as has been noted for candidemia.24,25
In this study population, CR BSI were more likely than non-CR BSI to be caused by gram-positive than gram-negative organisms. Gram-positive skin flora may be more likely to colonize the catheter during catheter insertion and use, whereas infections with gram-negative organisms may be more commonly associated with translocation of flora from the gut or respiratory or urinary tract. Neonates in NICU 1 were more likely to have a BSI with gram-positive organisms than neonates in NICU 2. This observation may have reflected an outbreak of S epidermidis in NICU 1.16
There were limitations to this study. We did not assess several potential confounding factors such as delays in enteral feeding, use of intralipids or antibiotics, crowding, or understaffing. No severity of illness indicator was used to assess neonatal acuity, but birth weight, the primary predictor of risk for infection, was assessed and included in analyses. It is possible that ventilator use, found to be predictive in our logistic model comparing neonates with CR- and non-CR BSI, was actually a surrogate marker for severity of illness. Despite the clear definitions and the interrater reliability assessment that we used, some misclassification of CR versus non-CR BSI may have occurred. Because NC-CPAP was used predominantly in NICU 1, this practice was excluded from the logistic regression models, but the use of NC-CPAP may have been partly responsible for the association between site and BSI rates because of intranasal trauma leading to translocation of bacteria into the bloodstream. Finally, we did not include fungal BSI in these analyses because those data have been reported elsewhere.26
In summary, this study confirmed the risk factors of central venous catheters and low birth weight for neonates with health care-associated bacterial BSI. This study further elucidated the potential increased risks associated with TPN and ventilation in subgroups of neonates. Additional studies are needed to examine the incremental risk of TPN among infants with central venous catheters and to understand the link between CR BSI and ventilator use. Preventive strategies for BSI in neonates in NICU should continue to focus on limiting the use of invasive devices.
Acknowledgments
Supported by grant 5 RO1 NR05197 from the National Institutes of Health National Institute for Nursing Research, Bethesda, MD.
The authors thank colleagues J. Cimiotti, P. Della-Latta, M. Nesin, and J. Haas, who were coinvestigators on the larger study from which this analysis was conducted, and the staff of the 2 neonatal intensive care units that participated in the study.
References
- 1.Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Semin Perinatol. 2003;27:293–301. doi: 10.1016/s0146-0005(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 2.Wright LL, Carlo WA, Ehrenkranz RA, Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(1 pt 1):285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 3.Brady MT. Healthcare-associated infections in the neonatal intensive care unit. Am J Infect Control. 2005;33:268–75. doi: 10.1016/j.ajic.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pessoa-Silva CL, Richtmann R, Calil R, Santos RM, Costa ML, Frota AC, et al. Healthcare-associated infections among neonates in Brazil. Infect Control Hosp Epidemiol. 2004;25:772–7. doi: 10.1086/502475. [DOI] [PubMed] [Google Scholar]
- 5.Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114:348–55. doi: 10.1542/peds.114.2.348. [DOI] [PubMed] [Google Scholar]
- 6.Sohn AH, Garrett DO, Sinkowitz-Cochran RL, et al. Prevalence of nosocomial infections in neonatal intensive care unit patients: results from the first national point-prevalence survey. Pediatrics. 2001;139:821–7. doi: 10.1067/mpd.2001.119442. [DOI] [PubMed] [Google Scholar]
- 7.Mahieu LM, De Muynck AO, Ieven MM, De Dooy JJ, Goossens HJ, Van Reempts PJ. Risk factors for central vascular catheter-associated bloodstream infections among patients in a neonatal intensive care unit. J Hosp Infect. 2001;48:108–16. doi: 10.1053/jhin.2001.0984. [DOI] [PubMed] [Google Scholar]
- 8.Auriti C, Maccallini A, Di Liso G, Di Ciommo V, Ronchetti MP, Orzalesi M. Risk factors for nosocomial infections in a neonatal intensive-care unit. J Hosp Infect. 2003;53:25–30. doi: 10.1053/jhin.2002.1341. [DOI] [PubMed] [Google Scholar]
- 9.A report from the NNIS System. National Nosocomial Infections Surveillance (NNIS) system report data summary from January 1992 through June 2004, issued October 2004 Am J Infect Control 200432470–85. [DOI] [PubMed] [Google Scholar]
- 10.Larson E, Cimiotti J, Haas J, et al. Effect of antiseptic handwashing vs alcohol sanitizer on health care-associated infections in neonatal intensive care units. Arch Pediatr Adolesc Med. 2005;159:377–83. doi: 10.1001/archpedi.159.4.377. [DOI] [PubMed] [Google Scholar]
- 11.Garner J, Dul M, Hoyen C, et al. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 12.Larson E, Cimiotti J, Haas J, et al. Gram-negative bacilli associated with catheter-associated and noncatheter-associated bloodstream infections and hand carriage by health care workers in neonatal intensive care units. Pediatr Crit Care Med. 2005;6:457–61. doi: 10.1097/01.PCC.0000163669.37340.91. [DOI] [PubMed] [Google Scholar]
- 13.Huang YF, Hsieh KS, Liu YC, Feldman RG. The predisposing factors of coagulase negative staphylococcal bacteraemia in neonatal intensive care unit. Acta Paediatr Taiwan. 2001;42:22–6. [PubMed] [Google Scholar]
- 14.Beck-Sague CM, Azimi P, Fonseca SN, et al. Bloodstream infections in neonatal intensive care unit patients: results of a multicenter study. Pediatr Infect Dis J. 1994;13:1110–6. [PubMed] [Google Scholar]
- 15.Gupta A, Della-Latta P, Todd B, et al. Outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit linked to artificial nails. Infect Control Hosp Epidemiol. 2004;25:210–5. doi: 10.1086/502380. [DOI] [PubMed] [Google Scholar]
- 16.Milisavljevic V, Wu F, Cimiotti J, et al. Genetic relatedness of Staphylococcus epidermidis from infected infants and staff in the neonatal intensive care unit. Am J Infect Control. 2005;33:341–7. doi: 10.1016/j.ajic.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Avila-Figueroa C, Goldmann DA, Richardson DK, Gray JE, Ferrari A, Freeman J. Intravenous lipid emulsions are the major determinant of coagulase-negative staphylococcal bacteremia in very low birth weight newborns. Pediatr Infect Dis J. 1998;17:10–7. doi: 10.1097/00006454-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Roilides E, Kyriakides G, Kadiltsoglou I, et al. Septicemia due to multi-resistant Klebsiella pneumoniae in a neonatal unit: a case-control study. Am J Perinatol. 2000;17:35–9. doi: 10.1055/s-2000-7290. [DOI] [PubMed] [Google Scholar]
- 19.Kansagra K, Stoll B, Rognerud C, et al. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1162–70. doi: 10.1152/ajpgi.00243.2003. [DOI] [PubMed] [Google Scholar]
- 20.Donowitz LG, Haley CE, Gregory WW, Wenzel RP. Neonatal intensive care unit bacteremia: emergence of gram-positive bacteria as major pathogens. Am J Infect Control. 1987;15:141–7. doi: 10.1016/0196-6553(87)90137-4. [DOI] [PubMed] [Google Scholar]
- 21.Okada Y, Klein NJ, van Saene HK, Webb G, Holzel H, Pierro A. Bactericidal activity against coagulase-negative staphylococci is impaired in infants receiving long-term parenteral nutrition. Ann Surg. 2000;231:276–81. doi: 10.1097/00000658-200002000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin CY, Zhang H, Cheng KC, Slutsky AS. Mechanical ventilation may increase susceptibility to the development of bacteremia. Crit Care Med. 2003;31:1429–34. doi: 10.1097/01.CCM.0000063449.58029.81. [DOI] [PubMed] [Google Scholar]
- 23.Raad II, Hanna HA, Boktour M, Jabbour N, Hachem RY, Darouiche RO. Catheter-related vancomycin-resistant Enterococcus faecium bacteremia: clinical and molecular epidemiology. Infect Control Hosp Epidemiol. 2005;26:658–61. doi: 10.1086/502598. [DOI] [PubMed] [Google Scholar]
- 24.Saiman L, Ludington E, Pfaller M, et al. Risk factors for candidemia in neonatal intensive care unit patients. The national epidemiology of mycosis survey study group. Pediatr Infect Dis J. 2000;19:319–24. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 26.Feja KN, Wu F, Roberts K, Loughrey M, Nesin M, Larson E, et al. Risk factors for candidemia in critically ill infants: a matched case-control study. J Pediatr. 2005;147:156–61. doi: 10.1016/j.jpeds.2005.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


