Worldwide, approximately 170 million people are chronically infected with hepatitis C virus (HCV) and another 350 million individuals are chronically infected with hepatitis B virus (HBV) (1,2). Canada is estimated to have 240,000 to 300,000 HCV and 200,000 to 280,000 HBV chronic carriers (3,4). Without intervention, over multiple decades, approximately 15% to 30% of chronic HBV- and HCV-infected individuals will develop cirrhosis, end-stage liver disease or liver cancer, or will require liver transplantation (1,2,5). From a public health perspective, the major challenge is how best to avoid acute (incident) infections in at-risk populations, and for those already chronically infected, how to prevent consequent morbidity and mortality.
For HCV, primary prevention is difficult, because a vaccine is not available nor is one likely to be available in the near future. Although the blood product transmission of HCV has been virtually eliminated by the effective serological screening of blood donors, parenteral substance abuse continues to be the major source of ongoing transmission (6-8).
Despite the lack of a HCV vaccine, new and effective but costly HCV treatments (interferon, pegylated interferons with and without ribavirin) can cure (ie, eliminate HCV RNA from the body) in 30% to 90% of treated individuals (1). Recently Jaeckel et al (9) reported that the early treatment of acute HCV with interferon monotherapy prevented chronic infection in 98% of treated cases. The efficacy of currently available treatments for HCV will drive future demands for treatment and the ensuing costs. Recent estimates suggest that the cost of HCV to our health care system is similar to the cost of asthma, or approximately US$5.4 billion/year in the United States (10).
For HBV, a safe and effective vaccine is available for primary prevention, but many decades of population-based vaccination will be required before the entire population is adequately protected because of the large numbers of chronic carriers in the global population (11). HBV vaccine escape mutants have been described; these refer to mutations in the hepatitis B surface antigen that may result in failed protection from the current HBV vaccine (12,13). In some cases, this may affect the ability of current enzyme immunoassays to detect active infection, ie, hepatitis B surface antigen in serum or plasma (12,13). Clearly, surveillance systems need to be in place to ensure that HBV vaccines remain effective and that vaccine escape mutants do not replace current HBV strains.
Treatment for HBV is also evolving rapidly (eg, lamivudine, adefovir dipivoxil, entecavir, fluorothiacytidine and interferons) (14). Both lamivudine and interferon can suppress HBV viral replication and improve the outcome of chronic HBV infection. Typically, 15% to 50% of treated HBV patients have a durable response after interferon or long term lamivudine treatment, but unlike HCV, complete viral elimination from the body may not be possible, because viral DNA is incorporated into the host genome (5). The patient seroconverting from being hepatitis B e antigen-positive to being anti-hepatitis B e antigen-positive typically indicates treatment response. The complete loss of hepatitis B surface antigen is rare, generally occurring less than 10% of the time (2,5). Some patients, however, may have viral mutations (eg, precore mutations) that prevent the expression of hepatitis B e antigen. For these patients, HBV DNA tests are required to monitor treatment response (5). At this point in time, there is a pressing need for more standardized serological and/or nucleic acid tests, or other markers that predict the patients who are most likely to have a durable treatment response and/or that define the optimal treatment duration (15-17). Another challenge to clinicians is that lamivudine monotherapy is associated with the rapid emergence of antiviral resistance in 15% to 60% of treated individuals. Therefore, the utility of combination therapy needs to be investigated (14-16).
For hepatitis A virus (HAV) infection, while it does not result in chronic disease, its public health impact relates to its ease of transmission (fecal-oral), the rare occurence of fulminant hepatitis and the fact that HAV shares some common risk factors for transmission with HBV and HCV. It is also readily vaccine preventable.
The current issue of The Canadian Journal of Infectious Diseases contains multiple articles reporting on the prevalence, incidence and impact of hepatitis A, B and C in Canada (Wu et al, pages 341-344; Zhang et al, pages 345-350; Minuk and Uhanova, pages 351-356; Zou et al, pages 357-363). These articles highlight significant limitations in our surveillance systems and the lack of standardized measurement tools to monitor the true burden of these infectious diseases in our population. Proper resource planning requires detailed information on the natural history of hepatitis, utility of preventive interventions and a better assessment of treatment cost and effectiveness. Garnet et al (18) recently highlighted how a combination of both prevention and treatment is required to minimize ongoing transmission of HIV in the population effectively. Developing best practice models for prevention and treatment requires sensitive surveillance systems. Incident cases need to be detected to define current risk factors for transmission, whereas prevalent cases need to be identified so that treatment or monitoring can be initiated. Given the fact that approximately 0.5% to 1.0% of Canada's population is infected with either HBV or HCV, and the plethora of new effective and expensive drugs in the pharmaceutical pipeline, it behooves us to develop cost effective care management strategies. Although the articles in the current issue provide the best available information on the status of hepatitis in Canada and outline interventions to improve outcomes, the articles illustrate the difficulty in obtaining reliable incidence, prevalence and burden of disease information.
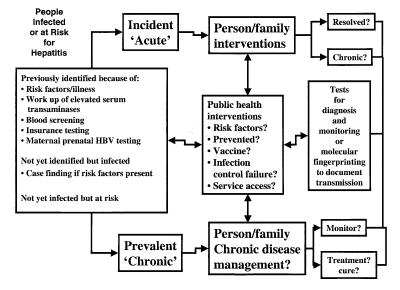
British Columbia has recently developed an integrated viral hepatitis program, details of which are available at <http://www.bccdc.org/hepatitis/index.shtml> (19). This program attempts to integrate hepatitis services across the continuum of prevention, surveillance, care management, education and support, information management and research. Part of the conceptual model is illustrated in the following paragraphs. For example, Figure 1 illustrates the population infected with or at risk for hepatitis, as well as the component activities relating to prevention and care management.
Figure 1.
The population infected with or at risk for hepatitis, and component activities relating to prevention and care management
Typically, hepatitis-infected individuals are identified after serological testing by physicians, because they presented with risk factors or illness, or were identified during blood donor testing, during insurance testing or after maternal screening to identify HBV carriers to prevent perinatal HBV transmission. Because most cases of hepatitis remain asymptomatic, it is estimated that only one- to two-thirds of infected individuals have been diagnosed. The identification of incident cases is important to determine whether the acute infection resolves or the individual becomes chronically infected (HBV and HCV). Risk factor information for incident infections is necessary to evaluate prevention strategy effectiveness, including vaccine efficacy for HBV and HAV. This information is also important for the quality assurance of infection control procedures and the evaluation of access to support services. Where appropriate, molecular fingerprinting may be necessary to support surveillance initiatives (20). Prevalent cases also need identification to ensure appropriate counselling and/or vaccination of contacts. Care management of chronic hepatitis would be similar to chronic disease management models in which long term monitoring or treatment interventions are offered, as appropriate.
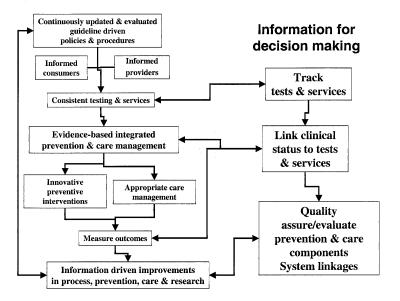
Current hepatitis service delivery in Canada is highly fragmented. Clinical and preventive guidelines are not regularly updated or evaluated, leading to inconsistent health care provider and consumer education. This results in variable policies and procedures that generate inconsistent testing and services. Prevention and care activities are also fragmented, and there are limited outcome measurements, which lead to ad hoc improvements in the process, prevention, care management and research. Our current hepatitis care system hampers evidence-based decision-making. At the root of the problem are the limited tracking of tests and services, and the fact that clinical status is not typically linked to test results or services. The lack of system linkages limits quality assurance, and evaluation of prevention and care management components.
Figure 2 illustrates an integrated model of hepatitis service delivery, which recognizes the need for continuously updated and evaluated guidelines as well as the need for the constant education of consumers and health care providers to support evidence-based prevention and care management. Tracking tests and services, and linking these to the clinical status of patients are required for the system linkages to allow quality assurance and evaluation of prevention and care.
Figure 2.
An integrated model of hepatitis service delivery
Because new and expensive treatments are now available to cure or improve hepatitis outcomes, it seems intuitive that cost effective strategies to optimize prevention and care management need to be developed. Developing a national hepatitis strategy to improve surveillance and prevention, care management, education and support, information management and research would help focus our efforts to reduce the burden of hepatitis illness in Canada. This will require an increased investment in informatics and the sharing of health care information. To achieve this, consumers need to be assured that their privacy and confidentiality will be protected while allowing the benefits of analyzing aggregate data to assess outcomes (21).
Canada is in a unique position to play a global leadership role in health care integration, because the country operates within a single-payer, publicly funded health care framework. Developing the system linkages and informatics supports necessary to track prevention and care management outcomes effectively is a challenge. Health Canada has recently funded the Canadian Viral Hepatitis Network to create national systems linkages, and British Columbia's integrated hepatitis services program will need to adopt a broader national perspective.
It is only by obtaining much better information on prevention and care management outcomes that we will be able to truly apply evidence-based policies to enhance the quality of hepatitis services. With a concerted and coordinated effort, five to 10 years from now, a similar series of articles outlining the hepatitis A, B and C in Canadians should be able to report more concrete information on the burden of these hepatitis viruses, and outline a path to their eventual elimination.
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection.N Engl J Med 2001;345:41-52. [DOI] [PubMed] [Google Scholar]
- 2.Lee WM. Hepatitis B virus infection.N Engl J Med 1997;337:1733-46. [DOI] [PubMed] [Google Scholar]
- 3.Medical Research Council of Canada. Identification of a Research Agenda for the Diagnosis, Care and Prevention of Hepatitis C in Canada. Report to the Minister of Health: The Honourable Allan Rock, June 1999. <http://www.cihr.ca/news/publications/publications/ehepc.pdf>(Version current at November 29, 2001) [Google Scholar]
- 4.M Sherman, CASL Hepatitis Consensus Group. Management of viral hepatitis: Clinical and public health perspectives - A Consensus Statement.Can J Gastroenterol 1997;11:407-16. [DOI] [PubMed] [Google Scholar]
- 5.Ganem D, Schneider RJ. Hepadnoaviridae: The viruses and their replication. In: Knipe DM, Howley PM, eds. Fields Virology, 4th edn. Philadelphia: Lippincott Williams & Wilkins,2001:2923-69. [Google Scholar]
- 6.Patrick DM, Tyndall MW, Cornelisse PG, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection.CMAJ 2001;165:889-95. [PMC free article] [PubMed] [Google Scholar]
- 7.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994.N Engl J Med 1999;341:556-62. [DOI] [PubMed] [Google Scholar]
- 8.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention.MMWR Morb Mortal Wkly Rep 1998;47:1-39. [PubMed] [Google Scholar]
- 9.Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b.N Engl J Med 2001;345:1452-7. [DOI] [PubMed] [Google Scholar]
- 10.Leigh JP, Bowlus CL, Leistikow BN, Schenker M. Costs of hepatitis C.Arch Intern Med 2001;161:2231-7. [DOI] [PubMed] [Google Scholar]
- 11.Margolis HS. Prevention of acute and chronic liver disease through immunization: hepatitis B and beyond.J Infect Dis 1993;168:9-14. [DOI] [PubMed] [Google Scholar]
- 12.Zuckerman AJ, Zuckerman JN. Molecular epidemiology of hepatitis B virus mutants.J Med Virol 1999;58:193-5. [DOI] [PubMed] [Google Scholar]
- 13.Zuckerman AJ. Effect of hepatitis B virus mutants on efficacy of vaccination.Lancet 2000;355:1382-4. [DOI] [PubMed] [Google Scholar]
- 14.Pianko S, McHutchison J. Chronic hepatitis B: new therapies on the horizon?Lancet 1999;354:1662-3. [DOI] [PubMed] [Google Scholar]
- 15.Liaw YF, Leung NW, Chang TT, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B.Gastroenterology 2000;119:172-80. [DOI] [PubMed] [Google Scholar]
- 16.Lai CL, Chien RN, Leung NWY, et al. A one-year trial of lamivudine for chronic hepatitis B.N Engl J Med 1998;339:61-8. [DOI] [PubMed] [Google Scholar]
- 17.Krajden M, Minor J, Cork L, Comanor L. Multi-measurement method comparison of three commercial hepatitis B virus DNA quantification assays.J Viral Hepat 1998;5:415-22. [DOI] [PubMed] [Google Scholar]
- 18.Garnett GP, Bartley LM, Cameron DW, Anderson RM. Both a 'magic bullet' and good aim are required to link public health interests and health care needs in HIV infection.Nat Med 2000;6:261-2. [DOI] [PubMed] [Google Scholar]
- 19.BC Centre for Disease Control. BC Hepatitis Services. <http://www.bccdc.org/hepatitis/index.shtml>(Version current at November 29, 2001) [Google Scholar]
- 20.Massari M, Petrosillo N, Ippolito G, et al. Transmission of hepatitis c virus in a gynecological surgery setting.J Clin Microbiol 2001;39:2860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melton LJ. The threat to medical-records research.N Engl J Med 1997;337:1466-70. [DOI] [PubMed] [Google Scholar]