Abstract
BACKGROUND:
A 1996 preproject survey among Canadian Hospital Epidemiology Committee (CHEC) sites revealed variations in the prevention, detection, management and surveillance of Clostridium difficile-associated diarrhea (CDAD). Facilities wanted to establish national rates of nosocomially acquired CDAD (N-CDAD) to understand the impact of control or prevention measures, and the burden of N-CDAD on health care resources. The CHEC, in collaboration with the Laboratory Centre for Disease Control (Health Canada) and under the Canadian Nosocomial Infection Surveillance Program, undertook a prevalence surveillance project among selected hospitals throughout Canada.
OBJECTIVE:
To establish national prevalence rates of N-CDAD.
METHODS:
For six weeks in 1997, selected CHEC sites tested all diarrheal stools from inpatients for either C difficile toxin or C difficile bacteria with evidence of toxin production. Questionnaires were completed for patients with positive stool assays who met the case definitions.
RESULTS:
Nineteen health care facilities in eight provinces participated in the project. The overall prevalence of N-CDAD was 13.0% (95% CI 9.5% to 16.5%). The mean number of N-CDAD cases were 66.3 cases/100,000 patient days (95% CI 37.5 to 95.1) and 5.9 cases/1000 patient admissions (95% CI 3.4 to 8.4). N-CDAD was found most frequently in older patients and those who had been hospitalized for longer than two weeks in medical or surgical wards.
CONCLUSIONS:
This national prevalence surveillance project, which established N-CDAD rates, is useful as 'benchmark' data for Canadian health care facilities, and in understanding the patterns and impact of N-CDAD.
Key Words: Canada, CDAD, Clostridium difficile-associated diarrhea, Hospital, Nosocomial diarrhea, Prevalence
Nosocomial acquisition and transmission of Clostridium difficile are well known (1-4). Despite efforts to control and prevent infections in health care facilities, nosocomially acquired C difficile-associated diarrhea (N-CDAD) persists; some have reported that the number of N-CDAD infections are increasing (5-10). Although the majority of patients remain asymptomatic following acquisition of C difficile (5), it is still the most commonly identified cause of nosocomial diarrhea (5,11,12). While specific antibiotic therapy for C difficile has reduced morbidity and mortality among people with CDAD (13-15), evidence exists that C difficile infection contributes to patient morbidity (7,10) and significantly impacts hospital costs (15-17).
Published literature related to the prevalence of CDAD primarily describes periodic outbreaks or endemic situations in health care facilities (7,10,16-19). Because elderly people and those exposed to large amounts of antibiotics have a higher risk of acquiring CDAD, they are commonly surveyed (15,20,21). Specific wards (eg, medical and surgical) where the rates of CDAD are higher are also more frequently studied (2,6,22,23). Multicentre and national surveillance of CDAD in North America and Europe is rare (24-28). In Canada, individual health centres have data on the prevalence and demographics of CDAD cases (6,9,21); however, no national data exist.
Many CDAD surveillance studies include community cases (16,17,24-28); however, it is useful to examine specifically N-CDAD cases, because they represent illness that may be prevented by hospital infection prevention and control practices. The primary reservoirs of C difficile in the hospital are humans and the environment (29). Consequently, the nosocomial acquisition of this organism may represent inadequate infection control practices (30). This underscores the importance of instigating measures to monitor the prevalence of N-CDAD, and implementing and assessing the efficacy of any prevention or control practices.
There is no Canadian literature that examines the hospital costs of C difficile infections. Worldwide, there are limited data regarding the hospital costs associated with CDAD (16,17). One British study specifically examined the costs of N-CDAD (15). However, all studies suggest that these costs are substantial, which include the expenses of caring for and treating patients with CDAD, combined with the costs associated with C difficile outbreaks (15-17).
An N-CDAD prevalence project was undertaken by the Canadian Nosocomial Infection Surveillance Program (CNISP) through participating Canadian health care facilities. CNISP is a collaborative national surveillance program among the Laboratory Centre for Disease Control, Health Canada and the Canadian Hospital Epidemiology Committee (CHEC), a subcommittee of the Canadian Infectious Disease Society. CHEC members participated voluntarily in the CNISP project. The intent of this project was to establish health care facility N-CDAD prevalence rates that could be used as 'benchmark' data for other Canadian health care facilities, and to assist with the development and evaluation of guidelines that may decrease the incidence and cost of N-CDAD within Canadian health care facilities. The project used standardized case definitions for CDAD and N-CDAD. Non-nominal data were collected and submitted to the Laboratory Centre for Disease Control for compilation, analysis and interpretation. To estimate the burden of N-CDAD on the Canadian health care system, it was necessary to first determine national N-CDAD prevalence rates through a multicentre, geographically diverse surveillance project.
BACKGROUND
A pilot survey was conducted among 18 CHEC members in June 1996 to provide information on the prevalence, prevention and control practices, and current surveillance activities of CDAD.
The results of this survey showed variation between specific control or prevention policies at CHEC sites. In addition, it revealed that facilities were unclear on whether or not their facility was experiencing problematic rates of CDAD; seven sites reported that they had a problem with CDAD, and 11 stated that they did not. However, despite the different perceptions of whether CDAD was a major issue, rates of N-CDAD in each of the two groups did not appear to be statistically significantly different. As a result of these findings, the CNISP 1997 N-CDAD Prevalence Surveillance Project was developed and implemented. N-CDAD was examined because of the need for comparison data to evaluate hospital infection control practices.
METHODS
At participating CHEC facilities and selected affiliated centres, all inpatient stools submitted to the hospital laboratory in a liquid or semiformed condition were screened for the presence of C difficile toxin by the method currently in use at that facility (ie, cytotoxin assay or growth of C difficile bacteria with evidence of toxin production), regardless of the clinical indication for the specimen. In addition, surveillors included the endoscopy laboratory, who looked for patients with atypical membranes on sigmoidoscopy or colonoscopy.
A C difficile 'potential' case was defined to be any person with stool containing C difficile cytotoxin or pseudomembranes on endoscopy. When a potential C difficile case was identified, the hospital's infection control practitioner reviewed the patient's chart to determine whether the patient met the project's case definition of N-CDAD (consistent with that recommended by the Society For Healthcare Epidemiology of America) (29).
An N-CDAD case was defined as a potential C difficile case with an acute onset of loose stools that persisted for at least two days without an alternative explanation for the diarrhea. In addition, all CDAD cases had to fulfill one of two criteria to ensure that it was a case of N-CDAD: the symptoms occurred three days or more after admission, or the symptoms caused readmission in a patient who was discharged from the partici-pating facility or any other health care facility within one month before the current admission date.
Participating sites collected data between January and April 1997, either for six continuous weeks or until 200 conconsecutive diarrhea stool samples had been tested at the site, whichever happened first.
For cases that met the project's definition of N-CDAD, further information was collected, including sex, date of birth, type of N-CDAD case (primary, relapse or reinfection), location of patient, whether patient was on antimicrobial medications at the time of stool specimen collection, and details on the treatments of or investigations for N-CDAD. Relapses of N-CDAD were defined as cases that recurred within two weeks after the end of the previous N-CDAD treatment, while reinfections were defined as cases that recurred after the patient had been asymptomatic for at least two weeks after the end of therapy. Primary cases were those that did not meet the definitions of relapse or reinfection.
The type of care an N-CDAD patient received was recorded. A long term care (LTC) patient was defined as a patient who was waiting for a LTC bed or who was already in a designated LTC bed. An acute care patient was defined as one that did not meet the definition for LTC.
Information was collected describing whether the patient had other morbidity due to N-CDAD or whether the infection control practitioner believed that the length of stay (LOS) in hospital was extended as a result of the N-CDAD episode. The patients' outcome at discharge or at the end of the study was recorded, as well as the date of discharge or death. Deaths directly or indirectly attributed to N-CDAD were defined respectively as due to colitis (eg, hemorrhage or perforation) or complications that would not have occurred if N-CDAD had not developed (eg, dehydration, debilitation). For these cases, the participating CHEC members consulted the attending physician, and a short narrative was written concerning the events surrounding the patient's death.
Laboratory information collected included the time period over which diarrheal stools were screened, the laboratory methods used for detecting C difficile toxin, and the total number of samples and patients tested. Duplicate specimens were not analyzed.
Information about each hospital was collected, including the type of health care facility (adult care, paediatric care, LTC), as well as the total number of hospital beds, admissions and person days in the survey period. These data were collected from the 1996 Canadian Hospital Association Guide to Canadian Health Care Facilities (31) and the 1995 CNISP CHEC site database.
Data were entered into Epi Info Version 6.03 (Centers for Disease Control and Prevention, USA), which was used to calculate descriptive statistics, odds ratios (ORs), P values and 95% CIs.
RESULTS
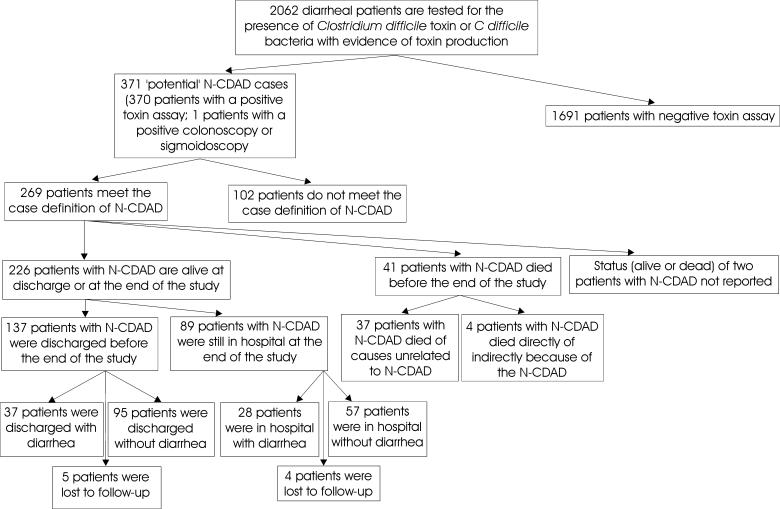
Nineteen health care facilities in eight provinces participated in the CNISP 1997 N-CDAD prevalence surveillance project, and their profiles can be seen in Table 1. One facility participated from British Columbia, two from Alberta, one from Manitoba, six from Ontario, three from Quebec, one from New Brunswick, one from Nova Scotia and four from Newfoundland. Eighteen of the centres had adult care facilities, five had LTC services and five had paediatric services. Of the 18 facilities that provided site demographic information, two had fewer than 200 beds, five had 200 to 399 beds, three had 400 to 599 beds, one had 600 to 799 beds and seven had 800 or more beds. The median duration of participation in the project was 42 days, while the range was 30 to 99 days. The results of the surveillance can be seen in (Figure 1). During the surveillance period, 2062 inpatients had diarrhea that was submitted to participating microbiology laboratories for analysis. Duplicate samples were eliminated, and samples were then screened for C difficile cytotoxin; 370 samples were positive. One additional patient without diarrhea had a colonoscopy or sigmoidoscopy, which revealed the typical pseudomembranes of C difficile infection. Thus, 18.0% (95% CI 13.7% to 22.3%) of the inpatients investigated for C difficile infection were potential C difficile cases.
TABLE 1.
Characteristics of health care facilities participating in the Canadian Nosocomial Infection Surveillance Program 1997 nosocomial Clostridium difficile-associated diarrhea prevalence surveillance project (n=19)
| Characteristic | Number of facilities |
|---|---|
| Province | |
| British Columbia | 1 |
| Alberta | 2 |
| Manitoba | 1 |
| Ontario | 6 |
| Quebec | 3 |
| New Brunswick | 1 |
| Nova Scotia | 1 |
| Newfoundland | 4 |
| Number of beds | |
| <200 | 2 |
| 200 to 399 | 5 |
| 400 to 599 | 3 |
| 600 to 799 | 1 |
| ≥800 | 7 |
| Did not report | 1 |
| Type of care | |
| Adult care only | 11 |
| Paediatric care only | 1 |
| Adult and paediatric care | 2 |
| Adult and long term care | 3 |
| Adult, paediatric and long term care | 2 |
Figure 1.
Breakdown of the results of the Canadian Nosocomial Infection Surveillance Program 1997 nosocomial Clostridirum difficile-associated diarrhea (N-CDAD) prevalence surveillance project
All 19 participating sites used a toxin assay to detect C difficile, but some used more than one type of assay. Eleven sites (58%) used the cytotoxin B assay by means of cell culture, nine sites (47%) used enzyme immunoassay (toxin A or A/B), and one site used latex agglutination (antigen detection) of C difficile bacterium. Also, two sites cultured for C difficile bacterium and then tested for toxin production.
Of the 371 potential C difficile cases, 269 (72.5%) met the case definitions for N-CDAD. One hundred two (27.5%) of the cases did not meet the case definition of N-CDAD because they did not meet the criteria to be considered nosocomially acquired, the diarrhea did not persist for at least two days or the diarrhea could be explained by other causes, such as chemotherapy. Two hundred sixty-two (98%) of the patients with N-CDAD were detected by toxin assay. Four were found by the detection of C difficile bacterium with evidence of toxin production, and one case was detected by sigmoidoscopy or colonoscopy. Thus, the overall prevalence of N-CDAD among inpatients was 13.0% (13.0 cases/100 diarrheal stools) (95% CI 9.5% to 16.5%, range 0% to 30.8%). The mean number of N-CDAD cases was 66.3 cases/100,000 patient days (95% CI 37.5 to 95.1) and 5.9 cases/1000 patient admissions (95% CI 3.4 to 8.4). The mean number of N-CDAD cases per health care facility in a six-week period was 13.7 (95% CI 7.2 to 20.2).
Multiple testing of patient diarrheal stool samples for C difficile varied between the 19 participating sites, ranging from 0% to 43.5% of all samples (median 13.7%). Multiple testing was more common among stools negative for C difficile (median 16.6%) and less common among stools positive for C difficile (median 2.5%).
Of the 269 N-CDAD cases, 250 (93%) occurred three days or more after admission, and 19 patients (7%) were readmitted for diarrhea after being discharged from a hospital within the previous month. Two hundred thirteen patients (80%) were acute care patients while 54 (20%) were LTC patients. Most of the N-CDAD cases were primary cases (85%), while the others were relapses (11%) or reinfections (4%).
The characteristics of the patients with N-CDAD can be seen in Table 2. One hundred fifty-three patients (57%) with N-CDAD were female, while 115 (43%) were male. The mean age was 70 years for females and 65.5 years for males (P not significant), with an overall range of younger than one year to 95 years.
TABLE 2.
Characteristics of patients of nosocomial Clostridium difficile-associated diarrhea (N-CDAD) participating in the Canadian Nosocomial Infection Surveillance Program 1997 N-CDAD prevalence surveillance project (n=269)
| Characteristic | Values |
|---|---|
| Mean age | 68.1 years (95% CI 65.6 to 70.6) |
| Sex | |
| Male | 153 (57%) |
| Female | 115 (43%) |
| Type of patient | |
| Acute care | 213 (80%) |
| Long term care | 54 (20%) |
| Length of time from admission to onset of N-CDAD symptoms | Mean 39.9 days (95% CI 29.8 to 50.2) Median 15.0 days |
| Average length of stay (surviving cases) | Mean 36.7 days (95% CI 27.1 to 46.3) Median 25.0 days 156 (59%) |
| Number receiving antibacterial medications at time of stool specimen collection for C difficile toxin testing | 156 (59%) |
| Patients whose morbidity was related to N-CDAD | 21 (8%) |
| Patients whose length of stay was reportedly extended due to N-CDAD | 23 (9%) |
The mean length of time from admission to onset of symptoms was 39.9 days (95 % CI 29.8 to 50.2), with a range of three days to 809 days and a median of 150 days. The difference in the time from admission to onset of symptoms between those who survived and those who died was not significant. The mean length of time from onset of symptoms to laboratory specimen collection was 2.5 days (95% CI 1.9 to 3.1). The average LOS in hospital for surviving cases was 36.7 days (95% CI 27.1 to 46.3).
At the time of onset of N-CDAD symptoms, 50% of patients with N-CDAD were on a medical ward, 25% were on a surgical ward, 9% were in the intensive care unit and 6% were on a LTC ward. One hundred fifty-six (59%) were on antibacterial medications at the time of stool specimen collection. The details related to the treatment and/or investigation of the cases can be found in Table 3. Thirty-four of the patients (13%) were given no antibiotics to treat N-CDAD. One hundred ninety-nine patients (75%) were given one antibiotic, while 33 patients (12%) were given two or three antibiotics. The mean duration of administration for each antibiotic was eight days. The most common antibiotic used as therapy, oral metronidazole, was administered in 212 (80%) N-CDAD cases. Intravenous metronidazole was administered in 24 cases (9%), oral vancomycin was given in 21 cases (8%) and intravenous vancomycin was given in 11 cases (4%).
TABLE 3.
Treatments of or investigations for nosocomial Clostrodium difficile-associated diarrhea (N-CDAD), as found in the Canadian Nosocomial Infection Surveillance Program 1997 N-CDAD prevalence surveillance project (n=266)
| Treatment or investigation | Number of cases |
|---|---|
| No treatment | 31 (12%) |
| Only one antibiotic | 199 (75%) |
| Two or three sequential antibiotic courses | 33 (12%) |
| Type of antibiotic therapy (all treatments): | 212 (80%) |
| Oral metronidazole | 24 (9%) |
| Intravenous metronidazole | 21 (8%) |
| Oral vancomycin | 11 (4%) |
| Intravenous vancomycin | |
| Other treatment or investigation (eg, ultrasound) | 7 (3%) |
| Endoscopy as workup for diarrhea | 5 (2%) |
| Discontinued inciting antibiotic | 24 (9%) |
Patients with N-CDAD who were not treated with antibiotics were more likely to be LTC patients (OR 2.85, 95% CI 1.23 to 6.57) and located on surgical wards (OR 3.44, 95% CI 1.56 to 7.57). In addition, patients not treated with antibiotics were more likely to die (OR 2.69, 95% CI 1.08 to 6.60). Among the 34 patients with N-CDAD who were not treated with antibiotics, 10 (29%) died compared with 31 (13%) of the 231 patients with N-CDAD treated with antibiotics (P<0.05). The time from onset of N-CDAD symptoms to death was shorter for those cases who received no treatment for N-CDAD (6.6 days) than for those who died during or after treatment (15.2 days), but this difference was not statistically significant.
Following the identification of N-CDAD, 24 (9%) patients with N-CDAD stopped taking the inciting antibiotic. Seven (3%) patients with N-CDAD had a procedure performed as part of the workup of the diarrhea (eg, abdominal ultrasound), and five (2%) patients underwent endoscopy specifically for the diarrhea. No patients underwent surgery as a result of N-CDAD.
Complications resulting from N-CDAD were reported among 21 patients (8%). The most common conditions reported were dehydration (eight cases), hypokalemia (six cases) and gastrointestinal hemorrhage (three cases). These patients did not have a significantly higher mortality rate compared with patients who did not report complications (P>0.05).
Twenty-three patients (9%) had an LOS extension because of the N-CDAD episode, while 228 (85%) did not. For 13 patients (5%), it was unknown whether their LOS was extended. The mean length of time of the LOS extension was 9.9 days (95% CI 6.0 to 13.8).
Two hundred twenty-six patients (84%) with N-CDAD were alive at discharge or at the end of study. Of the 137 patients discharged, 28% had diarrhea and 72% did not have diarrhea at the time of discharge. Patients 65 years old or younger were more likely to be discharged with diarrhea than those older than 65 years old (OR 2.20, 95% CI 0.94 to 5.19, P=0.05).
Among the 89 inpatients who were alive and still in the hospital at the end of the study, 31% had diarrhea and 64% did not. The number of cases of diarrhea were not statistically significantly different between those who were still in the hospital at the end of the study and those who had been discharged (P=0.44).
Forty-one patients (15%) with N-CDAD died; 37 reportedly died from non-CDAD causes, while four reportedly died directly or indirectly because of N-CDAD. Of these four patients who died because of N-CDAD, two were dehydrated, one was suspected to have toxic megacolon and one became septic, with N-CDAD as the only inciting factor. Of the 41 N-CDAD patients who died, 10 (24%) received no treatment for N-CDAD compared with 21 (9%) of the 224 N-CDAD cases who did not die (OR 3.12, P<0.05).
DISCUSSION
The CNISP 1997 prevalence surveillance project identified 269 cases of N-CDAD among 2062 diarrheal patients tested, thus yielding a prevalence rate of 13% (95% CI 9.5% to 16.5%). The CNISP study is the first Canadian, multicentre, national point prevalence project to examine N-CDAD. The rates of CDAD reported in other multicentre, national studies are not comparable because they are based on less specific definitions of CDAD (24-28).
The CNISP project only included nosocomial patients who had had diarrhea for at least two days that could not be explained by another cause. Our selective case definition decreased the probability of false positives. According to Gerding et al (32), 10% or more of inpatients may be colonized with C difficile; however, only a minority of these patients develop CDAD even when the cytotoxin is found (33-35). Consequently, testing formed stools for C difficile or its toxins (29), or including patients whose diarrhea can be explained by other causes, can decrease the specificity of the diagnosis of CDAD.
Poor infection control practices have been associated with nosocomial outbreaks of CDAD (19). In one report, the use of disposable vinyl gloves was observed to reduce the incidence of CDAD from 7.7 to 1.5 cases/1000 patient discharges (36). Antibiotic control policies have also been associated with reducing the incidence of CDAD (7). Despite this finding, almost 30% of the sites in this study responded that they had no antibiotic restriction policies or had antibiotic restriction policies that were not fully enforced.
Some authors feel that antimicrobial exposure is necessary for the development of CDAD (29,37). While antibiotics appear to be one of the most common agents that predispose individuals to CDAD, infections can occur in cases related to other factors, such as chemotherapy. The present study found that 59% of patients with N-CDAD were on antimicrobial medications at the time of stool specimen collection. Patients were excluded if there were reasons that could account for the diarrhea, such as chemotherapy or gastrointestinal disease. Thus, it is likely that the 41% of patients who were not on antibiotics at the time of stool collection may have been exposed previously, rather than having N-CDAD arising de novo in all cases. This issue would be interesting to investigate further because it is important for clinicians to recognize that diarrhea occurring after antibiotics have been discontinued may be related to N-CDAD.
CDAD is reportedly more common among older individuals (6,10,20,21,24-26). In this study, 176 patients (67%) were older than age 65 years; this high proportion of older patients may reflect that there are larger numbers of elderly hospitalized patients or that older individuals are more susceptible to C difficile infections. Elderly individuals have decreased humoral immunity to C difficile (38), which may increase their susceptibility to C difficile infections. Furthermore, the National Corporation of Swedish Pharmacies has reported research findings that the increased consumption of antibiotics among the elderly is not always clearly correlated with the higher prevalence of CDAD infections (26).
LOS in hospital has been found to be significant in determining whether patients become colonized, and subsequently symptomatic, with C difficile (20). People with a longer LOS are generally older, sicker and on antibiotics, which may render them more susceptible to acquiring C difficile colonization or infection (15). Because of the longer LOS, they also have a greater likelihood of being exposed to C difficile (15). An alternative theory is that patients may develop N-CDAD and subsequently require further days of hospitalization (15).
Two case control studies found that patients with CDAD stayed between 18 and 21 days longer in hospital than controls (15,17). The median length of time from admission to onset of symptoms in the present study was 15 days, and the median LOS for discharged CDAD cases was 25 days (range two to 602 days). A case control study is necessary to examine whether CDAD may be responsible in prolonging the LOS for patients with CDAD. Some feel that the increase in LOS due to CDAD illness is the 'most important consequence' of C difficile infections. It has been estimated that 94% of the increased cost in managing a CDAD patient is due to the increased LOS (15).
While it was difficult to determine clearly all of the costs associated with hospitalized N-CDAD cases, these costs can more easily be estimated for N-CDAD readmissions. Overall, 19 readmissions in the present study were due to N-CDAD acquisition, with a mean LOS of 13.6 days. This information was used to estimate that, on average, 10 readmissions due to N-CDAD should occur per site per year. The cost of basic services associated with a Canadian hospital bed was approximately $900/day, and the yearly cost of antibiotics to treat N-CDAD readmissions at each site was $5,800/year, assuming that 80% of patients received oral metronidazole. Overall, the estimated cost for readmissions per site per year is $128,200. This figure is an underestimate of the cost of N-CDAD readmissions because it does not include physician time, laboratory and diagnostic expenses, or the cost of N-CDAD related complications.
The morbidity and mortality associated with CDAD infections can be significant (39). In the present project, 21 patients (8%) reported additional morbidity associated with N-CDAD. Four (1.5%) of the patients with N-CDAD died directly or indirectly because of CDAD. This percentage is somewhat higher than that found in a 10-year surveillance project at one centre in the United States, where five of the 9008 (0.6%) CDAD cases had identified CDAD as the primary cause of death (7).
Interestingly, it was found that those who died were more likely to not have received treatment for CDAD than those who lived. Individuals who received no specific treatment for CDAD may have died before treatment could be initiated or may have only received palliative measures.
Thirteen per cent of patients received more than one antibiotic for CDAD therapy. Another study reported that 12% of CDAD cases on a geriatric ward received repeat courses of antibiotic treatment (15). Clinical failure rates of antibiotic treatment may increase the LOS and the costs of CDAD therapy. Eighty per cent of patients were treated with oral metronidazole and 7% were treated with oral vancomycin. This is similar to the percentage observed by Wilcox et al (15). In that study, 70% of patients received oral metronidazole and 13% received oral vancomycin. The mean duration of treatment observed in the present study (8 days) was also similar to that reported by Wilcox et al (15).
The diagnosis of CDAD is a 'controversial area' (29); there is much discussion about the optimal laboratory testing methods of stools for C difficile. Testing diarrheal stools for C difficile using cultures and subsequent testing for toxin production is the 'gold standard' laboratory test that has been recommended for the diagnosis of CDAD because of its greater sensitivity than the cytotoxin B and enzyme immunoassay tests (10,29). However, these methods are slow, labour intensive and expensive. The authors found much diversity in the methods used at different centres.
Although differences in diagnostic methodologies may influence the identification of CDAD cases at separate sites, this cannot fully explain why the prevalences of CDAD at the different health care facilities ranged from 0% to 30.8% (29).
In the authors' 1996 pilot survey, it was found that health care facilities did not know whether their rates of N-CDAD were 'high' or 'low'. Not understanding what should be acceptable rates of CDAD requires good benchmark data for comparisons and education, because the perception of whether N-CDAD is a problem does not appear to correspond with the observed rates of N-CDAD. The present CNISP 1997 N-CDAD Point Prevalence Surveillance Project has established N-CDAD rates to aid facilities in 'benchmarking' their data against other Canadian facilities. These 'benchmark' rates should assist hospital administration in decision making regarding the necessary infection control measures within their institutions.
Acknowledgments
This project was funded by The Division of Nosocomial and Occupational Infections, Bureau of Infectious Diseases, Laboratory Centre for Disease, Ottawa, Ontario.
References
- 1.Facade R, Kim K-H, Brown D, et al. Epidemiology of antibiotic-associated colitis: isolation of Clostridium difficile from the hospital environment.Am J Med 1981;70:906-8. [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, Cabots CR, Linn FV, et al. Nosocomial Clostridium difficile colonization and disease.Lancet 1990;336:97-100. [DOI] [PubMed] [Google Scholar]
- 3.Kim K-H, Facade R, Batts DH, et al. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis.J Infect Dis 1981;143:42-50. [DOI] [PubMed] [Google Scholar]
- 4.McFarland LV, Mulligan ME, Kwok RYY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection.N Engl J Med 1989;320:204-10. [DOI] [PubMed] [Google Scholar]
- 5.Silva J Jr. Clostridium difficile nosocomial infections - still lethal and persistent.Infect Control Hosp Epidemiol 1994;15:368-70. [DOI] [PubMed] [Google Scholar]
- 6.Nath SK, Thornley JH, Kelly M, et al. A sustained outbreak of Clostridium difficile in a general hospital; persistence of a toxigenic clone in four units.Infect Control Hosp Epidemiol 1994;15:382-9. [DOI] [PubMed] [Google Scholar]
- 7.Olson MM, Shanhotzer MT, Lee JT JR, Gerding DN. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982-1991.Infect Control Hosp Epidmiol 1994;15:371-81. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health and Public Health Laboratory Service Joint Working Group. Clostridium difficile infection. Prevention and management. London, 1994.
- 9.Orenstein P, Amihod B, Miller MA. Clostridium difficile - do we just have to live with it?Can J Infect Control 1996;11:73 (Abst) [Google Scholar]
- 10.Riley TV, O'Neill GL, Bowman RA, Golledge CL. Clostridium difficile-associated diarrhoea: epidemiological data from Western Australia.Epidemiol Infect 1994;113:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegal DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital.JAMA 1990;263:979-82. [PubMed] [Google Scholar]
- 12.Yannelli B, Gurevich I, Schoch PE, Cunha BA. Yield of stool cultures, ova and parasite tests, and Clostridium difficile determinations in nosocomial diarrhea.Am J Infect Control 1988;16:246-9. [DOI] [PubMed] [Google Scholar]
- 13.Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin producing clostridia.N Engl J Med 1978;298:531-4. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett JG, Gorbach SL. Pseudo membranous enterocolitis (antibiotic-related colitis).Adv Intern Med 1977;22:455-76. [PubMed] [Google Scholar]
- 15.Wilcox MH, Cunniffe JG, Trundle C, Redpath C. Financial burden of hospital-acquired Clostridium difficile infection.J Hosp Infect 1996;34:23-30. [DOI] [PubMed] [Google Scholar]
- 16.Kofsky P, Rosen L, Reed J, et al. Clostridium difficile - A common and costly colitis.Dis Colon Rectum 1991;34:244-8. [DOI] [PubMed] [Google Scholar]
- 17.Riley TV, Codde JP, Rouse IL. Increased length of hospital stay due to Clostridium difficile associated diarrhea.Lancet 1995;345:455-6. [DOI] [PubMed] [Google Scholar]
- 18.Yablon SA, Krotenberg R, Fruhmann K. Clostridium difficile-related disease: evaluation and prevalence among inpatients with diarrhea in two freestanding rehabilitation hospitalsArch Phys Med Rehabil 1993;74:9-13. [PubMed] [Google Scholar]
- 19.Manian FA, Meyer L, Jenne J. Clostridium difficile contamination of blood pressure cuffs: a call for a closer look at gloving practices in the era of universal precautions.Infect Control Hosp Epidemiol 1996;17:180-2. [PubMed] [Google Scholar]
- 20.Rudensky B, Rosner S, Sonnenblick M, et al. The prevalence and nosocomial acquisition of Clostridium difficile in elderly hospitalized patients.Postgrad Med J 1993;69:45-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simor AE, Yake SL, Tsimidis K. Infection due to Clostridium difficile among elderly residents of a long-term care facility.Clin Infect Dis 1993;17:672-8. [DOI] [PubMed] [Google Scholar]
- 22.McFarland LV, Stamm WE. Review of Clostridium difficile-associated diseases.Am J Infect Control 1986;14:99-109. [DOI] [PubMed] [Google Scholar]
- 23.Cabots CR, Johnson S, Olson MM, et al. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection.J Infect Dis 1992;166:561-7. [DOI] [PubMed] [Google Scholar]
- 24.Barbut F, Corthier G, Charpak Y, et al. Prevalence and pathology of Clostridium difficile in hospitalized patients.Arch Intern Med 1996;156:1449-54. [PubMed] [Google Scholar]
- 25.Aronsson B, Mollby R, Nord CE. Diagnosis and epidemiology of Clostridium difficile enterocolitis in Sweden.J Antimicrob Chemother 1984;14(Suppl D):85-95. [DOI] [PubMed] [Google Scholar]
- 26.Aronsson B, Mollby R, Nord CE. Antimicrobial agents and Clostridium difficile in acute enteric disease: epidemiologic data from Sweden, 1980-82.J Infect Dis 1985;151:476-81. [DOI] [PubMed] [Google Scholar]
- 27.Liesenfeld O, Weinke T, Hahn H. Three-year prevalence of enteropathogenic bacteria in an urban patient population in Germany.Infection 1993;21:101-5. [DOI] [PubMed] [Google Scholar]
- 28.Mollby R, Nord CE, Aronsson B. Diagnosis of Clostridium difficile-associated enterocolitis in Sweden. Laboratory and epidemiological aspects.Scand J Infect Dis 1980;22(Suppl):30-6. [PubMed] [Google Scholar]
- 29.Gerding DN, Johnson S, Peterson LR, et al. Shea position paper - Clostridium difficile-associated diarrhea and colitis.Infect Control Hosp Epidemiol 1995;16:459-77. [DOI] [PubMed] [Google Scholar]
- 30.Mylotte JM. Laboratory surveillance method for nosocomial Clostridium difficile diarrhea.Am J Infect Control 1998;26:16-23. [DOI] [PubMed] [Google Scholar]
- 31.Canadian Healthcare Association. Guide to Canadian Healthcare Facilities, Volume 4, 1996-1997. Ottawa: CHA Press, 1996. [Google Scholar]
- 32.Gerding D, Olson MM, Peterson LR, et al. Clostridium difficile-associated diarrhea and colitis in adults.Arch Intern Med 1986;146:95-100. [PubMed] [Google Scholar]
- 33.Bender BS, Bennett R, Laughon BE, et al. Is Clostridium difficile endemic in chronic-care facilities?Lancet 1986;ii:11-3. [DOI] [PubMed] [Google Scholar]
- 34.Thomas DR, Bennett RG, Laughon BE, et al. Postantibiotic colonization with Clostridium difficile in nursing home patients.J Am Geriatr Soc 1990;38:415-20. [DOI] [PubMed] [Google Scholar]
- 35.Johnson S, Homann SR, Bettin KM, et al. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole.Ann Intern Med 1992;117:297-302. [DOI] [PubMed] [Google Scholar]
- 36.Johnson S, Gerding DN, Olson MM, et al. Prospective, controlled study of vinyl glove use to interrupt Clostridium difficile nosocomial transmission.Am J Med 1990;88:137-40. [DOI] [PubMed] [Google Scholar]
- 37.Thibault A, Miller M, Gaese C. Risk factors for the development of Clostridium difficile-associated diarrhea during a hospital outbreak.Infect Control Hosp Epidemiol 1991;12:345-9. [DOI] [PubMed] [Google Scholar]
- 38.Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium difficile.J Infect Dis 1983;148:93-100. [DOI] [PubMed] [Google Scholar]
- 39.Eriksson S, Aronsson B. Medical implications of nosocomial infection with Clostridium difficile. Scand JInfect Dis 1989;21:733-4. [DOI] [PubMed] [Google Scholar]