Abstract
A community-based, single strain cluster of methicillin-resistant Staphylococcus aureus (MRSA) is described. The cluster of cases was identified in a small rural community in southwestern Manitoba, although some spread of the MRSA strain to neighbouring communities was observed. The majority of people were otherwise healthy, had no contact with the hospital system and did not fit the profile of those at risk for MRSA infection.
Key Words: Community cluster, Methicillin-resistant Staphylococcus aureus, MRSA
Methicillin-resistant Staphylococcus aureus (MRSA) is recognized worldwide as a major nosocomial pathogen in hospitals. Guidelines have been established to provide a framework for the management of patients with MRSA in health care facilities. However, the epidemiology and control of MRSA in the community is not clear. We recently investigated a single strain community outbreak of MRSA in a small rural community. This report describes the community spread of this strain among otherwise healthy individuals with no clear predisposing risk factors.
DATA AND METHODS
Since 1995, Cadham Provincial Laboratory, Winnipeg, Manitoba, has been conducting molecular surveillance of MRSA isolates in rural areas of the province. From June to September 1997, isolates of MRSA with identical pulsed field gel electrophoresis (PFGE) patterns (type 1) were identified in four residents of a small rural community in southwestern Manitoba (community A). MRSA isolates with this PFGE pattern and antibiogram had not been seen previously in southern Manitoba during the surveillance period. Isolation of identical MRSA within this short timeframe and small geographic area was unusual compared with the baseline data that had been gathered over the previous two years.
An epidemiological investigation was initiated to characterize further the extent of the outbreak in the area. All isolates of MRSA from rural laboratories in Manitoba were sent to Cadham Provincial Laboratory for molecular surveillance. People from community A in which MRSA isolates previously had been identified were contacted either in person or by telephone to attempt to identify a source of the infection and any associated risk factors. Parents or guardians were interviewed in the case of minors. A patient was considered to have an MRSA infection if he or she was culture-positive, and showed signs and symptoms of infection caused by the organism. A person was considered to be colonized with MRSA if he or she tested culture-positive, and had no signs or symptoms of infection caused by the organism. The cases were interviewed using a questionnaire collecting information on demographic characteristics and exposure to potential risk factors for MRSA. The risk factors explored were an individual's underlying medical conditions, contact with hospitals or long term care facilities, occupation, travel history, contacts in northern Manitoba, previous antibiotic use, contact with other known cases of MRSA, alcohol or substance abuse, number of people in household, and type of household drinking water and sewage system. Clinical information was also obtained with regard to symptoms, physician visits, hospitalizations and treatment associated with the illness.
Antibiotic susceptibility patterns were ifdentified with the Vitek testing system (bioMérieux, USA) using a GPS-SB card (bioMérieux, USA) according to the manufacturer's instructions. Methicillin susceptibility testing was also performed by disk diffusion on Mueller-Hinton agar with a 1 mg disk. Quality control strains were tested routinely and yielded expected results. PFGE was performed using standard methods. Initial cell density was adjusted to 25% transmittance in saline using a Vitek colorimeter. Cells were resuspended in Tris-EDTA acid buffer and lysed with lysostaphin for 5 h. Agarose blocks were washed four times, for 30 min per wash, with Tris-EDTA buffer (10 mM Tris, 1 mM EDTA) at room temperature. Cleavage was performed overnight with 30 U of SmaI at 30°C. PFGE was performed with a CHEF-DRII system (Bio-Rad, Canada) with 1% agarose gels in 0.5x Tris-borate acid buffer. Electrophoresis was performed at 14°C with a constant voltage of 5 V cm-1 and a pulse time of 5 to 45 s for 22 h.
RESULTS
Community A is a small rural agricultural town in southwestern Manitoba with a population of approximately 1400 including the surrounding rural municipality. The community has a 16-bed hospital and a 20-bed long term care facility.
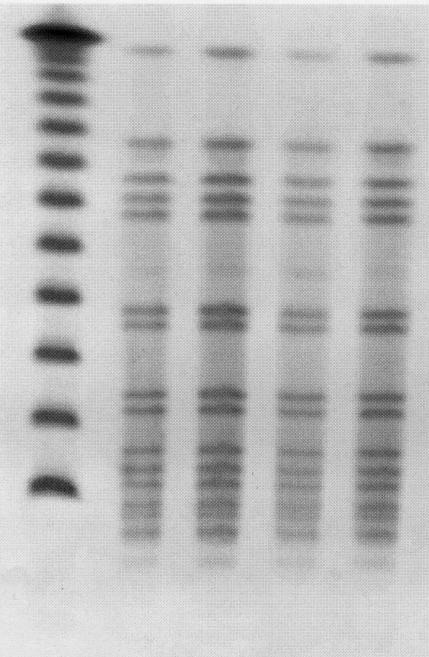
From June 1997 to November 1998, a total of 15 MRSA-infected cases from community A were identified. The MRSA isolates were identical by PFGE (Figure 1). The same strain had been isolated in five individuals from two isolated communities in northern Manitoba between 1995 and 1997. Following its emergence in community A, PFGE type 1 MRSA was also identified in 10 individuals in seven other rural communities in southern Manitoba (October 1997 to March 1998). The investigation focused on community A cases only. Thirteen of the 15 cases were interviewed.
Figure 1.
SmaI-generated pattern obtained by pulsed field gel electrophoresis of the methicillin-resistant Staphylococcus aureus (MRSA) isolates from community cases in southwestern Manitoba. Lane 1, lambda ladder standard; lanes 2 to 5, representative MRSA isolates of four different individuals from community A.
All isolates were resistant to penicillin, oxacillin, ceph-alothin, erythromycin and imipenum, and initially were sensitive to vancomycin, cotrimoxazole, clindamycin, ciprofloxacin and gentamicin. One case developed resistance to cotrimoxazole in subsequent isolates after treatment with a variety of antibiotics, including cotrimoxazole.
Characteristics of the cases are shown in Table 1 and Table 2. Seven of the cases were under the age of 18 years, and only two cases were over the age of 50 years. There were 12 cases of MRSA infection and one case of MRSA colonization. Skin infections accounted for most of the infections, presenting as furuncles. Seven people described problems with recurrent furuncles at various sites. Various family physicians in the community were involved in the management of these infections.
TABLE 1.
Characteristics of methicillin-resistant Staphylococcus aureus (MRSA) cases
| Variable | Result |
|---|---|
| Total number of cases | 13 |
| Number of MRSA infections | 12 |
| Number colonized with MRSA | 1 |
| Mean age (years) | 31 (range 6 to 84) |
| Sex (male:female) | 8:5 |
| Site of MRSA infection | |
| Skin (furuncle) | 10 |
| Eye | 1 |
| Skin and eye | 1 |
TABLE 2.
Occurrence of risk factors for methicillin-resistant Staphylococcus aureus (MRSA)
| Risk factor for MRSA | Number of cases |
|---|---|
| Underlying chronic illness | 2 |
| Hospital impatient | 1 |
| Prior hospitalization | 1 |
| Prior hospital outpatient visits | 2 |
| Contact with previous MRSA case | 10 |
| Family member with MRSA | 7 (3 families) |
| Prior antibiotic use | 8 |
| Hospital or long term case employee | 1 |
Only one person acquired the infection in hospital. This individual was a long term inpatient and had contact (frequent visits) with a previous case. Specimens taken from other inpatients in this hospital were negative for MRSA. The majority of patients did not have any contact with the hospital system, including family members. One person who was colonized with MRSA worked in the long term care facility. Two cases had a history of occasional outpatient visits to the local hospital.
Two individuals had a history of chronic disease (cardiovascular and cerebrovascular disease). Eight of the interviewed cases described prior antibiotic use in the preceding year. However, the majority (five cases) had only one course of antibiotics. None of the patients were known to have a history of alcohol or substance abuse. No contact with the northern Manitoba communities where this strain had previously been isolated could be found. Crowded living conditions were not identified, and all patients had running water and sewage systems.
Ten cases identified casual contact with other previous cases. Three families (six cases) had frequent social contact. Two of these families reported recurrent furuncles in multiple family members. Three members (60%) of one family were either colonized or infected with MRSA. All of these persons had problems with furuncles during the preceding year. One otherwise healthy child remained colonized with MRSA more than five months after the initial infection.
DISCUSSION
Current information on MRSA in the community stems largely from the surveillance of hospital admissions. Community acquisition is usually defined as a positive culture obtained within the first 72 h of hospital admission. Studies have revealed that a substantial proportion of MRSA isolates in Canadian and American hospitals are community cases (1,2). Recently, concern has been expressed about how nosocomial pathogens are transmitted in the community (3-5). MRSA-colonized patients who are discharged home are thought to pose little risk of transmission to healthy household contacts. However, factors such as shorter hospital stays, day procedures and community-based care may lead to increased nosocomial transmission in the community.
Previous reports of community-acquired MRSA have suggested that patients with a history of injection drug use, antibiotic therapy, residing in a nursing home or chronic underlying illness, or employed by an institution with endemic MRSA are most at risk (6-9). A community outbreak of MRSA associated with close physical contact recently has been identified in a high school wrestling team (10). Four paediatric deaths from community-acquired MRSA have been identified in Minnesota and North Dakota (11). In contrast to the above reports, the majority of people affected in the cluster of cases described above did not have any of these risk factors. Although 62% of cases reported previous antibiotic use, the majority had received only one course of antibiotics and were being treated empirically for furuncles. It is unknown whether these previous furuncles were caused initially by MRSA, and the prior antibiotic use may have been incorrect empirical treatment.
The isolation of a single strain of MRSA suggests person-to-person transmission in the community setting. The spread of MRSA appeared to occur predominantly through casual social contact. Studies evaluating the risk of transmission of MRSA in healthy family members show minimal transmission and a low frequency of persistent MRSA carriage (12,13). Current guidelines reflect this belief, and suggest that there is minimal risk to healthy family members upon discharge of an individual colonized with MRSA (9). In this incident, both transmission among healthy family members and carriage of the organism over many months were seen.
This strain of MRSA was previously known to be endemic in two communities in northern Manitoba. Factors such as crowded living conditions, high rates of skin sepsis, limited access to water and sewage facilities, and increased use of antibiotics are thought to contribute to endemic transmission within a community (14). These factors did not appear to be a concern in community A.
The apparent transmission of this organism among healthy community members raises several infection control issues. Guidelines for the treatment of MRSA in the community may need to be re-evaluated. At present, decolonization and surveillance cultures for MRSA in the community are generally not recommended. However, in a community outbreak setting, eradication and surveillance may be indicated in families experiencing recurrent problems with MRSA infection.
There appears to be a growing reservoir of MRSA in the community, resulting in increased numbers of hospital isolates. This has significant implications for infection control in hospitals. Individuals colonized with MRSA in the community are difficult to identify and may be the source of hospital outbreaks when admitted as patients. In addition, transmission may occur via visitors, as was suggested in this cluster. Further education about MRSA in outpatient settings and about the potential for transmission to susceptible persons may be necessary. The increased prevalence of MRSA in this community also has implications for the treatment of common staphylococcal infections. Empirical therapy with an antibiotic against methicillin-susceptible S aureus may no longer be effective. Culture and sensitivity testing is necessary for lesions that do not respond as expected to therapy.
CONCLUSIONS
No additional risk factors for MRSA infection were identified in this investigation. Most of the individuals affected were otherwise healthy and did not fit the profile for those at risk of acquiring MRSA infection. The investigation does suggest that transmission of MRSA in the community may occur more readily than previously thought. Hospital-based infection control policies may limit the nosocomial transmission of MRSA in facilities. However, interventions to prevent community-acquired organisms from entering and spreading in facilities and the community may need to be reassessed.
References
- 1.Embil J, Ramotar K, Romance L, et al. MRSA in tertiary care institutions on the Canadian prairies 1990-1992.Infect Control Hosp Epidemiol 1994;15:646-51. [DOI] [PubMed] [Google Scholar]
- 2.Moreno F, Crisp C, Jorgensen JH, Patterson JE. Methicillin-resistant Staphylococcus aureus as a community organism.Clin Infect Dis 1995;21:1308-12. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg J. Methicillin-resistant Staphylococcus aureus (MRSA) in the community: who's watching?Lancet 1995;346:132-3. [DOI] [PubMed] [Google Scholar]
- 4.Hollyoak V, Gunn A. Methicillin-resistant Staphylococcus aureus (MRSA) in the community.Lancet 1995;346:513. [PubMed] [Google Scholar]
- 5.Bowman C, Mitchell J, Tillotson G. Methicillin-resistant Staphylococcus aureus (MRSA) in the community.Lancet 1995;346:513-4. [PubMed] [Google Scholar]
- 6.Saravolatz LD, Pohlod DJ, Arking LM. Community-acquired methicillin-resistant Staphylococcus aureus infections: A new source for nosocomial outbreaks.Ann Intern Med 1982;97:325-9. [DOI] [PubMed] [Google Scholar]
- 7.Taylor G, Kirkland T, Kowalewska-Grochowska K, Wang Y. A multistrain cluster of methicillin-resistant Staphylococcus aureus based in a native community.Can J Infect Dis 1990;1:121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dammann TA, Wiens RM, Taylor GD. Methicillin-resistant Staphylococcus aureus: Identification of a community outbreak by monitoring of hospital isolates.Can J Pub Health 1988;79:312-4. [PubMed] [Google Scholar]
- 9.Mulligan ME, Murray-Leisure KA, Ribner BS, et al. Methicillin-resistant Staphylococcus aureus: A consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management.Am J Med 1993;94:313-28. [DOI] [PubMed] [Google Scholar]
- 10.Lindenmayer, JM, Schoenfeld S, O'Grady R, Carney JK. Methicillin-resistant Staphylococcus aureus in a high school wrestling team and the surrounding community.Arch Intern Med 1998;158:895-9. [DOI] [PubMed] [Google Scholar]
- 11.Hunt C, Dionne M, Delorme M, et al. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus - Minnesota and North Dakota, 1997-1999.MMWR Morb Mortal Wkly Rep 1999;48:707-10. [PubMed] [Google Scholar]
- 12.Cox RA, Mallaghan C, Conquest C, King J. Epidemic methicillin-resistant Staphylococcus aureus: controlling the spread outside hospital.J Hosp Infect 1995;29:107-19. [DOI] [PubMed] [Google Scholar]
- 13.MJCH, Verhoef J. Long-term carriage, and transmission of methicillin-resistant Staphylococcus aureus after discharge from hospital.J Hosp Infect 1992;22:207-15. [DOI] [PubMed] [Google Scholar]
- 14.Maguire GP, Arthur AD, Boustead PJ, Dwyer B, Currie BJ. Emerging epidemic of community-acquired methicillin-resistant Staphylococcus aureus infection in the Northern Territory.Med J Aust 1996;164:721-3. [DOI] [PubMed] [Google Scholar]