Cytokines and related signaling molecules lead to profound regulatory changes in differentiated cell function, modulating immune functions, the stress response, energy metabolism, growth, and reproduction. A network of intracellular molecules that dampen or inhibit the effects of these pleiotropic factors provides a crucial counterbalance to cytokine signals. Recent studies have shown that negative feedback, initiated in the various target tissues by the cytokines themselves, is central to endocrine homeostasis. Cytokine-mediated adrenocorticotropin hormone (ACTH) and cortisol overproduction, for instance, is prevented by tightly regulated cytokine-induced intracellular negative control systems. Likewise, growth hormone (GH) signaling is abrogated by cytokine-induced proteins, providing an explanation for GH resistance and stunted growth observed in states of elevated cytokine activity, including inflammation, starvation, and chronic illness. This article explores the role of the signal suppressor SOCS-3 in inhibiting the actions of neuro-endocrine cytokines and hormones, while maintaining the plasticity of the ultimate neuro-immune endocrine responses.
SOCS proteins as inhibitors of the JAK-STAT cascade
The JAK-STAT cascade is an intracellular signaling pathway shared by a variety of cytokines, including gp130 cytokines (IL-6, IL-11, leukemia inhibitory factor [LIF], oncostatin M [OSM], ciliary neurotrophic factor [CNTF], cardiotropin-1 [CT-1], cardiotropin-like cytokine [CLC]), as well as leptin, GH, and prolactin. Ligand binding to cytokine receptors, which themselves lack intrinsic kinase activity, activates receptor-associated Janus kinases (JAK’s) by autophosphorylation and subsequent tyrosine phosphorylation of the receptor’s cytoplasmic domain and of associated proteins termed STATs for signal transducers and activators of transcription. Tyrosine phosphorylation of STATs enables homo- or heterodimerization of various STAT proteins. The dimerized STAT complexes translocate to the nucleus, where they transactivate their target genes by binding to specific promoter elements (1, 2).
A family of proteins able to inhibit the JAK-STAT signaling cascade has synonymously been described as suppressor of cytokine signaling (SOCS) protein (3), JAK-binding protein (4), and STAT-induced STAT inhibitor. The SOCS protein family (5) encompasses SOCS-1 (3, 4, 6), SOCS-2 (3), SOCS-3 (3), CIS (7), and SOCS-4 to SOCS-7 (5). While the role of the former four proteins is increasingly well understood, the roles of SOCS-4 to SOCS-7 remain poorly characterized. In vitro overexpression studies demonstrate that SOCS-1 and SOCS-3 exert similar effects and represent the most potent and broadly acting suppressors of cytokine signaling. These factors potently inhibit JAK-STAT signaling of several gp130 cytokines (see Arzt, this Perspective series, ref. 8; and refs. 3, 4, 9–12), GH (13–15), and prolactin (16).
The common protein structure of SOCS proteins is a variable N-terminal region, a central SH2 domain, and a C-terminal domain, termed SOCS-box motif (5). The central SH2 domain alone is not sufficient for inhibiting JAK-STAT signaling (4), as part of the N-terminal region, termed pre-SH2 domain/kinase inhibitory region, is also required (10, 17, 18). The C-terminal SOCS-box interacts with elongin BC complex (19), although the physiological significance of this interaction is still unclear. Some observations suggest that this interaction protects SOCS proteins from degradation in the ubiquitin-proteasome pathway, whereas others suggest that it actually directs these proteins to the ubiquitin-proteasome pathway and sets the stage for their degradation (19, 20). Conversely, the significance of the proteasome in the turnover of these molecules is not in doubt. The half-lives of SOCS-1 and SOCS-3 in COS-7 cells have been calculated to be as short as 1.5 hours (21), but incubation with proteasome inhibitors stabilizes them significantly (18, 19). Therefore, the C-terminal SOCS-box also appears not to be essential for direct inhibitory interaction with JAK (10, 17) but might be required to control SOCS protein degradation and thus might modulate the intracellular level of the SOCS protein (18).
SOCS-1 and SOCS-3 suppress JAK-STAT signaling by similar mechanisms. SOCS-1 associates with and inhibits JAK1 (4, 22), JAK2 (4, 6, 15–17), JAK3 (4, 22), Tyk 2 (4), and Tec (6). Following early autophosphorylation of Tyr1007 in the JH1 domain of JAK2 (4, 17), SOCS-1 binds via its SH2 domain to the catalytic JH1 domain of JAK2 (4), thus inhibiting JH1 activity. SOCS-3 also coimmunoprecipitates with JAK2 (18, 23) and binds Tyr1007. SOCS-3 is distinguished from SOCS-1 by its lower affinity for JAK2 (16). In addition to direct JAK interaction, SOCS-3’s action is also mediated by binding to phosphorylated tyrosine residues in the intracellular domain of various receptors — Tyr759 of gp130 (24), Tyr333 and Tyr338 of GH receptor (15), or Tyr985 and Tyr1077 of the long receptor of leptin (25, 26) — which allows it to suppress receptor function directly as well as indirectly. Similarly, CIS has been demonstrated to exert its inhibitory role by binding to tyrosine residues of intracellular receptor domains (7).
Despite in vitro overexpression data demonstrating similar suppressor activities for SOCS-1 and SOCS-3, these proteins are induced specifically by different cytokines and in different cell types, suggesting that they play different roles in vivo. Induction of SOCS expression by various cytokines is STAT-dependent, and indeed, the promoter regions of the cis (7, 27), SOCS1, and SOCS3 genes (28) show functional STAT-binding elements. Cytokine-induced expression of SOCS-1 and SOCS-3 can be inhibited by STAT3 dominant negative mutants (28), while gene expression of CIS can be inhibited by STAT5 dominant negative mutants (29).
LIF, a modulator of hypothalamic-pituitary-adrenal axis function
LIF is a potent neuro-immunoendocrine modulator of pituitary corticotroph function in vivo and in vitro. Both alone and in additive or synergistic actions with CRH, LIF induces proopiomelanocortin (POMC) gene expression and ACTH secretion by cultured murine (30, 31) and human (32) corticotrophs. In these cells, LIF stimulates the JAK-STAT signaling cascade, causing phosphorylation of JAK2 (18), gp130 (33), STAT3 (31, 33, 34), and STAT1 (31). LIF-induced POMC gene expression is critically STAT3-dependent, as dominant negative STAT3 mutants significantly decrease LIF-induced POMC promoter activity and gene expression (34).
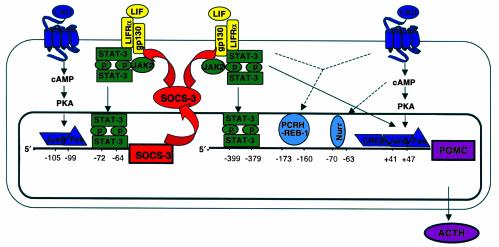
Increase of POMC promoter activity following STAT3 activation is mediated by direct and indirect mechanisms (35). The rat POMC promoter region contains two juxtaposed STAT3-binding elements (–399 -TTTACCTCCAAATGCCAGGAA- –379), their sequences only distantly related to the classic STAT3 consensus DNA-binding sequence (TTCCA). While each of these elements can bind STAT3, both are required for high-affinity binding (35). Mutation of the STAT3 DNA-binding sequence in this region reduces LIF-induced POMC promoter activity by half. In addition, STAT3 (either alone or in synergy with CRH) can stimulate POMC promoter activity indirectly by stimulating c-fos and JunB expression (35), thus allowing the formation of the Fos/Jun heterodimer, AP-1 (36). Interestingly, the genes for each of these transcription factors harbor STAT-binding elements in their promoters. In addition, binding of c-fos and JunB to an AP-1 site in the POMC exon 1 occurs in vitro and appears to participate in LIF-induced transactivation of POMC in vivo, since mutation or deletion of this site modestly reduces promoter activity in cultured corticotrophs (35). To summarize, activation of the JAK-STAT cascade activates several genes in the corticotroph cell by direct binding of activated STAT3 to the respective promoter regions of POMC, Fos, and JunB. The transcription factors c-fos and JunB subsequently bind to the POMC promoter themselves. The direct and indirect mechanisms by which STATs induce POMC promoter activity constitute the molecular basis of the neuro-immunoendocrine regulation of corticotroph POMC expression by LIF and CRH (Figure 1).
Figure 1.
Corticotroph SOCS-3 as an intracellular suppressor of cytokine signaling. Corticotroph SOCS-3 inhibits STAT-dependent POMC gene expression by negatively interfering with LIF-induced activation of the JAK-STAT cascade. In contrast, CRH-induced POMC gene expression is not affected by SOCS-3. LIF stimulation of the corticotroph results in rapid upregulation of SOCS-3 by a STAT-dependent mechanism. Thus, LIF-induced activation of corticotroph POMC gene activation is limited by parallel induction of SOCS-3 expression, rendering the cell resistant to further JAK-STAT activation. On the other hand, autoregulation of STAT-dependent SOCS-3 gene expression and rapid degradation of SOCS-3 protein by the proteasome pathway enable the cell to restore its functional status. LIF and CRH synergistically induce POMC promoter activity. LIF activates POMC promoter activity not only by direct binding of activated STATs, but also indirectly by inducing STAT-dependent expression of transcription factors c-fos and JunB. CRH also induces SOCS3 promoter activity by binding of c-fos and JunB. Thus, CRH indirectly inhibits LIF-induced POMC promoter activation and downregulates the synergistic cross-talk of CRH and LIF on POMC promoter activity. Adapted from ref. 34 and ref. 47 with permission.
In vivo, systemic LIF administration induces pituitary POMC transcription and ACTH secretion either alone or in synergy with CRH (37, 38). Hypothalamic and pituitary LIF expression is induced by systemic inflammatory stimuli such as LPS (39), IL-1β (40), and CFA (41), as well as by local inflammatory stimuli, such as turpentine (41). LIF knockout animals show a corresponding decrease in hypothalamic-pituitary-adrenal (HPA) axis response to stress induced by immobilization (38, 42) or systemic inflammation (41). Conversely, pituitary-directed LIF overexpression in transgenic mice results in corticotroph hyperplasia and the usual sequelae of hypercortisonism, including obesity and failed dexamethasone suppression (43). Thus, LIF is required for both appropriate corticotroph development and function.
SOCS-3 as a suppressor of STAT-dependent POMC expression
Corticotroph SOCS-3 coimmunoprecipitates with JAK2 (18). Furthermore, the overexpression of SOCS-3 inhibits LIF-induced phosphorylation of JAK2 (18), gp130 (33), and STAT3 (33). The physiological importance of this interaction is suggested by the phenotype of corticotroph cells stably overexpressing wild-type SOCS-3, which show significant inhibition of LIF-induced POMC promoter activation, POMC transcription, and ACTH secretion (33). Inhibition of LIF-induced STAT3 phosphorylation by SOCS-3 overexpression also blocks STAT3 protein binding to the bipartite STAT3-binding element in the POMC promoter (35). Consistent with the in vitro analysis described above, corticotroph SOCS-3 overexpression also abrogates LIF-induced c-fos and JunB expression (35). Thus, inhibition of LIF-induced STAT3 phosphorylation by SOCS-3 overexpression causes inhibition of corticotroph POMC promoter activity by direct negative regulation of STAT3 function, and indirectly by suppression of the STAT-dependent transcription factors c-fos and JunB (Figure 1).
Murine corticotroph AtT-20 cells incubated with 0.1–10.0 ng/ml LIF exhibit rapid and potent stimulation of SOCS-3 mRNA expression, peaking at 30 minutes and persisting for as long as 8 hours (28, 33). The related inhibitors SOCS-2 and CIS are not significantly induced under these circumstances. Interestingly, SOCS-3 mRNA is also induced, albeit at a lower magnitude, in corticotroph AtT-20 cells following incubation with IL-11 (28, 44), IL-6 (28), or IL-1β (33). Hence, in the corticotroph, the termination of signaling by multiple cytokines appears to depend on SOCS-3.
In vivo, the hypothalamus (33, 41, 45, 46) and pituitary (33, 41) of intact mice show very low basal expression of SOCS-3 mRNA but can induce SOCS-3 expression within 30–60 minutes following systemic administration of LIF or IL-1β (33), respectively. Similarly, systemic administration of LPS endotoxin rapidly induces hypothalamic SOCS-3 expression (46). In other models of inflammation, subcutaneous injection of CFA or intramuscular administration of turpentine causes increased SOCS-3 mRNA pituitary expression, and to a lesser extent in the hypothalamus (41). This response in part reflects the action of an important STAT1/STAT3- binding element located in the SOCS3 promoter (28). Deletion or inactivating mutation of this binding element in the murine SOCS3 promoter almost completely abrogates promoter activity. Likewise, overexpression of dominant negative STAT3 mutants decreases LIF-induced SOCS3 promoter activity and gene expression (28), confirming that LIF-induced SOCS-3 expression in the corticotroph cell is critically STAT3-dependent. On the other hand, since SOCS-3 is a potent inhibitor of LIF-induced JAK-STAT signaling, SOCS-3 negatively regulates its own cytokine-induced expression at the mRNA level by a short intracellular inhibitory feedback loop (Figures 1 and 2).
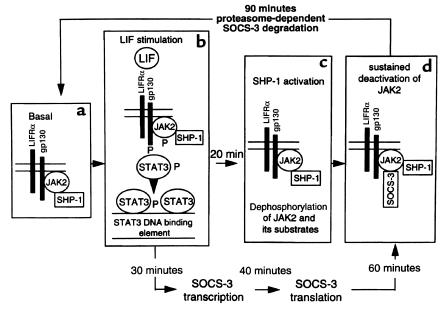
Figure 2.
Inhibitory effects of SOCS-3 and SHP-1 on LIF-mediated gene expression. (a) The tyrosine kinase JAK2 and tyrosine phosphatase SHP-1 are constitutively expressed but remain inactive in the unstimulated corticotroph. In contrast, SOCS-3 expression in the unstimulated corticotroph is minimal. (b) LIF binding rapidly induces the LIF receptor (LIFR) and gp130 subunits to form a heterodimer receptor complex. Receptor complex formation leads to autophosphorylation of receptor-associated JAK2, followed by tyrosine phosphorylation of the receptor’s cytoplasmic domain and recruitment of STAT proteins to the receptor complex. Subsequent tyrosine phosphorylation of STATs enables homo- or heterodimerization of STAT proteins. The dimerized STAT complexes translocate to the nucleus and bind to specific STAT-binding elements in the promoter region of various genes, among them SOCS3. (c) The tyrosine phosphatase SHP-1 is activated by LIF, showing maximum catalytic activity at 30 minutes. JAK2 and its substrates are dephosphorylated by SHP-1. Thus, SHP-1 is a constitutively expressed and rapidly activated inhibitor of JAK-STAT signaling in the corticotroph. (d) STAT-dependent SOCS3 gene expression is induced severalfold by LIF within 30 minutes. SOCS-3 protein associated with JAK2 is detectable 40–60 minutes after LIF stimulation. Association of SOCS-3 with JAK2 inhibits JAK2 activity. Thus, SOCS-3 inhibits JAK-STAT signaling in the corticotroph, its expression rapidly up- and downregulated by LIF and negative autoregulation of its own STAT-dependent gene expression. SOCS-3 is rapidly degraded by a proteasome-dependent pathway, allowing the corticotroph to return to its basal state, in which it can once again be activated by LIF or other gp130 cytokines (see a). Reproduced from ref. 18 with permission.
Corticotroph SOCS-3 gene expression is also induced by cAMP analogues, such as pituitary adenylate cyclase–activating polypeptide (PACAP), CRH, or epinephrine, which can act either alone or cooperatively with LIF (47). These effects on corticotroph SOCS-3 expression are protein kinase A–dependent (PKA-dependent), as overexpression of a dominant negative PKA isoform inhibits cAMP-mediated SOCS promoter activation (47). Following stimulation of AtT-20 cells with dibutyryl-cAMP, c-fos and JunB bind specifically to the AP-1 site in the SOCS3 promoter (47). Mutation of this AP-1 site inhibits dibutyryl-cAMP–mediated SOCS3 promoter activation by approximately 40%, consistent with evidence that dibutyryl-cAMP’s effects on the SOCS3 promoter are mediated by several other elements in addition to the AP-1 site (47). As shown in Figure 2, corticotroph SOCS-3 protein expression is rapidly regulated and short-lived. LIF-induced SOCS-3 protein expression occurs as early as 20 minutes, peaking after 40–60 minutes, and disappearing at 90 minutes. Preincubation of AtT-20 cells with the proteasome inhibitor LLnL resulted in LIF-induced SOCS-3 protein remaining detectable for as long as 180 minutes (18).
Deletion analysis of SOCS-3 has shed some light on the mechanism by which this protein suppresses JAK-mediated LIF signaling. A truncated recombinant form of SOCS-3 containing the pre-SH2 domain, the SH2 domain, and the SOCS-box motif inhibits LIF-induced POMC promoter activity. In contrast, constructs in which the SOCS-box motif or the pre-SH2 domain deletions are deleted are inactive (18). These results are consistent with other studies, demonstrating the essential roles of pre-SH2 and SH2 domains of SOCS-3 and SOCS-1, respectively, for suppression of the JAK-STAT cascade. The SOCS-box motif alone is sufficient to inhibit corticotroph LIF signaling by SOCS-3 (18). However, the requirement for the SOCS-box and other motifs for the suppressive action of SOCS proteins is controversial (19, 20) and requires further investigation.
SOCS-3 as a negative regulator of leptin signaling
SOCS-3 is a candidate leptin resistance factor. Overexpression of SOCS-3 results in inhibition of leptin-induced tyrosine phosphorylation of JAK2 (23). Mutational analysis shows that Tyr985, which is essential for recruitment of SHP-2 (48) and SOCS-3 (25, 26) to the intracellular domain of the long leptin receptor isoform, is not required for STAT3 signaling. Binding of SOCS-3 to this site is nevertheless required for the inhibition of leptin signaling (25), at least when SOCS-3 is present at normal levels; higher, possibly supraphysiological concentrations of SOCS-3 may also inhibit leptin signaling by direct association with JAK2 (23, 25). In vitro incubation with leptin induces expression of SOCS-3 but not SOCS-1, SOCS-2, or CIS (23).
Following systemic leptin administration, leptin-deficient ob/ob mice (23) as well as Wistar rats show an increase in hypothalamic SOCS-3 expression. Several other in vivo findings suggest that SOCS-3 reduces leptin sensitivity. Hypothalamic SOCS-3 expression is increased in the lethal yellow (Ay/a) mouse, which is subject to both hyperleptinemia and leptin resistance (45). Hypothalamic SOCS-3 expression also increased in 18-month-old versus 2-month-old rats, the former exhibiting relative leptin resistance (49).
SOCS-3 as a negative regulator of GH signaling
GH rapidly and potently induces hepatic SOCS-3 expression (13, 50), suggesting that signaling by this factor, too, may be terminated by SOCS-3, and raising the possibility that heightened expression of this regulator could lead to the GH resistance seen in chronic illness or inflammatory states. Induction of hepatic SOCS-1 following GH is weak (13) or undetectable (50); CIS and SOCS-2 are induced in the liver, although with much slower kinetics than SOCS-3 (13, 50).
Overexpression of SOCS-3 and SOCS-1 (but not of CIS or SOCS-2; refs. 13–16, 51) strongly inhibits GH (13–15) and prolactin (16) signaling. Interestingly, high SOCS-2 expression has been reported to superinduce GH signaling (13, 14). SOCS-1–inhibited GH and prolactin signaling is restored in a concentration-dependent manner by SOCS-2 coexpression (14, 16). The same phenomenon is not observed during coexpression of SOCS-3 and SOCS-2 (14, 16). Thus, the rapidly induced SOCS-3 and SOCS-1 genes might switch off GH signaling, which could then be restored by later induction of SOCS-2, which partially antagonizes SOCS-1 action.
Bacterial endotoxin and IL-1β induce hepatic SOCS-3 gene expression (52, 53) and inhibit GH signaling. GH resistance, characteristic of several inflammatory states, could thus be mediated by hepatic SOCS-3 expression, induced by endotoxin and various inflammatory cytokines, e.g., IL-6, IL-1β, and TNF-α (52, 53). SOCS-3 subsequently mediates GH resistance by inhibition of intracellular GH signaling. The stunted growth observed in children with chronic illness is likely caused by GH resistance mediated by cytokine-induced intracellular inhibition of GH action.
Summary
SOCS-3 is a potent inhibitor of the JAK-STAT signaling cascade, negatively regulating signal transduction of a variety of cytokines, including gp130 cytokines, leptin, and GH.
Several gp130 cytokines are neuro-immune modulators of HPA axis function. LIF action on corticotroph cell function is well characterized and has been demonstrated to be important for the HPA axis response. LIF upregulates POMC gene expression as well as SOCS-3 gene expression by STAT-dependent mechanisms. Following LIF stimulation, corticotroph SOCS-3 expression is rapidly upregulated. Cellular SOCS-3 expression is tightly controlled by negative autoregulation of its own STAT-dependent promoter activity as well as short protein half-life enabling rapid “on” and “off” mechanisms, subserving corticotroph plasticity toward various neuro-immune stimuli. SOCS-3 also contributes to central leptin resistance and hepatic GH resistance. Thus, SOCS-3 plays a critical role in integrating the neuro-immunoendocrine interface in the HPA axis as well as other neuro-immunoendocrine circuits.
References
- 1.Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37:1–11. doi: 10.1016/s0161-5890(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 2.Auernhammer CJ, Melmed S. Leukemia-inhibitory-factor: neuroimmmune modulator of endocrine function. Endocr Rev. 2000;21:313–345. doi: 10.1210/edrv.21.3.0400. [DOI] [PubMed] [Google Scholar]
- 3.Starr R, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 4.Endo TA, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 5.Hilton DJ, et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohya K, et al. SOCS-1/JAB/SSI-1 can bind to and suppress Tec protein-tyrosine kinase. J Biol Chem. 1997;272:27178–27182. doi: 10.1074/jbc.272.43.27178. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto A, et al. CIS, a cytokine inducible SH2 protein, is a target of the Jak-STAT5 pathway and modulates STAT5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- 8.Artz E. gp130 cytokine signaling in the pituitary gland: a paradigm for cytokine–neuro-endocrine pathways. J Clin Invest. 2001;108:1729–1733. doi: 10.1172/JCI14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson SE, et al. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duval D, Reinhardt B, Kedinger C, Boeuf H. Role of suppressors of cytokine signaling (Socs) in leukemia inhibitory factor (LIF)-dependent embryonic stem cell survival. FASEB J. 2000;14:1577–1584. doi: 10.1096/fj.14.11.1577. [DOI] [PubMed] [Google Scholar]
- 12.Hamanaka I, et al. Induction of JAB/SOCS-1/SSI-1 and CIS3/SOCS-3/SSI-3 is involved in gp130 resistance in cardiovascular system in rat treated with cardiotrophin-1 in vivo. Circ Res. 2001;88:727–732. doi: 10.1161/hh0701.088512. [DOI] [PubMed] [Google Scholar]
- 13.Adams TE, et al. Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem. 1998;273:1285–1287. doi: 10.1074/jbc.273.3.1285. [DOI] [PubMed] [Google Scholar]
- 14.Favre H, Benhamou A, Finidori J, Kelly PA, Edery M. Dual effects of suppressor of cytokine signaling (SOCS-2) on growth hormone signal transduction. FEBS Lett. 1999;453:63–66. doi: 10.1016/s0014-5793(99)00681-x. [DOI] [PubMed] [Google Scholar]
- 15.Ram PA, Waxman DJ. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem. 1999;274:35553–35561. doi: 10.1074/jbc.274.50.35553. [DOI] [PubMed] [Google Scholar]
- 16.Pezet A, Favre H, Kelly PA, Edery M. Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J Biol Chem. 1999;274:24497–24502. doi: 10.1074/jbc.274.35.24497. [DOI] [PubMed] [Google Scholar]
- 17.Yasukawa H, et al. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bousquet C, Susini C, Melmed S. Inhibitory roles for SHP-1 and SOCS-3 following pituitary proopiomelanocortin induction by leukemia inhibitory factor. J Clin Invest. 1999;104:1277–1285. doi: 10.1172/JCI7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JG, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okabe S, et al. Thrombopoietin induces an SH2-containing protein, CIS1, which binds to Mpl: involvement of the ubiquitin proteosome pathway. Exp Hematol. 1999;27:1542–1547. doi: 10.1016/s0301-472x(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 21.Siewert E, Muller-Esterl W, Starr R, Heinrich PC, Schaper F. Different protein turnover of interleukin-6-type cytokine signalling components. Eur J Biochem. 1999;265:251–257. doi: 10.1046/j.1432-1327.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 22.Sporri B, Kovanen PE, Sasaki A, Yoshimura A, Leonard WJ. JAB/SOCS1/SSI-1 is an interleukin-2-induced inhibitor of IL-2 signaling. Blood. 2001;97:221–226. doi: 10.1182/blood.v97.1.221. [DOI] [PubMed] [Google Scholar]
- 23.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson SE, et al. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci USA. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjorbak C, et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275:40649–40657. doi: 10.1074/jbc.M007577200. [DOI] [PubMed] [Google Scholar]
- 26.Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett. 2000;486:33–37. doi: 10.1016/s0014-5793(00)02205-5. [DOI] [PubMed] [Google Scholar]
- 27.Verdier F, et al. A sequence of the CIS gene promoter interacts preferentially with two associated STAT5A dimers: a distinct biochemical difference between STAT5A and STAT5B. Mol Cell Biol. 1998;18:5852–5860. doi: 10.1128/mcb.18.10.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auernhammer CJ, Bousquet C, Melmed S. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc Natl Acad Sci USA. 1999;96:6964–6969. doi: 10.1073/pnas.96.12.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Stravopodis D, Teglund S, Kitazawa J, Ihle JN. Naturally occurring dominant negative variants of Stat5. Mol Cell Biol. 1996;16:6141–6148. doi: 10.1128/mcb.16.11.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akita S, et al. Human and murine pituitary expression of leukemia inhibitory factor. Novel intrapituitary regulation of adrenocorticotropin hormone synthesis and secretion. J Clin Invest. 1995;95:1288–1298. doi: 10.1172/JCI117779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray DW, Ren SG, Melmed S. Leukemia inhibitory factor (LIF) stimulates proopiomelanocortin (POMC) expression in a corticotroph cell line. Role of STAT pathway. J Clin Invest. 1996;97:1852–1859. doi: 10.1172/JCI118615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimon I, Yan X, Ray DW, Melmed S. Cytokine-dependent gp130 receptor subunit regulates human fetal pituitary adrenocorticotropin hormone and growth hormone secretion. J Clin Invest. 1997;100:357–363. doi: 10.1172/JCI119541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auernhammer CJ, Chesnokova V, Bousquet C, Melmed S. Pituitary corticotroph SOCS-3: novel intracellular regulation of leukemia-inhibitory factor-mediated proopiomelanocortin gene expression and adrenocorticotropin secretion. Mol Endocrinol. 1998;12:954–961. doi: 10.1210/mend.12.7.0140. [DOI] [PubMed] [Google Scholar]
- 34.Bousquet C, Melmed S. Critical role for STAT3 in murine pituitary adrenocorticotropin hormone leukemia inhibitory factor signaling. J Biol Chem. 1999;274:10723–10730. doi: 10.1074/jbc.274.16.10723. [DOI] [PubMed] [Google Scholar]
- 35.Bousquet C, Zatelli MC, Melmed S. Direct regulation of pituitary proopiomelanocortin by STAT3 provides a novel mechanism for immuno-neuroendocrine interfacing. J Clin Invest. 2000;106:1417–1425. doi: 10.1172/JCI11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bousquet C, Ray DW, Melmed S. A common pro-opiomelanocortin-binding element mediates leukemia inhibitory factor and corticotropin-releasing hormone transcriptional synergy. J Biol Chem. 1997;272:10551–10557. doi: 10.1074/jbc.272.16.10551. [DOI] [PubMed] [Google Scholar]
- 37.Akita S, Conn PM, Melmed S. Leukemia inhibitory factor (LIF) induces acute adrenocorticotrophic hormone (ACTH) secretion in fetal rhesus macaque primates: a novel dynamic test of pituitary function. J Clin Endocrinol Metab. 1996;81:4170–4178. doi: 10.1210/jcem.81.11.8923879. [DOI] [PubMed] [Google Scholar]
- 38.Chesnokova V, Auernhammer CJ, Melmed S. Murine leukemia inhibitory factor gene disruption attenuates the hypothalamo-pituitary-adrenal axis stress response. Endocrinology. 1998;139:2209–2216. doi: 10.1210/endo.139.5.6016. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Ren SG, Melmed S. Hypothalamic and pituitary leukemia inhibitory factor gene expression in vivo: a novel endotoxin-inducible neuro-endocrine interface. Endocrinology. 1996;137:2947–2953. doi: 10.1210/endo.137.7.8770918. [DOI] [PubMed] [Google Scholar]
- 40.Auernhammer CJ, Chesnokova V, Melmed S. Leukemia inhibitory factor modulates interleukin-1beta-induced activation of the hypothalamo-pituitary-adrenal axis. Endocrinology. 1998;139:2201–2208. doi: 10.1210/endo.139.5.6017. [DOI] [PubMed] [Google Scholar]
- 41.Chesnokova V, Melmed S. Leukemia inhibitory factor mediates the hypothalamic pituitary adrenal axis response to inflammation. Endocrinology. 2000;141:4032–4040. doi: 10.1210/endo.141.11.7778. [DOI] [PubMed] [Google Scholar]
- 42.Akita S, Malkin J, Melmed S. Disrupted murine leukemia inhibitory factor (LIF) gene attenuates adrenocorticotropic hormone (ACTH) secretion. Endocrinology. 1996;137:3140–3143. doi: 10.1210/endo.137.7.8770940. [DOI] [PubMed] [Google Scholar]
- 43.Yano H, Readhead C, Nakashima M, Ren SG, Melmed S. Pituitary-directed leukemia inhibitory factor transgene causes Cushing’s syndrome: neuro-immune-endocrine modulation of pituitary development. Mol Endocrinol. 1998;12:1708–1720. doi: 10.1210/mend.12.11.0200. [DOI] [PubMed] [Google Scholar]
- 44.Auernhammer CJ, Melmed S. Interleukin-11 stimulates POMC gene expression and ACTH secretion in corticotroph cells: evidence for a redundant cytokine network in the HPA axis. Endocrinology. 1999;140:1559–1566. doi: 10.1210/endo.140.4.6636. [DOI] [PubMed] [Google Scholar]
- 45.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 46.Lebel E, Vallieres L, Rivest S. Selective involvement of interleukin-6 in the transcriptional activation of the suppressor of cytokine signaling-3 in the brain during systemic immune challenges. Endocrinology. 2000;141:3749–3763. doi: 10.1210/endo.141.10.7695. [DOI] [PubMed] [Google Scholar]
- 47.Bousquet C, Chesnokova V, Kariagina A, Ferrand A, Melmed S. cAMP neuropeptide agonists induce pituitary suppressor of cytokine signaling-3: novel negative feedback mechanism for corticotroph cytokine action. Mol Endocrinol. 2001;15:1880–1890. doi: 10.1210/mend.15.11.0733. [DOI] [PubMed] [Google Scholar]
- 48.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZW, et al. The role of leptin resistance in the lipid abnormalities of aging. FASEB J. 2001;15:108–114. doi: 10.1096/fj.00-0310com. [DOI] [PubMed] [Google Scholar]
- 50.Davey HW, McLachlan MJ, Wilkins RJ, Hilton DJ, Adams TE. STAT5b mediates the GH-induced expression of SOCS-2 and SOCS-3 mRNA in the liver. Mol Cell Endocrinol. 1999;158:111–116. doi: 10.1016/s0303-7207(99)00175-6. [DOI] [PubMed] [Google Scholar]
- 51.Karlsson H, Gustafsson JA, Mode A. Cis desensitizes GH induced Stat5 signaling in rat liver cells. Mol Cell Endocrinol. 1999;154:37–43. doi: 10.1016/s0303-7207(99)00101-x. [DOI] [PubMed] [Google Scholar]
- 52.Mao Y, et al. Endotoxin-induced inhibition of growth hormone receptor signaling in rat liver in vivo. Endocrinology. 1999;140:5505–5515. doi: 10.1210/endo.140.12.7212. [DOI] [PubMed] [Google Scholar]
- 53.Colson A, Le Cam A, Maiter D, Edery M, Thissen JP. Potentiation of growth hormone-induced liver suppressors of cytokine signaling messenger ribonucleic acid by cytokines. Endocrinology. 2000;141:3687–3695. doi: 10.1210/endo.141.10.7724. [DOI] [PubMed] [Google Scholar]