Abstract
OBJECTIVE:
To determine, over time, the rate and serotypes of pneumococci with reduced penicillin susceptibility obtained from children with invasive infection.
DESIGN:
Active, hospital-based, multicentre surveillance spanning from 1991 to 1998.
SETTING:
Eleven Canadian tertiary care paediatric facilities located from coast to coast.
POPULATION STUDIED:
1847 children with invasive pneumococcal infection whose isolates (from a normally sterile site) were available for serotyping and standardized testing for penicillin susceptibility at the National Centre for Streptococcus.
MAIN RESULTS:
The prevalence of reduced penicillin susceptibility increased from 2.5% of 197 cases in 1991 to 13.0% of 276 cases in 1998. In the latter year, 8.7% of isolates had intermediate level resistance, and 4.3% had high level resistance. Since they were first detected in 1992, strains with high level resistance have been encountered only sporadically at most centres, but by 1998, all centres but two had encountered examples. Of 40 isolates with high level resistance and 101 isolates with intermediate level resistance, serotypes matched those included in new seven-valent conjugate vaccines for children in 97.5% and 79.2% of cases, respectively.
CONCLUSIONS:
Pneumococci with reduced susceptibility to penicillin are increasing in frequency across Canada among children with invasive infection. The Immunization Monitoring Program, Active data indicate that new conjugate vaccines could help to curb infections due to pneumococci with reduced susceptibility to penicillin but are unlikely to control completely the problem of antibiotic resistance.
Key Words: Children, Penicillin resistance, Pneumococcus, Steptococcus pneumoniae
Infections with Streptococcus pneumoniae (pneumococcus) are a leading cause of infection-related morbidity and mortality in children (1,2). Manifestations include middle ear and upper respiratory infections, pneumonia and invasive infections, including bacteremia and meningitis. The latter most often affect young children (1,2) and those with various chronic conditions (2,3). The recent emergence of resistance to penicillin and other antibiotics (4) adds to the challenge of treating pneumococcal infections.
In Canada, antibiotic resistance has emerged more slowly than it did in the United States, but the gap is narrowing with time. National surveillance in the United States indicated rates of reduced susceptibility to penicillin (minimal inhibitory concentration [MIC] 0.1 μg/mL or greater) averaging 5.0% during 1979 to 1987 (5), but by 1993 to 1994, the rate had increased to 14.1% (6). During 1997 to 1998, the rate had increased further to 29.5%, with 12.1% having high level penicillin resistance (MIC 2.0 μg/mL or greater) (7). Another national survey (8) in 1997, which was limited to invasive isolates, reported that 25.0% had reduced susceptibility to penicillin (referring to both intermediate and high level resistance), and 13.6% were highly resistant. In both recent studies (7,8), the proportion of isolates with reduced susceptibility to penicillin varied significantly by region of the country, from 12.8% to 64.6%, and among hospitals within a region.
Canadian reports from the 1970s indicated a low rate of intermediate level penicillin resistance (MIC 0.1 to 1.0 μg/mL) among collected strains (9) and cases in children (10). No increase was evident in surveys from Ontario (11) and Quebec (12) reported in 1989. By 1994, the rate of reduced penicillin susceptibility had increased in the Toronto area to 7.3%, and high level resistance was evident in 2.2% of isolates (13). Canada-wide surveys subsequently showed further increases, with at least one reporting a statistically significant increase in high level resistance between 1992 and 1995 (14). In serial surveys of respiratory isolates from 50 hospitals across the country, rates increased between 1994 and 1996 from 8.5% to 13.3% for reduced susceptibility, and from 2.1% to 4.4% for high level resistance (15). Allowing for sample size effects, resistance rates were similar across the country. Another survey in 1997 (16) involving lower respiratory tract isolates from seven large Canadian hospitals showed that 30.2% of isolates had reduced penicillin susceptibility, and 8.4% were highly resistant. All of the hospitals encountered isolates with intermediate level resistance (rates ranging from 15.0% to 33.3%) but differed markedly in experiences with highly resistant isolates (rates ranging from 0% to 17%). Another survey (17) in 1997 involving eight Canadian tertiary care hospitals focused on bloodstream isolates, and 30.5% of isolates were reported to have reduced penicillin susceptibility, while American centres in the same survey reported a rate of 41%.
Limited information is available about invasive infections in children. A study of such infections carried out in 1995 in the Toronto area (18) reported reduced penicillin susceptibility in 11% of isolates. The Canadian Paediatric Society/Laboratory Centre for Disease Control Immunization Monitoring Program, Active (IMPACT) network of 10 children's hospitals across Canada (19) reported that 3.4% of invasive isolates encountered in 1994 had reduced penicillin susceptibility, with high level resistance in 1.3%, but almost all of the latter isolates were from hospitals in Montreal. Surveillance has continued at IMPACT centres, and results through 1998 are presented in this report. Of particular interest were the extent of increase in rates of intermediate and high level resistance, geographic distribution of nonsusceptible isolates, and their association with particular clinical syndromes and serotypes.
DATA AND METHODS
The survey was conducted by the 11 paediatric centres of the IMPACT (20,21). These academic centres are located from coast to coast, encompass about 85% of the tertiary care paediatric beds in Canada and manage over 90,000 inpatient admissions annually. Surveillance began at 10 centres in 1993, and extended retrospectively to January 1, 1991 (at nine centres) and prospectively to December 31, 1998. An 11th centre contributed cases from January 1995.
Invasive infection was defined by the isolation of S pneumoniae (pneumococcus) from a normally sterile site by the laboratory of a reporting centre. Eligible cases included outpatients, inpatients and those with hospital-acquired infections. Only one isolate per case was included.
At each centre, the IMPACT nurse monitor searched for cases using multiple overlapping methods, including periodic audits of hospital records. Details of case finding and reporting methods were previously published (20). Monitors completed a standardized report for each case that included demographic information, nature of the presenting infection, sources of positive cultures, course in hospital and outcome. Case reports were assembled at the IMPACT data centre in Vancouver, where clinical and reference laboratory data were collated in a database.
Isolates were stored by hospital laboratories and forwarded regularly to the National Centre for Streptococcus in Edmonton, Alberta, where they were serogrouped and typed (14) according to the Danish nomenclature and screened for reduced susceptibility to penicillin using a 1 μg oxacillin disk (22). Isolates with zone diameters 19 mm or less were tested further by broth microdilution to determine the MIC of penicillin G (23). Isolates were defined as having intermediate level resistance with MICs 0.1 to 1.0 μg/mL, high level resistance with values 2.0 μg/mL or greater and reduced susceptibility with values 0.1 μg/mL or greater (24). Only isolates that were tested at the reference centre are included in this report, representing 90.5% of cases encountered. Isolate referral rates were similar among the IMPACT centres and were consistent from year to year (data not shown).
Statistical analyses were performed using SAS/STAT (SAS Institute, USA). χ2 test, two-tailed, was used to compare proportional data. The study was approved by the research ethics board of each participating centre.
RESULTS
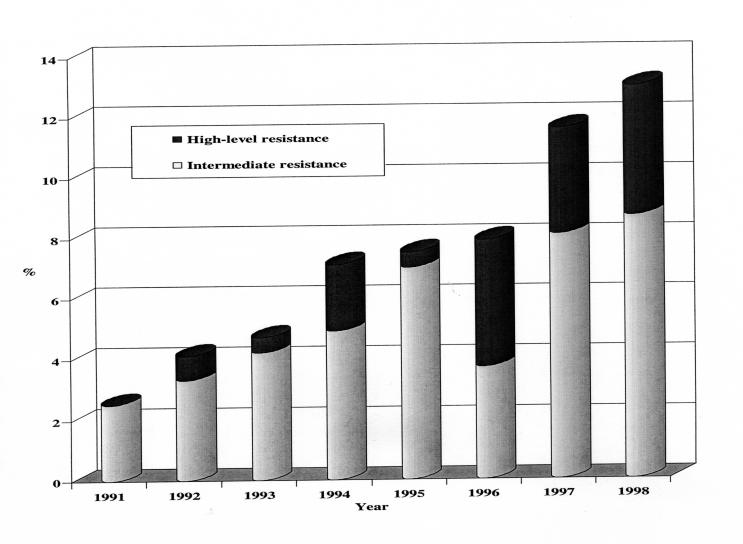
During the eight years surveyed, 1847 isolates were tested at the national reference centre, and 141 were classified as having reduced susceptibility to penicillin. As detailed in Figure 1, isolates with intermediate level resistance were present at low frequency (2.5%) in 1991 and steadily increased to 8.7% of isolates in 1998. Isolates with high level resistance were detected first in 1992 and accounted for 4.3% of isolates in 1998. By 1998, the rate of reduced susceptibility to penicillin had increased to 13.0%.
Figure 1.
Rates of reduced penicillin susceptibility among pneumonococcal isolates from Canadian Paediatric Society/Laboratory Centre for Disease Control Immunization Monitoring Program, Active (IMPACT) centres, 1991 to 1998
Table 1 summarizes the experience of individual centres. During 1997 to 1998, isolates with reduced susceptibility were consistently detected at every centre with an annual caseload of 15 or more. Isolates with high level resistance were present only sporadically during the survey period, but by 1998, all centres but two had encountered at least one example. The exceptions were Winnipeg, with a case total of 148 during the survey period, and St John's, with a case total of 15. Once detected at a particular centre, highly resistant strains did not necessarily reappear in following years. Only in Montreal and Toronto were such isolates consistently present during the last three years studied.
TABLE 1.
Distribution of isolates with reduced susceptibility to penicillin (PenR) among Canadian Paediatric Society/Laboratory Centre for Disease Control Immunization Monitoring Program, Active (IMPACT) centres, 1991 to 1998
| Centre | 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Vancouver | |||||||||
| PenR isolates | 1 (0)* | 1 | 2 | 2 | 1 | 3 (3) | 1 | 3 (2) | 14 (5)* |
| Total isolates | 40 | 23 | 29 | 22 | 30 | 42 | 37 | 44 | 267 |
| Calgary | |||||||||
| PenR isolates | 1 | 3 (1) | 2 (1) | 1 | 2 (1) | 2 (1) | 5 (4) | 0 | 16 (8) |
| Total isolates | 27 | 34 | 14 | 10 | 18 | 12 | 24 | 8 | 147 |
| Edmonton | |||||||||
| PenR isolates | - | - | - | - | 3 | 4 (2) | 4 | 2 | 13 (2) |
| Total isolates | - | - | - | - | 27 | 27 | 18 | 21 | 93 |
| Winnipeg | |||||||||
| PenR isolates | 1 | 1 | 0 | 1 | 1 | 0 | 3 | 1 | 8 (0) |
| Total isolates | 16 | 23 | 17 | 32 | 13 | 14 | 23 | 10 | 148 |
| Ottawa | |||||||||
| PenR isolates | 0 | 0 | 0 | 2 (1) | 0 | 2 | 1 | 1 | 6 (1) |
| Total isolates | 2 | 19 | 18 | 15 | 10 | 25 | 11 | 26 | 126 |
| Toronto | |||||||||
| PenR isolates | 0 | 2 | 1 | 1 | 4 | 2 (1) | 4 (1) | 8 (1) | 22 (3) |
| Total isolates | 24 | 22 | 25 | 22 | 38 | 27 | 36 | 34 | 228 |
| Montreal A | |||||||||
| PenR isolates | 1 | 1 (1) | 2 | 1 (1) | 4 | 1 | 5 (1) | 4 (2) | 19 (5) |
| Total isolates | 15 | 33 | 23 | 24 | 21 | 18 | 32 | 38 | 204 |
| Montreal B | |||||||||
| PenR isolates | 1 | 2 | 1 | 5 (2) | 1 | 5 (3) | 5 (2) | 13 (6) | 33 (13) |
| Total isolates | 45 | 55 | 29 | 59 | 21 | 42 | 48 | 62 | 361 |
| Quebec City | |||||||||
| PenR isolates | 0 | 0 | 1 | 2 (1) | 0 | 0 | 0 | 3 (1) | 6 (2) |
| Total isolates | 20 | 15 | 17 | 12 | 9 | 13 | 13 | 16 | 115 |
| Halifax | |||||||||
| PenR isolates | 0 | 0 | 0 | 1 | 0 | 0 | 2 (1) | 1 | 4 (1) |
| Total isolates | 8 | 17 | 16 | 30 | 25 | 18 | 14 | 15 | 143 |
| St John's | |||||||||
| PenR isolates | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total isolates | - | - | 5 | 1 | 1 | 3 | 3 | 2 | 15 |
| All centres | |||||||||
| PenR isolates | 5 (0) | 10 (2) | 9 (1) | 16 (5) | 16 (1) | 19 (10) | 30 (9) | 36 (12) | 141 (40) |
| Total isolates | 197 | 241 | 193 | 227 | 213 | 241 | 259 | 276 | 1847 |
Number of isolates with reduced susceptibility (number with high level resistance)
The proportion of isolates with reduced susceptibility to penicillin differed among the major presenting syndromes (Table 2) and was lowest among cases with bacteremia alone. The rate of high level resistance was higher in those presenting with pneumonia and bacteremia compared with those presenting with meningitis or bacteremia alone, a difference that was statistically significant. The presenting syndrome mix did not vary appreciably from year to year (data not shown).
TABLE 2.
Rates of reduced penicillin susceptibility among pneumococci by major presenting syndromes, Canadian Paediatric Society/Laboratory Centre for Disease Control Immunization Monitoring Program, Active (IMPACT) centres, 1991 to 1998
| Penicillin susceptibility* | Bacteremia alone (%) | Meningitis (%) | Pneumonia with bacteremia (%) |
|---|---|---|---|
| Intermediate | 30 (4.0) | 23 (8.4) | 19 (5.3) |
| Highly resistant | 7 (1.0)† | 3 (1.1)‡ | 17 (4.7)†,‡ |
| Reduced | 37 (5.0)§,¶ | 26 (9.5)§ | 36 (10.0)¶ |
| Total | 739 (100) | 275 (100) | 361 (100) |
See text for definitions;
Paired comparions, P<0.01, χ2 test
Paired comparions, P<0.01, χ2 test
Paired comparions, P<0.01, χ2 test
Paired comparions, P<0.01, χ2 test
Of the 40 isolates with high level penicillin resistance, 39 (97.5%) matched types in the new seven-valent conjugate vaccine (Table 3), the exception being serotype 19A. Among the vaccine-type strains, high level resistance was most prevalent in serotypes 9V (13.9% of isolates) and 23F (9.2%), and was absent in types 4 and 18C (Table 3).
TABLE 3.
Rates of reduced penicillin susceptibility among seven serotypes included in new conjugate pneumococcal vaccines
| Serotype | Intermediate level resistance (%) | High level resistance (%) | Reduced susceptibility (%) | Total |
|---|---|---|---|---|
| 4 | 0 | 0 | 0 | 115 |
| 6B | 25 (9.4) | 4 (1.5) | 29 (10.9) | 267 |
| 9V | 8 (10.1) | 11 (13.9) | 19 (24.0) | 79 |
| 14 | 19 (3.6) | 11 (2.1) | 30 (5.8) | 520 |
| 18C | 0 | 0 | 0 | 179 |
| 19F | 14 (6.4) | 2 (0.9) | 16 (7.3) | 219 |
| 23F | 14 (11.7) | 11 (9.2) | 25 (20.8) | 120 |
Among 101 isolates with intermediate level penicillin resistance from 1992 to 1998, 80 (79.2%) matched types in the seven-valent vaccine. Exceptions belonged to types 6A, 15B, 17F or 19A, with the latter accounting for most (15 of 21) exceptions. Among vaccine-type isolates, intermediate level resistance was most prevalent in serotypes 23F (11.7% of isolates), 9V (10.1%) and 6B (9.4%), and was absent in types 4 or 18C (Table 3).
DISCUSSION
The IMPACT data provide unique insight into the penicillin susceptibility of pneumococci causing invasive infection in children. It is the only surveillance system in Canada to focus exclusively on invasive pneumococcal infections in children. The data were collected actively by trained monitors in an effort to identify all cases, including those managed as outpatients. Participating centres admit over 90,000 children annually and have broad referral bases that span the nation from St John's to Vancouver. While all are tertiary care centres, most also provide all or most of the paediatric hospital services in the communities in which they are located, a fact that reduces referral bias in the observations. All available isolates were included in the survey to reduce selection bias. Including only isolates from normally sterile sites avoids the problem of inconsistent inclusion of pneumococci from sputum or the nasopharynx, which may possess different serotype and antibiotic resistance profiles. Bacteriological data are based on the results of standardized testing at the national reference centre, unlike our earlier report that relied on local test results (19).
While the IMPACT data show a progressive increase in penicillin nonsusceptibility rates akin to other recent Canada-wide surveys (14-17), the rate observed in 1998 (Figure 1) was lower by more than half compared with the other studies. Such a large discrepancy is unexpected, because population-based studies generally report similar (25) or higher (6) rates of penicillin nonsusceptibility in invasive isolates from children compared with isolates from adults. The other Canadian study of bloodstream isolates (17) involved eight tertiary care centres dealing primarily with adult patients and tracked susceptibility of a range of pathogens based on samples of the first 20 blood isolates (all types) encountered per month during a six-month period in 1997. Referral and selection biases, as well as sample size limitations, could have influenced the reported nonsusceptibility rate of 30.1% among 59 blood isolates, compared with 11.6% of 259 IMPACT isolates from the same year.
The rates of intermediate level penicillin resistance increased steadily between 1991 and 1998, but a substantial dip occurred in 1996, for which there was no apparent explanation. No similar dip was reported in other Canadian surveys, suggesting a sampling artifact within the IMPACT study.
The rate of high level resistance to penicillin increased abruptly in 1996, although highly resistant isolates were first obtained in 1992. Initially, such isolates were limited to a few centres (Table 1) and a few serotypes, principally 9V and 14. Nearly half of all highly resistant isolates reported during the survey were from the two centres in Montreal, which reported 30.6% of all cases. By 1998, all centres except Winnipeg and St John's had encountered instances. The Winnipeg centre subsequently encountered its first highly resistant isolate in 1999, based on local test results. Once detected at a centre, isolates with high level resistance typically were encountered sporadically in subsequent years. Only two centres reported such isolates during each of the final three years of surveillance (Table 1). Despite these variabilities, the IMPACT data indicate that it is reasonable to expect a rising rate of penicillin nonsusceptibility in pneumococci from children with invasive infection, and that risk appears to apply across Canada.
Caution is especially warranted with meningitis cases, because reduced susceptibility to penicillin was more prevalent among such cases than in children with bacteremia alone (Table 2), consistent with other large paediatric series (26,27). Limited penetration of antibiotics into cerebrospinal fluid increases the odds that meningitis caused by strains with intermediate level resistance will be inadequately treated by penicillin or ampicillin (28). Empirical coverage with vancomycin and one of cefotaxime or ceftriaxone has been recommended when pneumococcal meningitis is suspected (28-30), pending culture results. The observation that high level resistance was most common among cases with bacteremic pneumonia may also be of practical value to clinicians. An explanation for this association was not apparent, because the serotypes in such cases were generally the same as in the other major syndromes (20). However, patients with pneumonia are somewhat older than those with other syndromes (20), possibly affording more opportunities to acquire resistant strains through exposure to antibiotics or other children.
A seven-valent pneumococcal conjugate vaccine suitable for use in infants and young children was recently licensed in Canada. As shown previously by the IMPACT surveillance system (20), the serotypes included in this vaccine (ie, serotypes 4, 6B, 9V, 14, 18C, 19F and 23F) account for over 80% of the serotypes causing invasive diseases in the target age group. It is noteworthy that nearly all of the isolates with high level resistance that IMPACT and another surveillance system (14) encountered belonged to serotypes included in the new vaccine. The coverage was less complete among the isolates with intermediate level resistance; only 80% belonged to vaccine serotypes. Because colonization with vaccine serotypes appears to be reduced among vaccinated children (31,32), universal immunization programs may curb the spread of resistant organisms. Other new vaccines with nine (adding types 1 and 5) or 11 serotypes (adding also types 3 and 7F) would not improve coverage of the nonsusceptible isolates observed. However, the possibility exists of some cross-protection between 6B and 6A, and 19F and 19A (33). If confirmed among vaccinated children, inhibition of colonization with 19A strains would add significantly to the containment of nonsusceptible pneumococci. The incomplete match with isolates of intermediate penicillin resistance means that new vaccines can provide only a partial remedy to the problem of antibiotic resistance.
Acknowledgments
We gratefully acknowledge the expert assistance provided by the IMPACT nurse monitors and the staff of the IMPACT data centre, Canadian Paediatric Society Secretariat and the National Centre for Streptococcus. James Kellner MD provided helpful editorial assistance. IMPACT was funded by the Laboratory Centre for Disease Control, Ottawa and Alberta Health, Lederle Pediatrics and Vaccines, Rochester, New York; Pasteur Mérieux Connaught, Toronto, Ontario; and Merck Frosst Canada, Kirkland, Quebec.
IMPACT INVESTIGATORS AND PARTICIPATING CENTRES
Scott Halperin MD, IWK Health Centre, Halifax, Nova Scotia; Robert Morris MD, The Dr Charles A Janeway Child Health Centre, St John's, Newfoundland; Pierre Déry MD, Centre Hospitalier Universitaire de Québec (Pavillon Centre hospitalier de l'Université Laval), Quebec, Quebec; Marc Lebel MD, Hôpital Ste-Justine pour les enfants, Montreal, Quebec; Elaine Mills MD, Montreal Children's Hospital, Montreal, Quebec; Noni MacDonald MD, Children's Hospital of Eastern Ontario, Ottawa, Ontario; Ron Gold MD, Elaine Wang MD, The Hospital for Sick Children, Toronto, Ontario; Barbara Law MD, Manitoba Children's Hospital, Winnipeg, Manitoba; Taj Jadavji MD, James Kellner MD, Alberta Children's Hospital, Calgary, Alberta; Wendy Vaudry MD, Stollery Children's Health Centre, Edmonton, Alberta; David Scheifele MD, British Columbia's Children's Hospital, Vancouver, British Columbia; Victor Marchessault MD, Gilles Delage MD, Canadian Paediatric Society Liaisons, Ottawa, Ontario; Louise Pelletier MD, Phillipe Duclos DVM PhD, Robert Pless MD, Daniel Kertesz MD, Laboratory Centre for Disease Control Liaisons, Ottawa, Ontario; John Waters MD, Alberta Health Liaison, Edmonton, Alberta; James Talbot MD PhD, Marguerite Lovgren ART, National Centre for Streptococcus, Edmonton, Alberta
References
- 1.Jacobs NM, Lerdkachornsuk S, Metzger WI. Pneumococcal bacteremia in infants and children: a ten-year experience at the Cook County hospital with special reference to the pneumococcal serotypes isolated.Pediatrics 1979;64:296-300. [PubMed] [Google Scholar]
- 2.Kaplan SL, Mason EO, Barson WJ, et al. Three-year multicenter surveillance of systemic pneumococcal infections in children.Pediatrics 1998;102:538-45. [DOI] [PubMed] [Google Scholar]
- 3.Wong WY, Overturf GD, Powers DR. Infection caused by Streptococcus pneumoniae in children with sickle cell disease: epidemiology, immunologic mechanisms, prophylaxis and vaccination.Clin Infect Dis 1992;14:1124-36. [DOI] [PubMed] [Google Scholar]
- 4.Campbell GD, Silberman R. Drug-resistant Streptococcus pneumoniae.Clin Infect Dis 1998;26:1188-95. [DOI] [PubMed] [Google Scholar]
- 5.Spika JS, Facklam RR, Plikaytis BD, Oxtoby MJ and the Pneumococcal Surveillance Working Group. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979-1987.J Infect Dis 1991;163:1273-8. [DOI] [PubMed] [Google Scholar]
- 6.Butler JC, Hofmann J, Cetron MS, Elliott JA, Facklam RR, Brieman RF, and the Pneumococcal Sentinel Surveillance Working Group. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System.J Infect Dis 1996;174:986-93. [DOI] [PubMed] [Google Scholar]
- 7.Doern GV, Brueggemann AB, Huynh H, Wingert E, Rhomberg P. Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997-98.Emerg Infect Dis 1999;5:757-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelling L, Rothwock G, Reingold A, et al. Geographic variation in penicillin resistance in Streptococcus pneumoniae - selected sites, United States, 1997.MMWR Morb Mortal Wkly Rep 1999;48:656-61. [PubMed] [Google Scholar]
- 9.Dixon JMS, Lipinski AE, Graham MEP. Detection and prevalence of pneumococci with increased resistance to penicillin.Can Med Assoc J 1977;117:1159-61. [PMC free article] [PubMed] [Google Scholar]
- 10.Ahronheim GA, Reich B, Marks MI. Penicillin-insensitive pneumococci - case report and review.Am J Dis Child 1979;133:187-91. [DOI] [PubMed] [Google Scholar]
- 11.Mazzulli T, Simor AE, Jaeger R, Fuller S, Low DE. Survey of antimicrobial susceptibility of community isolates of Streptococcus pneumoniae in Ontario.Can Dis Wkly Rep 1989;15:131-3. [PubMed] [Google Scholar]
- 12.Jetté LP, Lamothe F and the Pneumococcus Study Group. Surveillance of invasive Streptococcus pneumoniae infection in Quebec, Canada from 1984-1986: serotype, distribution, antimicrobial susceptibility and clinical characteristics.J Clin Microbiol 1989;27:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simor AE, Rachlis A, Louie L, Goodfellow J, Louie M. Emergence of penicillin-resistant Streptococcus pneumoniae in southern Ontario, 1993-1994.Can J Infect Dis 1995;6:157-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovgren M, Spika JS, Talbot JA. Invasive Streptococcus pneumoniae infections: serotype distribution and antimicrobial resistance in Canada, 1992-1995.Can Med Assoc J 1998;158:327-31. [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson RJ, Canadian Bacterial Surveillance Network, Low DE. A cross-Canada surveillance of antimicrobial resistance in respiratory tract pathogens.Can J Infect Dis 1999;10:128-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doern GV, Pfaller MA, Kugler K, Freeman J, Jones RN. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the SENTRY antimicrobial surveillance program.Clin Infect Dis 1998;27:764-70. [DOI] [PubMed] [Google Scholar]
- 17.Pfaller MA, Jones RN, Doern GV, et al. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997).Antimicrob Agents Chemother 1998;42:1762-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellner J, McGeer A, Cetron MS, et al. The use of Streptococcus pneumoniae nasopharyngeal isolates from healthy children to predict features of invasive disease.Pediatr Infect Dis J 1998;17:279-86. [DOI] [PubMed] [Google Scholar]
- 19.Scheifele D, Gold R, Marchessault V, Talbot J, et al. Penicillin resistance among invasive pneumococcal isolates at 10 children's hospitals, 1991-1994.Can Commun Dis Rep 1996;22:157-62. [PubMed] [Google Scholar]
- 20.Scheifele D, Halperin S, Pelletier L, Talbot J and Members of the CPS/LCDC IMPACT Monitoring Program. Invasive pneumococcal infections in Canadian children, 1991-1998: implications for new vaccination strategies.Clin Infect Dis 2000;31:58-64. [DOI] [PubMed] [Google Scholar]
- 21.Morris R, Halperin S, Déry P, et al. IMPACT monitoring network: a better mousetrap.Can J Infect Dis 1993;4:194-5. [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests, 6th edn. Approved Standard. NCCLS Document M2-A6. Wayne: NCCLS, 1997.
- 23.National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 4th edn. Approved Standard. NCCLS Document M7-A4. Wayne: NCCLS, 1997.
- 24.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing. 8th Informational Supplement. NCCLS Document M100-S8. Wayne: NCCLS, 1998
- 25.Hofmann J, Cetron MS, Farley MM, et al. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta.N Engl J Med 1995;333:481-6. [DOI] [PubMed] [Google Scholar]
- 26.Bedos JP, Chevret S, Chastang C, Geslin P, Regnier B and the French Cooperative Pneumococcus Study Group. Epidemiological features of and risk factors for infection by Streptococcus pneumoniae strains with diminished susceptibility to penicillin: Findings of a French survey.Clin Infect Dis 1996;22:63-72. [DOI] [PubMed] [Google Scholar]
- 27.Tan TQ, Mason EO, Kaplan SL. Systemic infections due to Streptococcus pneumoniae relatively resistant to penicillin in a children's hospital: clinical management and outcome.Pediatrics 1992;90:928-33. [PubMed] [Google Scholar]
- 28.Kaplan SL, Mason EO. Management of infections due to antibiotic-resistant Streptococcus pneumoniae.Clin Microbiol Rev 1998;11:628-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley JS, Scheld WM. The challenge of penicillin-resistant Streptococcus pneumoniae meningitis: current antibiotic therapy in the 1990s.Clin Infect Dis 1997;24(Suppl 2):S213-21. [DOI] [PubMed] [Google Scholar]
- 30.Fiore AE, Moroney JF, Farley MM, et al. Clinical outcomes of meningitis caused by Streptococcus pneumoniae in the era of antibiotic resistance.Clin Infect Dis 2000;30:71-7. [DOI] [PubMed] [Google Scholar]
- 31.Dagan R, Melamed R, Muallem M, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine.J Infect Dis 1996;174:1271-8. [DOI] [PubMed] [Google Scholar]
- 32.Mbelle N, Heubner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine.J Infect Dis 1999;180:1171-6. [DOI] [PubMed] [Google Scholar]
- 33.Yu X, Gray B, Chang S, Ward JI, Edwards KM, Nahm MH. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants.J Infect Dis 1999;180:1569-76. [DOI] [PubMed] [Google Scholar]