Abstract
Organisms of the genus Gemella can, on occasion, cause serious systemic illness. The present paper reports a successfully treated case of endocarditis in a 12-year-old girl with congenital heart disease caused by species of Gemella. The child presented with cough, fatigue and decreased appetite without fever. Echocardiogram demonstrated marked mitral insufficiency with flail posterior mitral valve leaflet, mitral valve vegetations, and an enlarged left atrium and ventricle. While being treated with vancomycin, the child initially had persistent bacteremia, which resolved after the addition of gentamycin; the course of therapy was completed with penicillin G and gentamycin once antimicrobial susceptibilities were available. Attempts to identify the species of Gemella were unsuccessful in the local laboratory, and at reference laboratories in Canada and the United States. The isolate is undergoing further evaluation to determine its taxonomic status.
Key Words: Endocarditis, Gemella species, Paediatrics
The genus Gemella has five known species: Gemella haemolysans, Gemella morbillorum, Gemella bergeri, Gemella sanguinis and Gemella palaticanis (1-4). All but the last are opportunistic human pathogens that may cause severe infections; G palaticanis has been identified only in dogs. These organisms have been implicated in serious systemic disease, including meningitis (5) and septic shock (6). In addition, several cases of endocarditis have been reported in the past 20 years, primarily in the adult population (4,7-9). The present article describes a case of endocarditis in a child caused by a species of Gemella.
CASE PRESENTATION
A 12-year-old girl with congenital heart disease (mitral stenosis, ventricular septal defect and patent ductus arteriosus) repaired at six months of age presented to her community paediatrician with a cough, decreased appetite and two-week history of fatigue. There was no history of fever, rash or symptoms of heart failure. There were no recent dental procedures. Several other members of her family had experienced a influenza-like illness with vomiting and diarrhea one week before the onset of her symptoms. A blood culture grew Gram-positive cocci in pairs within 24 h. The patient was given intravenous vancomycin 500 mg every 6 h and was referred to the cardiology service at the IWK Grace Health Centre (Halifax, Nova Scotia).
On physical examination, she was not toxic but appeared pale. She was afebrile but tachycardic with a resting heart rate of 100 beats/min. General examination was within normal limits. There was no evidence of infection, rash, lymphadenopathy, hepatosplenomegaly, splinter hemorrhages, Osler nodes, Janeway lesions or Roth's spots. There was no evidence of dental disease; however, there was granulation tissue around some loose primary teeth. Her cardiovascular examination revealed a grade 3/6 pansystolic murmur of mitral insufficiency at the apex and a grade 2/6 late diastolic murmur of mitral stenosis at the apex.
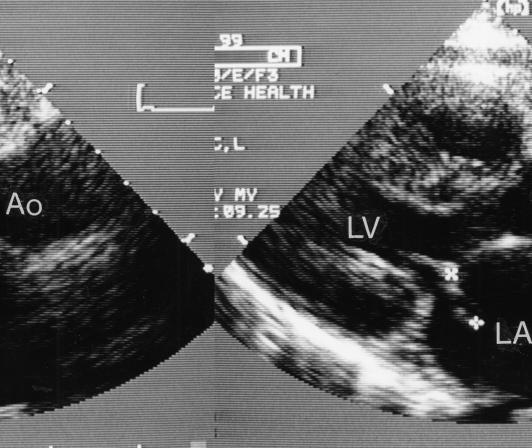
Investigations included a complete blood cell count that revealed anemia (hemoglobin 92 g/L) and a normal white blood cell count (6.6x109 cells/L with 62% neutrophils). She had an elevated erythrocyte sedimentation rate of 53 mm/L. Chest x-ray showed mild cardiomegaly with normal lungs. An echocardiogram revealed marked mitral insufficiency with flail posterior mitral leaflet, thickened mitral leaflets with vegetations, and an enlarged left atrium and ventricle (Figure 1). A diagnosis of infective endocarditis and ruptured chordae tendinae of the posterior mitral leaflet was made.
Figure 1.
Echocardiogram; parasternal long axis view of the heart showing flail mitral leaflet (between crosses) extending into left atrium (LA) and thickened. Ao Aorta; LV Left ventricle
Gram stain of the positive blood cultures (Bactec 9240; Becton Dickinson Diagnostic Systems, Canada) revealed Gram-positive cocci in pairs. On subculture, the organism grew best at 35°C on chocolate agar incubated in an anaerobic jar using the BBL GasPak Anaerobic System (Becton Dickinson Diagnostic Systems, Canada); all cultures required 48 h of incubation before individual colonies could be seen. A slow growing Streptococcus species-like organism was recovered that was identified as Gemella species by the laboratory at the IWK Grace Health Centre. Identification was made using the MicroScan Walkaway 40 (Dade Behring, USA) with a Rapid Positive Combo 1 panel and a variety of conventional tests (Table 1). Susceptibility testing was performed on Mueller-Hinton agar with 5% defribrinated sheep blood and was incubated for 48 h in 5% to 10% carbon dioxide using the Kirby-Bauer method for streptococci. Susceptibility results took several days because of the slow growth of the organism.
TABLE 1.
Characteristics useful for Gremella speciation compared with the reactions of the case isolate
| Gremella haemolysans* | Gemella morbillorum† | Gamella bergeri‡ | Gemella sanguinis§ | Gemella palaticanis¶ | Case isolate | |
|---|---|---|---|---|---|---|
| Beta-hemolysis | + (rabbit blood) | - (rabbit blood) | V (horse blood) | V (horse blood) | - (sheep blood) | - (rabbit blood) |
| Voges-Proskauer | V | - | - | V | - | - |
| Nitrite reduction | + | - | NR | NR | NR | - |
| Lactose | - | - | - | V | + | - |
| Mannitol | - | + | V | + | - | - |
| Sorbitol | - | + | - | + | - | - |
| Sucrose | + | + | - | V | + (weak) | + |
| Trehalose | + | + | - | - | + | + |
| Alkaline phosphatase** | + | - | - | + | - | - |
| Alanine phenylalanine proline arylamidase** | - | V | - | V | + | + |
| Glycyl-tryptophane arylamidase** | V | V | - | - | + | + |
Reactions based on references 3 and 11, and Berger reference;
Reactions based on references 3 and 11, and Berger reference;
Reactions based on references 1 and 3;
Reactions based on references 2 and 3;
Reactions based on reference 3;
Determined by API rapid ID 32 Strep (bioMérieux, USA).
NR Not reported; V Variable reaction. + Positive for substance; - Negative for substance
The organism was referred to the National Centre for Streptococcus, Edmonton, Alberta for further investigation. Laboratory testing by classical methods indicated that the organism belonged to the Gemella genus (10), but the profile was not typical of any known Gemella species (1-4). (Table 1) compares the reactions of this strain with those of the five currently recognized Gemella species. The isolate was forwarded to the Centers for Disease Control and Prevention (Atlanta, USA), where the genus was confirmed as Gemella without further speciation. Biochemical results obtained at the Centers for Disease Control and Prevention were consistent with those reported by the National Centre for Streptococcus. Molecular investigation of this isolate was not performed by either reference laboratory.
A total of three blood cultures were positive during the first week of antibiotic treatment. Gentamicin 70 mg given intravenously every 8 h was added on the third day for synergism after consultation with the infectious diseases service. Twelve days after the initial diagnosis, when the organism was identified as a Gemella species sensitive to penicillin, the vancomycin was discontinued, and penicillin 2,200,000 IU was given intravenously every 6 h. Blood cultures were negative after one week of antibiotic treatment.
The patient's hospital course was uneventful. She remained afebrile and her fatigue resolved. She was discharged home after two weeks in hospital on six weeks of penicillin (2,200,000 IU intravenously every 6 h) and gentamicin (70 mg intravenously every 8 h). At an eight-week follow-up visit with the IWK Grace Health Centre (Halifax, Nova Scotia), she was doing well with normal strength and activity levels. Cardiology follow-up is ongoing because of her underlying cardiac condition.
DISCUSSION
To our knowledge, this is only the third report of endocarditis in a child caused by Gemella species. In 1994, a six-year-old boy with congenital heart disease was diagnosed with endocarditis caused by G haemolysans, and was successfully treated with amoxicillin and gentamicin (7). In 1999, a nine-year-old girl with dental disease and recent dental procedures was diagnosed with endocarditis caused by G morbillorum (8). There are three reports of other serious infections caused by Gemella species in children, including two fatalities. A 15-year-old boy developed meningitis caused by G morbillorum and died within hours of admission to hospital (5). One other fatality resulted from septic shock in a two-year-old girl with Down's syndrome and complex cardiac disease (6). The child had undergone a Fontan procedure and died postoperatively after developing septic shock caused by G morbillorum. A second case of septic shock caused by G morbillorum occurred in an 11-year-old girl with nasopharyngeal Burkitt's lymphoma (6). These cases serve as a reminder that Gemella species can cause very serious infection and can be fatal, dispelling the common belief that it is a harmless commensal of the human pharynx.
Dental disease was often associated with previously reported cases of endocarditis caused by Gemella species (4,8,10). In other cases, there was an invasive procedure, concurrent infection or history of trauma that was the presumed portal of entry (6,10). In our patient, there was no identifiable source of infection. It is possible that the granulation tissue around her loose primary teeth may have been a site for bacterial invasion. She had pre-existing cardiac disease, a known risk factor for endocarditis that has been reported in other cases of Gemella species endocarditis (4,7-10).
Most cases of Gemella species endocarditis have been successfully treated with a combination of penicillin or vancomycin and an aminoglycoside two to four weeks (4,6-10). Our patient had an excellent response to vancomycin once gentamicin was added for synergism and responded well to penicillin when the organism was identified as penicillin-susceptible. She was treated for six weeks with both penicillin and gentamicin because of the difficulty in identifying the organism, the unusual nature of the organism and the persistently positive blood cultures for the first week of antibiotic therapy. Although there have been reports of resistance (6), it is still recommended that empirical therapy be started with penicillin or vancomycin and an aminoglycoside.
Gemella species are faculatively anaerobic, catalase-negative, Gram-positive cocci. These organisms often grow poorly on blood agar, and after 24 to 48 h of incubation, colonies are tiny and nonhemolytic or weakly alpha-hemolytic (11). Growth is enhanced by 5% carbon dioxide. Gemella species is easily overdecolourized in the Gram stain; cells are arranged in pairs, often with adjacent sides flattened, and short chains are observed (12). All Gemella species have a typical biochemical profile that includes positive leucine aminopeptidase and pyrrolidonylarylamidase reactions, and negative reactions for catalase, esculin, arginine, urease, hippurate and growth in 6.5% sodium chloride (11). The isolate from our patient was morphologically characteristic of Gemella species, and showed biochemical reactions that were consistent with this genus, but none of the three laboratories that examined this isolate were able to classify it further. This may indicate that this is a new species, not previously identified.
The colonial morphology of Gemella species resembles that of Streptococcus species. A positive pyrrolidonylarylamidase will rule out Streptococcus species, but the reaction for this test is typically weak and may be negative unless a heavy inoculum is used (11). Speciation presents a challenge for most routine clinical laboratories because only G haemolysans and G morbillorum are included in the databases of commercially available identification systems. Even when additional testing is performed, interpretation may be difficult, because comparisons of the published biochemical profiles are limited by the testing methodology that was used by the investigator. The three most recently described Gemella species (1-3) were characterized using API Rapid ID 32 Strep (bioMérieux, USA) and API ZYM (bioMérieux, USA) systems rather than by traditional methodologies that were used in the descriptions of the original two species, G haemolysans and G morbillorum (11-13). (Table 1) summarizes the published phenotypic characteristics of Gemella species identified (1-3,12,13), but testing methodologies must be considered when interpreting these data, because there may be poor correlation between the use of dehydrated substrates and conventional media (11). Beta-hemolysis may also be useful for Gemella speciation, but the animal source of the blood will affect the demonstration of this characteristic (12). G haemolysans, the original Gemella species, was described as beta-hemolytic on Mueller-Hinton agar supplemented with rabbit blood, but showed "greening" on sheep blood agar plates (12). Beta-hemolysis has also been described as a variable characteristic for both G bergeri and G sanguinis, but horse blood, rather than rabbit blood, was used (1,2).
Recognition of new bacterial species is facilitated by referral of unusual isolates to reference laboratories. Even with extensive testing, the reference laboratory may be unable to fully identify the organism, as we observed for this isolate. Typically molecular investigation of unidentified organisms is only initiated when a common pattern of biochemical reactions is observed for a number of clinical isolates. Both 16S ribosomal RNA gene sequencing and polyacrylamide gel electrophoresis analysis of whole cell proteins contribute to the verification of a unique new strain. The isolate recovered from our patient is currently being examined further to determine its taxonomic status.
Acknowledgments
The authors thank Drs Minoli Amit, Ross Anderson, Joanne Langley and Joanne Robichaud for their help in treating this patient.
References
- 1.Collins MD, Hutson RA, Falsen E, Sjöden B, Facklam RR. Gemella bergeriae sp nov, isolated from human clinical specimens.J Clin Microbiol 1998;36:1290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins MD, Hutson RA, Falsen E, Sjöden B, Facklam RR. Description of Gemella sanguinis sp nov, isolated from human clinical specimens.J Clin Microbiol 1998;36:3090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins MD, Jovita MR, Foster G, Sjöden B, Falsen E. Characterization of a Gemella-like organism from the oral cavity of a dog: description of Gemella palaticanis sp nov.Int J Syst Bacteriol 1999;49:1523-6. [DOI] [PubMed] [Google Scholar]
- 4.La Scola B, Raoult D. Molecular identification of Gemella species from three patients with endocarditis.J Clin Microbiol 1998;36:866-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debast SB, Koot R, Meis JFGM. Infections caused by Gemella morbillorum. Lancet 1993;342:560 (Lett) [PubMed] [Google Scholar]
- 6.Vasishtha S, Isenberg HD, Sood SK. Gemella morbillorum as a cause of septic shock.Clin Infect Dis 1996;22:1084-6. [DOI] [PubMed] [Google Scholar]
- 7.Breathnach AS, Gould FK, Bain HH, Aucken HM. Gemella haemolysans endocarditis associated with a raised anti-streptolysin-O titre.J Infect 1997;34:87-8. [DOI] [PubMed] [Google Scholar]
- 8.Farmaki E, Roilides E, Darilis E, Tsivitanidou M, Panteliadis C, Sofianou D. Gemella morbillorum endocarditis in a child.Pediatr Infect Dis J 2000;19:751-3. [DOI] [PubMed] [Google Scholar]
- 9.Kaufhold A, Franzen D, Lutticken R. Endocarditis caused by Gemella haemolysans. Infection 1989;17:385-7. [DOI] [PubMed] [Google Scholar]
- 10.Fresard A, Michel VP, Rueda X, Aubert G, Dorche G, Lucht F. Gemella haemolysans endocarditis.Clin Infect Dis 1993;16:586-7. (Lett) [DOI] [PubMed] [Google Scholar]
- 11.Facklam R, Elliot JA. Identification, classification and clinical relevance of catalase-negative gram-positive cocci, excluding the streptococci and enterococci.Clin Microbiol Rev 1995;8:479-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger U, Pervanidis A. Differentiation of Gemella haemolysans (Thotta and Boe 1938) Berger 1960, from Streptococcus morbillorum (Prevot 1933) Holdeman and Moore 1974.Zentralbl Bakteriol Mikrobiol Hyg [A] 1986;261:311-21. [DOI] [PubMed] [Google Scholar]
- 13.Kilpper-Balz R, Schleifer KH. Transfer of Streptococcus morbillorum to the Genus Gemella morbillorum comb.Int J Syst Bacteriol 1998;38:442-3. [Google Scholar]