Abstract
Objective
To characterize ground reaction forces (GRFs) and determine whether there were correlations between forces and passive coxofemoral joint laxity in puppies.
Animals
Fifty-one 16-week-old hound-breed dogs.
Procedure
Force-plate gait evaluation and distraction radiographic imaging were performed. Ground reaction forces evaluated included x (mediolateral), y (craniocaudal breaking and propulsion), and z (vertical) peak force and impulse. Z-plane limb loading and unloading rates, loading interval, and weight distribution and y-plane stance time breaking and propulsion percentages were calculated. One-way ANOVA with the Duncan multiple range test was used to evaluate differences in gait variables among limbs. The relationships of left, right, highest, and mean distraction index (DI) with individual limb data of each dog were evaluated with the Spearman rank correlation. Left and right DIs were compared by means of linear regression analysis.
Results
Mean ± SEM DI was 0.67 ± 0.02. Left and right DIs were strongly correlated, but there were no significant relationships between DIs and gait variables. Most fore- and hind limb gait variables differed significantly, whereas paired fore- and hind limb gait variables did not. Asymmetry was most pronounced in the x- and y-planes.
Conclusions and Clinical Relevance
GRFs were consistent with those of clinically normal mature dogs, supporting an absence of association between GRF and DI in young dogs. The GRFs and elucidation of the relationship between GRFs and DI may be useful for future studies in immature dogs.
Hip dysplasia is a leading cause of CFJ arthrosis and concomitant pain and disability in dogs and humans.1–4 The condition in both species appears to be encoded by multiple genes, the phenotypic expressions of which are attributed to multiple physiologic and environmental factors.3,5–10 There is a plethora of information regarding the various factors that contribute to transformation of healthy CFJs in the neonate into malformed, osteoarthritic joints with changes characteristic of CHD and developmental dysplasia of the hip in adults. 5,11,12 Coxofemoral joint laxity in puppies, quantified by a radiographic DI,a is associated with development of arthrosis in adults.8 Similarly, CFJ laxity in children has been associated with developmental dysplasia of the hip in humans.13 The exact nature of the role that excessive joint laxity plays in the initiation and progression of CFJ osteoarthritis has yet to be fully elucidated, although exaggerated motion between joint articular surfaces resulting in trauma and abnormal wear is presumed to be a major component.4,14 The similarities between the 2 conditions make CDH a useful animal model for the study of developmental dysplasia of the hip in humans.12
Use of GRFs in objective characterization of the gait in dogs is well established.15–19 The technique is routinely used to evaluate the efficacy of pharmaceutical or surgical treatments for CHD, although most available information is from studies16,20–24 of adult dogs with arthritic CFJs. Pain secondary to osteoarthritis is known to cause specific types of gait alterations in dogs and humans.2,16,20,21,25 Similarly, excessive joint laxity contributes to alterations in GRF in both species.26–28 To the authors’ knowledge, associations between canine CFJ laxity and GRFs have not been investigated. The purpose of this study was to describe GRFs in puppies with radiographically lax but osteoarthritis-free CFJs and to determine whether there were relationships between GRFs and DIs in individual limbs in each dog. We hypothesized that GRFs in puppies with moderate to severe CFJ laxity would differ from those reported for dogs free of orthopedic disease and that there would be predictable relationships between GRFs and CFJ DIs in individual dogs.
Materials and Methods
This study was performed in accordance with institutional and National Institutes of Health regulations governing the treatment of vertebrate animals. Procedures were initiated after approval by the University of Wisconsin-Madison Animal Care Committee.
Animals
Fifty-one 16-week-old mixed-breed hounds (the progeny of 6 dams and a single sire) from 9 litters were used for the study. There were 19 males and 32 females with a mean ± SEM body weight of 12.7 ± 0.28 kg (range, 8.0 to 18.5 kg). Litters were housed together in 3 × 4-m rooms until puppies reached 16 weeks of age. Dams remained with the litters from birth until weaning at 6 weeks. All puppies received a complete physical examination prior to inclusion in the study, including evaluation of CFJs and manual manipulation to establish the range of motion and pain-free extent of flexion and extension.
GRF evaluations
Ground reaction forces were recorded from a force platformb and processed with commercially available software.c The platform was mounted in the center of and level with the surface of a concrete runway. A series of 3 retroflective photocell sensorsd was used to determine the velocity and acceleration in each trial. The middle sensor was positioned so that it bisected the center of the platform. Three handlers experienced in canine force platform gait analysis and with whom the puppies were familiar participated equally in the gait trials; variance in results attributable to trained handlers is negligible.19 A trial was considered successful if a forefoot contacted the force platform followed by contact of the ipsilateral hind foot at a velocity of 1.70 to 2.40 m/s and acceleration of 0.9 to −0.9 m/s2, a comfortable trotting pace for the study dogs. An observer monitored all trials to confirm foot strikes. The first 3 valid trials for each side (306 trials) were recorded for statistical analysis. Measured forces included x (mediolateral), y (craniocaudal), and z (vertical) peak force and impulse area; y-plane forces were divided into percentage of stance time spent in braking and propulsion. Recorded forces were normalized to body weight. The signs (positive or negative) of x-plane forces in the left limbs were reversed so that they corresponded to the same direction (+ = lateral, − = medial) for left and right limbs.
Calculation of GRF
Maximum and mean limb loading and unloading rates, loading interval, and percentage weight distribution for each limb in the z-plane and percentage of stance time spent in braking and propulsion in the y-plane were calculated according to a described method.15,29–33 The breaking-to-propulsion ratio was calculated as follows: B:P = B(%)/P(%). An SI for each GRF was calculated for all fore-and hind limbs by use of the following equation15:
where nk is the number of trials measured, Lk is the mean GRF in the left limb, Rk is the mean GRF in the right limb, and wk is the weighing factor (equal to 1.0 for the purposes of this study, given that both limbs were considered to be equally affected). A value of 1.0 indicated perfect symmetry between limbs. Values > 1.0 indicated higher forces on the left limb, whereas values < 1.0 indicated higher forces on the right limb.
Radiographic evaluations
Each dog was anesthetized, and distraction radiographs of the CFJs were obtained via established techniques.34 The DI and osteoarthritis score for each CFJ were measured and reported.a For the purposes of this study, DI was considered to be an objective measure of CFJ laxity.8,34,35
Statistical analysis
Descriptive statistics for gait variables, including velocity and acceleration, were calculated. Values were compared between limbs with 1-way ANOVA. Significant differences were further analyzed with Duncan multiple range tests. When comparisons were not significant, the difference (δ) between populations that would have been necessary to detect a significant difference was calculated.36 The relationships among left, right, highest, and mean DI and the individual limb gait data of each dog were evaluated by use of the Spearman rank correlation. Left and right DIs were compared with least squares linear regression. Analyses were performed with commercially available software programs.e, f For all analyses, values of P < 0.05 were considered significant. Results were reported as mean ± SEM.
Results
There was no detectable lameness in any of the puppies, and none had signs of pain during manipulation of the CFJs. A full range of motion was detected in the CFJs of all puppies. Similarly, there was no radiographic evidence of osteoarthritis in any CFJs. Mean DI score for all CFJs was 0.67 ± 0.02 (range, 0.38 to 1.05). There was a strong linear correlation between DI scores in left and right limbs (R2 = 0.80; P < 0.001; Figure 1). Left, right, mean, and highest DIs were not significantly correlated with any of the gait variables evaluated. Velocity (1.99 ± 0.05 m/s; P = 0.71) and acceleration (−0.183 ± 0.07 m/s2; P = 0.86) did not vary significantly between individual trials. Gait variables were not significantly different between paired forelimbs or hind limbs (Table 1). Symmetry indexes ranged from approximately 0% to nearly 177% asymmetry. Values for stance time asymmetry (0% to 3%) and vertical GRF asymmetry (1% to 56%) were less pronounced than were values for craniocaudal (3% to 177%) and mediolateral (23% to 83%) asymmetry. Mean asymmetry values were higher in the forelimbs (87%) than in the hind limbs (26%) in the x-plane, approximately equal in the y-plane (forelimb, 61%; hind limb, 59%), and higher in the hind limbs (16%) than in the forelimbs (2%) in the z-plane. There were significant differences between fore- and hind limbs in all variables except y-plane propulsion peak force (δ = 154% at power = 0.8), z-plane impulse (δ = 28.1% at power = 0.8), and percent weight distribution (δ = 2.98% at power = 0.8; Table 2). Stance time, peak craniocaudal braking force, craniocaudal breaking impulse, percentage braking, braking-to-propulsion ratio, peak vertical force, loading interval, maximum loading rate, mean unloading rate, and maximum unloading rate were higher in the forelimb, whereas mediolateral peak force, mediolateral impulse, craniocaudal propulsion impulse, percentage propulsion, and mean loading rate were higher in the hind limbs.
Figure 1.
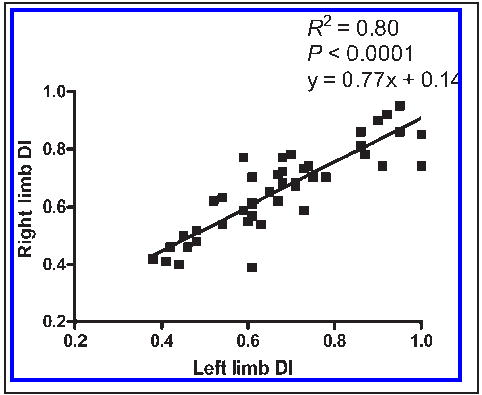
Relationship between DIs from right and left limbs of 16-week-old puppies (n = 51) with moderate to severe CFJ laxity. The least squares linear regression line indicates the highly predictable intra-animal relationship of the values.
Table 1.
Mean ± SEM values for GRFs measured in 16-week-old puppies with moderate to severe CFJ laxity and that were trotted at a velocity of 1.99 ± 0.05 m/s.
| Variable | Limb | Left | Right | P | δ (%) | SI |
|---|---|---|---|---|---|---|
| Stance time (ms) | F | 217 ± 4.4 | 215 ± 3.6 | 0.72 | 5.18 | 1.00 |
| H | 194 ± 5.1 | 199 ± 4.0 | 0.46 | 6.50 | 0.97 | |
| PFx (% bw) | F | 0.894 ± 0.265 | 1.12 ± 0.29 | 0.56 | 76.7 | 0.17 |
| H | 1.62 ± 0.27 | 1.65 ± 0.30 | 0.94 | 48.1 | 1.29 | |
| IMPx (% bw·s) | F | 0.085 ± 0.028 | 0.091 ± 0.030 | 0.89 | 92.3 | 0.10 |
| H | 0.141 ± 0.200 | 0.147 ± 0.031 | 0.89 | 225 | 0.77 | |
| PFyB (% bw) | F | −20.4 ± 1.2 | −19.8 ± 1.2 | 0.72 | 16.5 | 1.46 |
| H | −7.06 ± 0.72 | −7.91 ± 0.84 | 0.45 | 29.3 | 1.69 | |
| IMPyB (% bw·s) | F | −1.9 ± 0.3 | −1.5 ± 0.2 | 0.21 | 38.3 | 1.23 |
| H | −0.704 ± 0.500 | −0.694 ± 0.449 | 0.98 | 190 | 1.74 | |
| PFyP (% bw) | F | 16.4 ± 11.0 | 5.72 ± 0.48 | 0.35 | 145 | 2.77 |
| H | 11.3 ± 0.82 | 38.7 ± 28.2 | 0.32 | 162 | 1.36 | |
| IMPyP (% bw·s) | F | 0.281 ± 0.031 | 0.269 ± 0.032 | 0.82 | 32.1 | 1.04 |
| H | 0.972 ± 0.071 | 0.944 ± 0.082 | 0.79 | 22.4 | 1.34 | |
| %B (% stance time) | F | 83.6 ± 2.4 | 82.6 ± 2.7 | 0.81 | 8.56 | 1.03 |
| H | 23.8 ± 4.0 | 24.2 ± 3.5 | 0.93 | 43.9 | 1.81 | |
| %P (% stance time) | F | 16.5 ± 2.4 | 17.4 ± 2.7 | 0.8 | 42.0 | 1.29 |
| H | 76.3 ± 4.0 | 75.8 ± 3.5 | 0.93 | 13.9 | 1.31 | |
| B:P | F | 10.8 ± 1.9 | 9.71 ± 1.84 | 0.69 | 51.1 | 2.17 |
| H | 0.756 ± 0.321 | 0.858 ± 0.316 | 0.82 | 111 | 1.91 | |
| PFz (% bw) | F | 111 ± 2.7 | 112 ± 2.3 | 0.57 | 6.32 | 1.01 |
| H | 79.8 ± 2.0 | 80.3 ± 1.9 | 0.85 | 6.90 | 1.02 | |
| IMPz (% bw·s) | F | 14.2 ± 0.31 | 14.4 ± 0.33 | 0.69 | 6.21 | 0.99 |
| H | 13.4 ± 4.6 | 8.98 ± 0.24 | 0.34 | 60.6 | 1.56 | |
| LI (% stance time) | F | 53 ± 0.86 | 53.1 ± 0.55 | 0.91 | 3.72 | 1.00 |
| H | 38.9 ± 0.78 | 37.7 ± 0.73 | 0.26 | 5.52 | 1.38 | |
| ML (% bw/s) | F | 1.43 ± 0.06 | 1.44 ± 0.05 | 0.95 | 10.7 | 1.05 |
| H | 1.58 ± 0.06 | 1.61 ± 0.03 | 0.79 | 6.85 | 1.05 | |
| Lmax (% bw/s) | F | 3.05 ± 0.12 | 3.00 ± 0.12 | 0.75 | 10.8 | 1.05 |
| H | 2.5 ± 0.12 | 2.5 ± 0.14 | 0.97 | 14.5 | 1.08 | |
| MU (% bw/s) | F | −1.54 ± 0.06 | −1.63 ± 0.06 | 0.3 | 10.1 | 1.00 |
| H | −0.995 ± 0.035 | −0.971 ± 0.032 | 0.62 | 9.58 | 1.08 | |
| Umax (% bw/s) | F | −2.1 ± −0.11 | −2.05 ± 0.11 | 0.81 | 0.324 | 1.04 |
| H | −1.7 ± −0.06 | −1.63 ± 0.06 | 0.37 | 0.202 | 1.10 | |
| Distribution (% bw) | F | 29.6 ± 0.79 | 29.1 ± 0.38 | 0.63 | 5.58 | 1.01 |
| H | 21.4 ± 0.56 | 20.9 ± 0.35 | 0.39 | 6.03 | 1.02 |
= Differences between populations necessary for significance at power = 0.8. PF = Peak force. bw = Body weight. IMP = Impulse area. B = Breaking. P = Propulsion. x, y, and z = Planes of evaluated ground reaction forces. LI = Loading interval. ML = Mean loading rate. Lmax = Maximum loading rate. MU = Mean unloading rate. Umax = Maximum unloading rate. F = Forelimb. H = Hind limb.
Table 2.
Mean ± SEM values for GRF variables measured in the fore- and hind limbs in the same 51 puppies and trials.
| Variable | Forelimb | Hind limb | P |
|---|---|---|---|
| Stance time (ms) | 216 ± 2.8 | 196 ± 3.2 | 0.001 |
| PFx (% bw) | 1.01 ± 0.19 | 1.64 ± 0.19 | 0.014 |
| IMPx (% bw·s) | 0.09 ± 0.02 | 0.14 ± 0.02 | 0.04 |
| PFyB (% bw) | −20.1 ± 0.83 | −7.48 ± 0.55 | 0.001 |
| IMPyB (% bw·s) | −1.72 ± 0.17 | −0.70 ± 0.33 | 0.002 |
| PFyP (% bw) | 11.2 ± 5.7 | 24.7 ± 14.0 | 0.37 |
| IMPyP (% bw·s) | 0.28 ± 0.02 | 0.96 ± 0.05 | 0.001 |
| %B (% stance time) | 83 ± 1.8 | 24 ± 2.6 | 0.001 |
| %P (% stance time) | 17 ± 1.8 | 76 ± 2.6 | 0.001 |
| B:P | 10.2 ± 1.3 | 0.81 ± 0.22 | 0.001 |
| PFz (% bw) | 111 ± 1.8 | 80 ± 1.4 | 0.001 |
| IMPz (% bw·s) | 14.3 ± 0.2 | 11.2 ± 2.3 | 0.21 |
| LI (% stance time) | 53.1 ± 0.5 | 38.3 ± 0.5 | 0.001 |
| ML (% bw/s) | 1.4 ± 0.04 | 1.6 ± 0.04 | 0.001 |
| Lmax (% bw/s) | 3.0 ± 0.08 | 2.5 ± 0.09 | 0.001 |
| MU (% bw/s) | −1.59 ± 0.04 | −0.99 ± 0.02 | 0.001 |
| Umax (% bw/s) | −2.08 ± 0.08 | −1.69 ± 0.04 | 0.001 |
| Distribution (% bw) | 58.1 ± 0.5 | 41.9 ± 0.5 | 0.63 |
See Table 1 for key.
Discussion
The study population consisted of puppies with DIs that are associated with the development of osteoarthritic changes characteristic of CHD in the CFJ. The frequency of CFJ osteoarthritis at maturity increases in proportion with DI scores ≥ 3.0.35 Distraction index scores used in our investigation were derived from radiographs obtained at the youngest age at which the scores are reported to be prognostic, although that age varies among breeds.5 The population of dogs used in our study had a mean DI score of 0.67, indicating that most puppies were likely to develop CHD. As such, these puppies were appropriate for use in evaluating the relationship between GRFs and CFJ laxity, although by strict definition, they did not have radiographically evident degenerative joint changes that were characteristic of the disease at the time of the study.
Normal and pathologic patterns in canine gait variables have been well documented, but most of the gait alterations reported are attributable to pain.16,18,20,21,30 Changes in GRFs that are specifically related to pathologic changes in CFJs of dogs and humans include reduced y- and z-plane peak force and impulse as well as maximum loading and unloading rates.16,20,21 Mean values for z-plane peak force and impulse in the hind limbs were higher than those reported21,33 in dogs with CHD, whereas mean loading and unloading rates were lower, although direct comparisons were difficult given the differences in gait velocity used between studies. Values for y- and z-plane fore- and hind limb GRFs in our study were comparable to those published29 for clinically normal dogs with similar stance times, except for hind limb craniocaudal propulsion peak force, which was complicated by high individual variability in the present study. Fore- and hind limb x-plane GRFs were < 6% of body weight and primarily directed laterally, comparable to values in clinically normal dogs.33 Similarly, forelimbs bore 60% of body weight, whereas hind limbs bore 40% of body weight in our puppies, similar to findings in clinically normal dogs.31 On the basis of this information, values for x-, y-, and z-plane peak force and impulse in our study were consistent with those reported in mature dogs unaffected by orthopedic disease. Differences from reported reference range values in mean loading and unloading rates in our study may have arisen from the fact that the values were calculated over the entire loading and unloading phases, rather than at intervals, a method that more accurately reflects limb function.32 Despite this fact, both rates were higher in the forelimbs, as expected, and values between limbs had limited asymmetry, making it unlikely that the differences reflected pathologic gait changes.
Values for asymmetry varied widely for the variables evaluated. In general, values for z-plane and stance time asymmetry were least pronounced, whereas values for y-plane asymmetries were most pronounced, findings that were in accordance with those from previous studies.15,37 Values for y-plane asymmetries in our study were slightly higher than those of a previous study15 in clinically normal dogs that were evaluated at the same velocity; however, the small differences do not necessarily indicate that there were gait abnormalities. Also notable in our study was the fact that asymmetry values in the fore- and hind limbs were comparable in the y-plane, whereas values for asymmetry were highest in the x- and z-planes for the fore-and hind limbs, respectively. Results of a previous study15 revealed comparable values for asymmetry between fore- and hind limbs in z-forces, whereas values of y-plane asymmetry were higher in the hind limb. According to the formula used in the present study, SI values are close to 1.0 if differences within a gait variable are small, compared with its absolute values, and vice versa. Values for SI that are significantly different from 1.0 may occur if values for a given gait variable are close to 0, if the differences in values between right and left limbs are large, or both.37 The limits of a normal degree of asymmetry vary among gait variables, and asymmetries in gait should be interpreted with caution. Our findings in the gaits of immature dogs were in agreement with those from a study15 of mature dogs. Z-plane and stance time asymmetry indices appear to be the most applicable for canine gait analysis.
In the present study, a range of canine gait variables with associated SEMs and detection powers were described for future reference. Intertrial variation may have contributed to some variability in measured values because the dogs were immature; in humans, intertrial variation is known to be more important in children than in adults.38 Every effort was made to control such variation by limiting the velocity and acceleration at which the trials were conducted and by evaluating all trials for consistency and validity. It is possible that variation could have been reduced by limiting the velocity and acceleration ranges further, but although the puppies were all the same age, their sizes varied widely. The ranges were chosen to include comfortable trot rates to avoid introduction of variation from artificially altering the gait. Gait constraints are not recommended in human gait assessments because they prohibit evaluation of representative gait kinematics and kinetics.39 Puppies were allowed to trot at their preferred velocities so that the most representative GRFs would be recorded. Had the velocity range been restricted, the overall variability in GRF may have been reduced, resulting in higher statistical power. However, had only the puppies that trotted comfortably within a limited velocity range been included, a number of individuals would have been excluded from the study, reducing the range of CFJ laxity. It was determined that study objectives were best met by inclusion of the widest range of CFJ laxities; therefore, data from all puppies were included in analyses.
To our knowledge, these data are the first obtained from evaluation of immature animals. Neuromuscular function and coordination may not have been fully developed and may have resulted in some of the gait inconsistencies observed. The stages of gait development in children have been extensively evaluated kinetically and kinematically, and mediolateral stability is the most important factor in gait development in clinically normal infants.40 Ground reaction forces in the x-plane are considered too variable to be useful for gait analysis.41,31 It is therefore difficult to assess whether animal development contributed to the high variation and low sensitivity of the x-plane GRFs in our study. Our results support that x-plane GRFs have little value in gait evaluation in mature or immature dogs.
In normal ambulation, the primary function of the canine forelimb is deceleration, whereas that of the hind limb is propulsion.31 Reported values29 from clinically normal dogs at a similar trot velocity were 57% of stance time spent in breaking and 43% spent in propulsion in the forelimb, compared with 30% of stance time in breaking and 70% of stance time in propulsion in the hind limb. Although values for hind limbs in our study were comparable (24% braking, 76% propulsion), the values in forelimbs (83% braking, 17% propulsion) were proportionately different. There were large trial variations in the propulsion and braking GRFs, as indicated by the SEMs. This has not been reported as a problem in the evaluation of mature dogs and may have been related to the young age of the dogs in our study. Although every attempt was made to ensure consistency between trials, there were some unavoidable obstacles that are inherent to the study of young animals. This does not diminish the importance of findings from such investigations, but consideration must be given to the age of the subjects. Further studies are necessary to assess whether there are differences in individual variation in braking and propulsion as dogs mature.
Stable joints are necessary for normal gait.26–28 To our knowledge, gait alteration related to joint laxity that is not complicated by arthrosis has not been previously investigated in dogs. A wide range of CFJ laxity, from slightly greater than normal to very severe,35 was evaluated against GRF gait variables for detection of potential associations in our study. It is important to distinguish between active laxity and DI passive laxity when considering that no associations between DIs and GRFs were detected in this study. Other methods of imaging the CFJ may be more representative of active CFJ laxity, and such laxity may be more closely correlated with GRF values.42 Although CFJs in our dogs had moderate to severe degrees of passive laxity, it is possible that dogs actively stabilize the joint or otherwise compensate for instability by altering joint angles, pelvic tilt, and muscle group activation during ambulation.1 Determining whether such mechanisms are used would require the acquisition of kinematic and electromyographic data and could serve as the basis for future studies.
Values for GRFs in the present study were consistent with those derived from evaluations of clinically normal adult dogs, supporting the finding that gait variables and passive CFJ laxity appear to be unrelated in young dogs. The results also validate the use of gait analysis in young dogs as a method of objective evaluation. This information is essential to future investigations of CHD, specifically in the role played by joint laxity in the pathophysiologic features of hip dysplasia and associated changes in gait.
Acknowledgments
The authors thank John Bogdanske, Jennifer Devitt, and Susan Linden for technical assistance.
Supported in part by the National Institutes of Health-NIAMS, Arthritis Foundation, and the University of Wisconsin-Madison Companion Animal Fund.
- CFJ
Coxofemoral joint
- CHD
Canine hip dysplasia
- DI
Distraction index
- GRF
Ground reaction force
- SI
Symmetry index
Footnotes
Synbiotics PennHIP Analysis Center, Malvern, Pa.
Model No. OR6-6-1000, Advanced Mechanical Technology Inc, Watertown, Mass.
VETDATA, version 2.03, Acquire, version 5.0, Mininet, version 4.0, Update, version1.1, Sharon Software Inc, Dewitt, Mich.
Mek 92-PAD retroflective photocell, Joslyn Clark Controls Inc, Lancaster, SC.
SAS, version 8.2, SAS Institute Inc, Cary, NC.
GraphPad Prism, version 3.0, GraphPad Software Inc, San Diego, Calif.
References
- 1.Pedersen EN, Simonsen EB, Alkjaer T, et al. Walking pattern in adults with congenital hip dysplasia: 14 women examined by inverse dynamics. Acta Orthop Scand. 2004;75:2–9. doi: 10.1080/00016470410001708010. [DOI] [PubMed] [Google Scholar]
- 2.Endo H, Mitani S, Senda M, et al. Three-dimensional gait analysis of adults with hip dysplasia after rotational acetabular osteotomy. J Orthop Sci. 2003;8:762–771. doi: 10.1007/s00776-003-0705-z. [DOI] [PubMed] [Google Scholar]
- 3.Powers MY, Biery DN, Lawler DE, et al. Use of the caudolateral curvilinear osteophyte as an early marker for future development of osteoarthritis associated with hip dysplasia in dogs. J Am Vet Med Assoc. 2004;225:233–237. doi: 10.2460/javma.2004.225.233. [DOI] [PubMed] [Google Scholar]
- 4.Smith GK. Advances in diagnosing canine hip dysplasia. J Am Vet Med Assoc. 1997;210:1451–1457. [PubMed] [Google Scholar]
- 5.Novacheck TF. Developmental dysplasia of the hip. Pediatr Clin North Am. 1996;43:829–848. doi: 10.1016/s0031-3955(05)70437-5. [DOI] [PubMed] [Google Scholar]
- 6.Rumph PF, Kincaid SA, Baird DK, et al. Vertical ground reaction force distribution during experimentally induced acute synovitis in dogs. Am J Vet Res. 1993;54:365–369. [PubMed] [Google Scholar]
- 7.Leighton EA. Genetics of canine hip dysplasia. J Am Vet Med Assoc. 1997;210:1474–1479. [PubMed] [Google Scholar]
- 8.Smith GK, Popovitch CA, Gregor TP, et al. Evaluation of risk factors for degenerative joint disease associated with hip dysplasia in dogs. J Am Vet Med Assoc. 1995;206:642–647. [PubMed] [Google Scholar]
- 9.Todhunter RJ, Casella G, Bliss SP, et al. Power of a Labrador Retriever-Greyhound pedigree for linkage analysis of hip dysplasia and osteoarthritis. Am J Vet Res. 2003;64:418–424. doi: 10.2460/ajvr.2003.64.418. [DOI] [PubMed] [Google Scholar]
- 10.Todhunter RJ, Bliss SP, Casella G, et al. Genetic structure of susceptibility traits for hip dysplasia and microsatellite informativeness of an outcrossed canine pedigree. J Hered. 2003;94:39–48. doi: 10.1093/jhered/esg006. [DOI] [PubMed] [Google Scholar]
- 11.Lust G, Rendano VT, Summers BA. Canine hip dysplasia: concepts and diagnosis. J Am Vet Med Assoc. 1985;187:638–640. [PubMed] [Google Scholar]
- 12.Riser WH. The dog as a model for the study of hip dysplasia. Growth, form, and development of the normal and dysplastic hip joint. Vet Pathol. 1975;12:234–334. doi: 10.1177/030098587501200401. [DOI] [PubMed] [Google Scholar]
- 13.Shefelbine SJ, Carter DR. Mechanobiological predictions of growth front morphology in developmental hip dysplasia. J Orthop Res. 2004;22:346–352. doi: 10.1016/j.orthres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Fries CL, Remedios AM. The pathogenesis and diagnosis of canine hip dysplasia: a review. Can Vet J. 1995;36:494–502. [PMC free article] [PubMed] [Google Scholar]
- 15.Budsberg SC, Jevens DJ, Brown J, et al. Evaluation of limb symmetry indices, using ground reaction forces in healthy dogs. Am J Vet Res. 1993;54:1569–1574. [PubMed] [Google Scholar]
- 16.Budsberg SC, Johnston SA, Schwarz PD, et al. Efficacy of etodolac for the treatment of osteoarthritis of the hip joints in dogs. J Am Vet Med Assoc. 1999;214:206–210. [PubMed] [Google Scholar]
- 17.DeCamp CE, Soutas-Little RW, Hauptman J, et al. Kinematic gait analysis of the trot in healthy Greyhounds. Am J Vet Res. 1993;54:627–634. [PubMed] [Google Scholar]
- 18.DeCamp CE. Kinetic and kinematic gait analysis and the assessment of lameness in the dog. Vet Clin North Am Small Anim Pract. 1997;27:825–840. doi: 10.1016/s0195-5616(97)50082-9. [DOI] [PubMed] [Google Scholar]
- 19.Jevens DJ, Hauptman JG, DeCamp CE, et al. Contributions to variance in force-plate analysis of gait in dogs. Am J Vet Res. 1993;54:612–615. [PubMed] [Google Scholar]
- 20.Bennett RL, DeCamp CE, Flo GL, et al. Kinematic gait analysis in dogs with hip dysplasia. Am J Vet Res. 1996;57:966–971. [PubMed] [Google Scholar]
- 21.Budsberg SC, Chambers JN, Lue SL, et al. Prospective evaluation of ground reaction forces in dogs undergoing unilateral total hip replacement. Am J Vet Res. 1996;57:1781–1785. [PubMed] [Google Scholar]
- 22.McLaughlin RM, Jr, Miller CW, Taves CL, et al. Force plate analysis of triple pelvic osteotomy for the treatment of canine hip dysplasia. Vet Surg. 1991;20:291–297. doi: 10.1111/j.1532-950x.1991.tb01270.x. [DOI] [PubMed] [Google Scholar]
- 23.Poy NS, DeCamp CE, Bennett RL, et al. Additional kinematic variables to describe differences in the trot between clinically normal dogs and dogs with hip dysplasia. Am J Vet Res. 2000;61:974–978. doi: 10.2460/ajvr.2000.61.974. [DOI] [PubMed] [Google Scholar]
- 24.Patricelli AJ, Dueland RT, Adams WM, et al. Juvenile pubic symphysiodesis in dysplastic puppies at 15 and 20 weeks of age. Vet Surg. 2002;31:435–444. doi: 10.1053/jvet.2002.34766. [DOI] [PubMed] [Google Scholar]
- 25.Romano CL, Frigo C, Randelli G, et al. Analysis of the gait of adults who had residua of congenital dysplasia of the hip. J Bone Joint Surg Am. 1996;78:1468–1479. doi: 10.2106/00004623-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor BL, Woodbury P. The primary articular nerves to the dog knee. J Anat. 1982;134:563–572. [PMC free article] [PubMed] [Google Scholar]
- 27.Ferber R, Osternig LR, Woollacott MH, et al. Gait perturbation response in chronic anterior cruciate ligament deficiency and repair. Clin Biomech (Bristol, Avon) 2003;18:132–141. doi: 10.1016/s0268-0033(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 28.Ferber R, Osternig LR, Woollacott MH, et al. Bilateral accommodations to anterior cruciate ligament deficiency and surgery. Clin Biomech (Bristol, Avon) 2004;19:136–144. doi: 10.1016/j.clinbiomech.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin RM, Jr, Roush JK. Effects of subject stance time and velocity on ground reaction forces in clinically normal Greyhounds at the trot. Am J Vet Res. 1994;55:1666–1671. [PubMed] [Google Scholar]
- 30.Budsberg SC, Verstraete MC, Soutas-Little RW, et al. Force plate analyses before and after stabilization of canine stifles for cruciate injury. Am J Vet Res. 1988;49:1522–1524. [PubMed] [Google Scholar]
- 31.Budsberg SC, Verstraete MC, Soutas-Little RW. Force plate analysis of the walking gait in healthy dogs. Am J Vet Res. 1987;48:915–918. [PubMed] [Google Scholar]
- 32.Budsberg SC, Verstraete MC, Brown J, et al. Vertical loading rates in clinically normal dogs at a trot. Am J Vet Res. 1995;56:1275–1280. [PubMed] [Google Scholar]
- 33.Rumph PF, Lander JE, Kincaid SA, et al. Ground reaction force profiles from force platform gait analyses of clinically normal mesomorphic dogs at the trot. Am J Vet Res. 1994;55:756–761. [PubMed] [Google Scholar]
- 34.Smith GK, Biery DN, Gregor TP. New concepts of coxo-femoral joint stability and the development of a clinical stress-radiographic method for quantitating hip joint laxity in the dog. J Am Vet Med Assoc. 1990;196:59–70. [PubMed] [Google Scholar]
- 35.Smith GK, Gregor TP, Rhodes WH, et al. Coxofemoral joint laxity from distraction radiography and its contemporaneous and prospective correlation with laxity, subjective score, and evidence of degenerative joint disease from conventional hip-extended radiography in dogs. Am J Vet Res. 1993;54:1021–1042. [PubMed] [Google Scholar]
- 36.Markel MD. The power of a statistical test. What does insignificance mean? Vet Surg. 1991;20:209–214. doi: 10.1111/j.1532-950x.1991.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 37.Herzog W, Nigg BM, Read LJ, et al. Asymmetries in ground reaction force patterns in normal human gait. Med Sci Sports Exerc. 1989;21:110–114. doi: 10.1249/00005768-198902000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Stolze H, Kuhtz-Buschbeck JP, Mondwurf C, et al. Retest reliability of spatiotemporal gait parameters in children and adults. Gait Posture. 1998;7:125–130. doi: 10.1016/s0966-6362(97)00043-x. [DOI] [PubMed] [Google Scholar]
- 39.Martin PE, Marsh AP. Step length and frequency effects on ground reaction forces during walking. J Biomech. 1992;25:1237–1239. doi: 10.1016/0021-9290(92)90081-b. [DOI] [PubMed] [Google Scholar]
- 40.Yaguramaki N, Kimura T. Acquirement of stability and mobility in infant gait. Gait Posture. 2002;16:69–77. doi: 10.1016/s0966-6362(01)00205-3. [DOI] [PubMed] [Google Scholar]
- 41.White R, Agouris I, Selbie RD, et al. The variability of force platform data in normal and cerebral palsy gait. Clin Biomech (Bristol, Avon) 1999;14:185–192. doi: 10.1016/s0268-0033(99)80003-5. [DOI] [PubMed] [Google Scholar]
- 42.Farese JP, Lust G, Williams AJ, et al. Comparison of measurements of dorsolateral subluxation of the femoral head and maximal passive laxity for evaluation of the coxofemoral joint in dogs. Am J Vet Res. 1999;60:1571–1576. [PubMed] [Google Scholar]


