Abstract
The fluoroquinolone class of antimicrobials has been in clinical use for over 13 years. During that period, some representatives of the class have been extensively prescribed, such as ciprofloxacin and levofloxacin, while others have seen minimal use and have been restricted or withdrawn, namely, trovafloxacin and grepafloxacin. Manipulation of the fluoroquinolone structure by substituting a range of moieties around the core has yielded enhanced antibacterial activity, but in some cases this has come at a price. Specific substitutions are discussed in relation to particular recognized adverse events. In the present paper, newly introduced fluoroquinolones, such as moxifloxacin and gatifloxacin, are examined in terms of anticipated class effects and recent clinical experience. These antimicrobials are associated with reactions such as diarrhea, nausea, headache and other typical antimicrobial phenomena at rates less than 5%. New fluoroquinolone agents should be examined carefully in light of structural findings until adequate clinical data are amassed.
Key Words: Adverse events, Fluoroquinolones, Safety, Tolerability
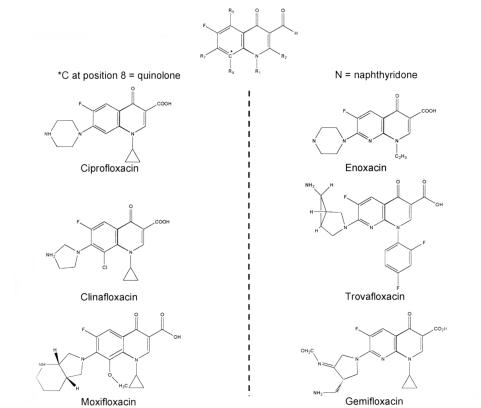
Although nalidixic acid had limited antibacterial activity and poor bioavailability, and was associated with the rapid development of bacterial resistance, its synthesis led to the evolution of the fluoroquinolones. The congeners of nalidixic acid are also synthetic antimicrobials that have a broad spectrum of antimicrobial activity, good absorption from the gastrointestinal (GI) tract, a unique mechanism of action resulting in the inhibition of bacterial DNA gyrase and topoisom-erase IV, favourable pharmacokinetic properties, and a good safety profile (1). Since the initial description of nalidixic acid in 1962 (2), more than 10,000 analogues have been synthesized, resulting in the addition of a fluorine atom at position 6 of the basic molecule and other molecular substitutions at positions 1, 5, 7 and 8 (3) (Figure 1). Currently, five fluoroquinolones are approved for clinical use in Canada, with others still in the investigational phase (Table 1).
Figure 1.
Analogues of the basic fluoroquinolone molecule
TABLE 1.
Fluoroquinolones approved in Canada
| Fluoroquinolone | Canadian trade name | Formulations | Comments |
|---|---|---|---|
| Norfloxacin | Noroxin (Merck Frosst) | • 400 mg tablets | • Limited to urinary tract infections |
| Ciprofloxacin | Cipro (Bayer) | • 100, 250, 500, 750 mg tablets | • Broadest range of indications, still only truly oral antipseudomonal agent |
| • IV infusion 200/400 mg | |||
| • 5%, 10% suspension ear drops | |||
| Ofloxacin | Floxin (Janssen-Ortho) | • 200, 300 400 mg tablets | • Tends to be used for urinary tract infections |
| • Iv solutions (4 mg/mL, 50 mL) | |||
| Levofloxacin | Levaquin (Janssen-Ortho) | • 250, 500, 750 mg tablets | • L-isomer of ofloxacin is more potent part |
| • IV infusion 500 mg/100 mL or 250 mg/50 mL | • Levofloxacin is only quinolone with FDA-approval for the treatment of DRSP | ||
| Moxifloxacin | Avelox (Bayer) | • 400 mg tablets | • Highest activity versus Streptococcus pneumoniae anaerobes and typicals, short course for AECB |
AECB Acute exacerbation of chronic bronchitis; DRSP Drug-resistant Streptococcus pneumoniae; FDA United States Food and Drug Administration; IV Intravenous.
The early fluoroquinolones - norfloxacin, ciprofloxacin, and ofloxacin - demonstrated excellent activity against a broad range of Gram-negative pathogens and achieved high concentrations in the urinary tract. They have emerged as effective therapies for urological infections, including uncomplicated and complicated urinary tract infection, prostatitis and pyelonephritis (4,5). Additionally, ciprofloxacin's antipseudomonal activity led to this agent becoming a mainstay of hospital Gram-negative therapy.
However, these early fluoroquinolones were marginally active in vitro against some Gram-positive pathogens, particularly Streptococcus pneumoniae. Against this background emerged anecdotal reports of clinical failures in severe or difficult pneumococcal infections (6,7), thus providing the impetus for the development of fluoroquinolones with improved activity against S pneumoniae and other Gram-positive organisms. Furthermore, some of these new variations possessed significant antianaerobic activity and many had superior pharmacokinetics.
Clear differences in the safety and tolerability of these agents are well recognized. For example, temafloxacin was withdrawn from use in June 1992 (8), the use of trovafloxacin was restricted to the treatment of only serious infections in June 1999 (9), and grepafloxacin was withdrawn globally in October 1999 (9). The safety profiles of these three drugs represent a significant divergence from other agents in this class. Consequently, fluoroquinolones cannot be considered interchangeable in terms of efficacy or tolerability and safety (11). In fact, concern has grown regarding the potential safety issues, with recently introduced drugs and those under current investigation. Assessment of the relationship between the molecular structure and the pathophysiological mechanisms of toxic effects should facilitate the understanding and prediction of fluoroquinolone-related adverse drug reactions.
The following discussion provides an overview of the safety and tolerability of fluoroquinolones. Some of the recently developed fluoroquinolones, such as moxifloxacin and gatifloxacin, are indicated for the treatment of respiratory tract infections because of the improved Gram-positive activity that is seen with gatifloxacin, as well as the additional anaerobic activity that is seen with moxifloxacin. Their safety profiles, based on clinical trials and actual empirical use, are examined. Possible explanations for the differing safety profiles of the fluoroquinolones are discussed, with a focus on molecular structure.
FLUOROQUINOLONE SAFETY: AN OVERVIEW
The fluoroquinolones as a class are generally well tolerated; most adverse effects are mild in severity, self-limiting and rarely result in treatment discontinuation (11). The most commonly occurring class effects are GI upset (nausea, vomiting, diarrhea, constipation and abdominal pain; less than 7% total). Less common effects may include central nervous system (CNS) events (less than 5%), blood disorders (approximately 5%), renal disturbances (approximately 4.5%), and skin hypersensitivity amd photosensitivity effects (approximately 2%) (12) (Table 2). Rare occurrences of convulsions, psychosis and tendinitis have also been reported (12). However, some of these events may not be directly attributable to fluoroquinolone therapy per se, and other underlying conditions of the patient, including additional drug therapy unrelated to the antimicrobial, may contribute to the reporting of side effects. Furthermore, phototoxicity, which is seen most often with lomefloxacin (13), sparfloxacin (14) and clinafloxacin therapy (15), is a dose-dependent phenomenon that requires exposure to direct or indirect ultraviolet A (UVA) light (16), and is linked most closely to the presence of a halide at the C-8 position.
TABLE 2.
Adverse reactions associated with fluoroquinolones
| Adverse reaction | Range of incidence (%) |
|---|---|
| Gastrointestinal (diarrhea, vomiting) | 0.8 - 6.8 |
| Central nervous system (dizziness, headache) | 0.9 - 11 |
| Skin (rashes) | 0.4 - 2.1 |
| Blood disorders | 0.5 - 5.3 |
| Cardiovascular (palpitations) | 0.5 - 2.0 |
| Musculoskeletal | 0.5 - 2.0 |
| Phototoxicity or photoallergy | 0.5 - 2.1 |
| Serious reactions, eg, hemolytic uremic syndrome, Stevens Johnson syndrome | <0.5 |
Adapted from reference 12
Serious toxic effects have developed with the use of three agents: temafloxacin, grepafloxacin and trovafloxacin. The 'temafloxacin syndrome' was characterized by hemolytic anemia, renal impairment, hepatotoxicity, disseminated intravascular coagulation and hypoglycemia (17). Nearly two-thirds of the patients with temafloxacin syndrome developed acute renal failure. In addition, mild hepatobiliary changes were observed in one-half of the patients and coagulopathy in one-third. The development of these adverse drug reactions resulted in the withdrawal of temafloxacin from the market in June 1992, within six months of the drug receiving United States Food and Drug Administration (FDA) approval (18).
The adverse effects of temafloxacin, which were not evident in developmental clinical trials, were observed at a rate of one of every 3500 patients during postmarketing surveillance. In contrast, adverse effects similar to those seen with temafloxacin were rarely reported for ciprofloxacin. This is significant considering that ciprofloxacin has the largest database of safety information of all the fluoroquinolones (from over 80,000 patients in clinical studies and more than 280 million prescriptions dispensed). Further analysis of these specific 'temafloxacin events' showed findings for norfloxacin and ofloxacin that were similar to those for ciprofloxacin (17). Specifically, these effects were seen in only one of every 17,000 ciprofloxacin-treated patients, one of every 25,000 patients who received norfloxacin and one of every 33,000 ofloxacin-treated patients (19).
Grepafloxacin, which was introduced to the market in August 1997, was withdrawn voluntarily from use in October 1999 due to reports of severe cardiovascular events among patients taking the drug (10). The serious cardiovascular events that were associated with grepafloxacin therapy became evident only after broad clinical use. Seven patients were observed to experience Torsade de Pointes from an estimated 3.7 million patients who received grepafloxacin from the time of its introduction to clinical use to its withdrawal in late 1999 (10).
Trovafloxacin was approved based on findings from equivalency-based clinical efficacy studies encompassing more than 6000 trovafloxacin-treated patients. In these studies, 5% of patients discontinued therapy because of adverse effects; the most frequently reported events involved the CNS and GI tract (20). As with temafloxacin, the toxic effects of trovafloxacin were not evident until after the drug was in widespread clinical use. Serious adverse events associated with the use of trovafloxacin, including hepatic eosinophilia and hypoglycemia (21), were identified during postmarketing surveillance after 2.5 million patients had been exposedto the drug. These events resulted in the United States and Canada restricting drug's use to the hospital (health care facility)-based treatment of serious life-or limb-threatening infections (9)
While the withdrawal of temafloxacin and grepafloxacin from the market and the significantly restricted use of trovafloxacin raise concerns about fluoroquinolone safety, it is necessary to recognize several important issues when assessing its efficacy and safety. Despite rigorous preclinical drug investigation, once a drug is introduced into widespread clinical practice, the likelihood of observing potentially rare but serious side effects is increased significantly (21). For example, in the 18 months from approval to restriction, approximately 2.5 million trovafloxacin prescriptions were written, which represented an increase in patient exposure of more than 400-fold over that of the clinical dossier study (9). However, this differs from the case of temafloxacin, in which serious adverse events were observed much earlier and with significantly fewer patients, approximately 180,000.
A class reaction is an effect that can be attributable solely to the drug in question or related to other factors (22). An understanding of the pathophysiological mechanism(s) of these adverse effects may help to clarify and explain some adverse reactions. Moreover, it is important to recognize the low incidence of side effects and significant adverse events in other agents in the class, which generally demonstrates the relative safety of this class of drugs (11).
EVALUATING SAFETY AND ANTIMICROBIAL ACTIVITY PROFILES BASED ON MOLECULAR STRUCTURE
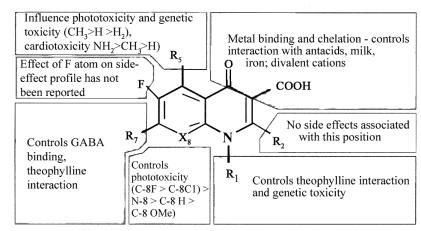
Potential reactions to fluoroquinolones may be predicted on the basis of differing molecular structures in this class. Such effects include GI, CNS and skin or cutaneous events. All fluoroquinolones are analogues of the basic quinolone pharmacore (Figure 1), and distinct antimicrobial and pharmacological activities have been defined for each modification in the molecular structure (3). Similarly, specific structural-side-effect relationships may also help to explain some of the adverse effects that have been observed with each drug (23) (Figure 2).
Figure 2.
Structure of adverse reaction relationships of fluroquinolone. Adapted from reference 3
The first alterations in the quinolone molecule included the addition of a fluorine atom at the 6-position and a piperazinyl moiety at the 7-position. These modifications resulted in the introduction of norfloxacin and ciprofloxacin, which offered a significantly broader spectrum of activity than that of nalidixic acid (23). The structure of ciprofloxacin differs from that of norfloxacin by the addition of a cyclopropyl ring at the 1-position, which is considered necessary for optimal Gram-negative activity (3,23). Ofloxacin and its active L-isomer levofloxacin are examples of 1,8-cyclo (N1 to C8 bridged) compounds. Both have an N-methyl piperazinyl moiety at the C7 position of a tricyclic benzoxazine nucleus. Similar to ciprofloxacin, these alterations in the base quinolone pharmacore resulted in enhanced Gram-negative activity, some activity against Gram-positive pathogens and improved pharmacokinetics. Furthermore, there is a lower potential for drug interaction with theophylline and other P450 metabolized compounds (3).
A C5-amino group substituent improves both Gram-positive and Gram-negative activity in the presence of N1 cyclopropyl. This improved activity is dependent on the C8 substituent and is most effective when this substituent is a halide, as is the case with sparfloxacin (24). Sparfloxacin also bears two cis-oriented methyl groups on the piperazinyl moiety. The cis-dimethyl substitution has been shown to increase in vivo antimicrobial potency, especially against Gram-positive organisms (25).
Selected agents among the most recently developed fluoroquinolones have demonstrated enhanced activity against Gram-positive organisms and anaerobes, eg, moxifloxacin. Their use may be more appropriate against respiratory tract and intra-abdominal pathogens (1). Again, their modified molecular structure may provide some insight regarding potential adverse effects and antimicrobial activity. Compared with ciprofloxacin, moxifloxacin and gatifloxacin have an additional methoxy side chain at C-8; moxifloxicin is differentiated further by a bulky, lipophilic azabicyclo modification at C-7, which increases activity against Gram-positive bacteria and reduces susceptibility to efflux pumps (26). Clinafloxacin bears a pyrrolidine ring at the 7-position, which also enhances activity against Gram-positive bacteria (3). Alkyl substitution of either the piperazine or pyrrolidine ring - for example, moxi-floxacin, gatifloxacin, levofloxacin and sparfloxacin - improves solubility (which, in turn, may decrease the risk for crystalluria), enhances the activity against Gram-positive bacteria, and prolong the drugs' half-life (Figure 2) (3).
CATEGORIZED ADVERSE EFFECTS
Many of the class side effects of fluoroquinolones are associated with modifications of the quinolone pharmacore at the 1-, 7- and 8-positions (Figure 2). The following discussion focuses on these specific class effects with respect to structural modifications at the 1-, 7- and 8-positions.
CNS effects
Although much about the pathophysiology of fluoroquinolone-related CNS effects remains ill defined, one hypothesis suggests that drug interactions with the gamma-aminobutyric acid receptor (GABAa), an inhibitory neurotransmitter, may explain CNS-stimulating effects. The R7 side chain substituent, particularly unsubstituted piperazinyl and pyrrolidinyl moieties (Figure 2), appears to dictate affinity for the GABA receptor. Thus, those agents with an unsubstituted piperazinyl ring (ciprofloxacin, enoxacin and norfloxacin) demonstrate high-affinity binding to GABAa and interfere with GABA binding to its receptor (24).
Furthermore, biphenyl acetic acid, an active metabolite of the nonsteroidal anti-inflammatory drug fenbufen, has been shown to enhance the binding of fluoroquinolones to GABA receptors (24). Coadministration of fenbufen and a fluoroquinolone has been shown to induce convulsive seizures in mice; yet, some investigators note that quinolone-mediated alterations in GABA receptor binding are weak and cannot fully explain these CNS effects (24). Furthermore, seven Japanese patients developed seizures as a result of taking enoxacin and fenbufen together (personal communication, Y Niki).
Studies have also shown that CNS penetration by quinolones does not appear to correlate with the reported incidence of CNS effects (27). A possible reconciliation of these discrepancies is that fluoroquinolones can also induce excitatory effects through direct activation of N-methyl-D-aspartate (NMDA) and adenosine-receptor mechanisms. Thus, it may be that only under specific conditions of sufficient CNS penetration, coupled with threshold antagonism of inhibitory pathways (GABA) and stimulation of excitatory pathways (NMDA, adenosine), that observable CNS symptoms are manifested.
Ofloxacin and its L-isomer, levofloxacin, have been observed to induce a range of CNS-related adverse reactions, including headaches (9% ofloxacin, 6% levo-floxacin), dizziness (5% ofloxacin, 3% levofloxacin), and less common events include confusion, impaired thinking, insomnia and rarely psychosis. These reactions have been induced even in the absence of concomitant drugs such as nonsteroidal anti-inflammatory drugs (28). These reactions tend to occur more frequently with ofloxacin than with levofloxacin.
CNS effects associated with quinolones range from the trivial to severe (dizziness to convulsions) (29), and vary among class members. For instance, with trovafloxacin, dizziness is the most frequently reported adverse event at 19% (30); however, with other new agents, dizziness is less common (moxifloxacin 2.9% [31], gatifloxacin 3% [32], gemifloxacin 2.8% [33].
Photosensitivity
Two types of photosensitivity reactions have been associated with fluoroquinolone therapy: photoallergic reactions, and phototoxic responses. Photoallergic reactions are rare and require previous exposure to a drug in the fluoroquinolone class. In contrast, phototoxic responses are more common and can develop without previous exposure to a fluoroquinolone if the dose of the photolabile drug and exposure to UVA light (around 350 to 360 nm) are sufficiently high, as demonstrated by the use of some fluoroquinolones in a murine model (Table 3) (33,34). Halogenation at the C-8 position is responsible for many of the photosensitivity reactions that occur during fluoroquinolone treatment (3). Some of the fluoroquinolones induce mild photosensitivity reactions, such as erythema of sun-exposed skin, with varying frequency; however, drugs such as lomefloxacin and sparfloxacin with a C-8-fluorine substituent and clinafloxacin with a C8-chlorine substituent, exhibit a greater incidence of phototoxic reactions than do drugs without this substituent (3). Corroboration of the halogen effect was provided by the marked phototoxicity of Bay 3118 compared with the virtual absence of such an effect with the use of moxifloxacin. The molecules are identical save for C-8 chlorine on Bay 3118 and a methoxy on moxifloxacin (35). The investigators concluded that moxifloxacin has almost no phototoxic potential.
TABLE 3.
Fluoroquinolones approved in Canada
| Fluoroquinolone | C-8 moiety | Highest 'no effect dose' (mg/kg) |
|---|---|---|
| Norfloxacin | C - H | >300 |
| Ciprofloxacin | C - H | >300 |
| Ofloxacin | C - OR | >300 |
| Moxifloxacin | CH3OH | >300 |
| Gatifloxacin | CH3OH | >100 |
| Gemifloxacin | N | >100 |
| Trovafloxacin | N | >100 |
| Enoxacin | N | 100 |
| Sparfloxacin | CF | 18 |
| Lomefloxacin | CF | 10 |
| Bay y 3118 | CF | 10 |
| Clinafloxacin | CF | 10 |
Photosensitivity reactions are postulated to occur as a result of fluoroquinolone photodegradation, and the molecule's ability to generate free monovalent oxygen radicals. In turn, these oxidative radicals may attack cellular lipid membranes, initiating inflammatory processes and eventually resulting in DNA damage (28). Evidence for photo-induced oxidative DNA damage is demonstrated by the development of tumours in mice treated with lomefloxacin (36).
Genetic toxicity
Quinolones have been shown to inhibit mammalian cellular topoisomerase II, which correlates with in vitro cytotoxicity in those cells (3). Substitutions at the 1-, 7- and 8-positions have the greatest potential for cytotoxicity, with the effect being additive. However, disruption of the chromosome, or clastogenicity, usually occurs only at very high drug concentrations (300 to 10,000 times the normal dose level), and postmarketing surveillance studies have not found any carcinogenic potential linked to fluoroquinolone use.
Cardiovascular effects
Cardiovascular effects, particularly prolongation of the QT interval corrected for heart rate (QTc interval), have been reported with quinolone therapy (22). Notably, sparfloxacin increased the QTc interval in up to 3% of patients (37). The significance of this finding may relate to the severe cardiac events that result in the withdrawal of grepafloxacin. It is recommended that sparfloxacin not be administered to patients with known QTc interval prolongation or to patients receiving concomitant pharmacotherapy that might increase the interval, induce bradycardia or promote Torsade de pointes (for example, class Ia and III anti-arrhythmics, bepridil, cisapride, erythromycin, terfenadine or tricyclic antidepressants). It appears that this effect may be more predictable with medications that are co-administered with quinolones that inhibit cytochrome P450-mediated metabolism due to increased drug accumulation. To date, no specific structural modification has been associated with cardiovascular effects, including those that might influence cytochrome P450-mediated metabolism. Currently, the only possible specific structural modifications that may be associated with the increased incidence of serious cardiovascular events associated with grepafloxacin and sparfloxacin therapy are a methyl or amino moiety at the C-5 position (grepafloxacin and sparfloxacin, respectively), although Phase III clinical studies did show associated QTc prolongation (22).
In light of the experience with sparfloxacin and grepafloxacin with respect to cardiovascular effects, more recent class members have been investigated to varying degrees, which is further highlighted by a recent report of a levofloxacin-associated ventricular tachycardia (38). In the opinion of the FDA, the investigations of moxifloxacin set a new standard in drug development (39). More than 2600 patients in clinical trials had paired, timed electrocardiogram evaluations that revealed a mean QTc prolongation of 6 ms for moxifloxacin, at a 2.8% frequency of the European Medicines Evaluation Agency - significant QTc changes. The comparable figures for the comparator agents were 1 ms and 2.2%; more specifically for clarithromycin 2 ms and 3.7%. (31). Extensive analyses of the QTc phenomena with levofloxacin (40), gatifloxacin (41) and gemifloxacin (42) have either not been completed or reported. Table 4 shows the reported QTc prolongation data that is currently available.
TABLE 4.
Reported QTc prolongation (ms) with four new fluoroquinolones
| Drug | Sample size | QTc prolongation | Reference | |
|---|---|---|---|---|
| Moxifloxacin | 2650 patients | 6 | 30 | |
| Levofloxacin | 21 patients | 4.3 | 44 | |
| Gatifloxacin | 55 volunteers | 2.9 | 41 | |
| Gemifloxacin | 121 patients | 5.1 | 42 |
Analysis of clinical trial and postmarketing data for moxifloxacin, encompassing over ten million patients, has revealed three cases of Torsade de Pointe, including an 83-year-old woman with a complex cardiological and pharmacological history, whose QTc interval was prolonged from 490 to 520 ms. The patient was successfully cardioverted. The two other cases were similar 'at risk' patients, ie, elderly, female, prior history of cardiac disease and various concomitant medications (Bayer AG, data on file). The incidence of significant ventricular tachyarrhythmias and torsade de pointes appears to be a class phenomenon, but at a rate that is similar to normal background reports (43).
Tendon and tendonitis
Rupture of tendons or tendonitis is a rare event associated with fluoroquinolones (44). Such events tend to affect the Achilles tendon, and are bilateral in 50% of cases. Predisposing factors are reported often, which can include corticosteroid therapy, renal disease, hemodialysis and transplantation (45,46). Usually symptoms resolve within weeks, but in a small proportion of patients, they may persist for months.
This problem was originally observed with pefloxacin, but has subsequently been reported with almost all class members. The reason for such an unusual event may be related to the serum concentrations of magnesium; low levels precipitate joint and tendon problems in animal models (47). Concomitant administration of corticosteroids and quinolones, especially in the elderly, is contraindicated.
Hepatic toxicity
The toxic effects associated with the use of temafloxacin and trovafloxacin therapy have not been definitively ascribed to specific molecular structure modifications. Investigators have hypothesized that increased halogenation, or a toxic metabolite, is the cause of the temafloxacin syndrome (18). The pathophysiology of adverse hepatic events (trovafloxacin) and hypogylcemia (trovafloxacin and temafloxacin) remains unknown. It has been suggested that the addition of a 2,4-difluorophenyl moiety at C-1 may be the culprit for the toxic effects associated with both of these agents, although no definitive evidence has proved this. A proposed mechanism suggests that this component may be metabolically cleaved off and may then act as a hapten, triggering an array of unusual immunological sequelae, including hepatic eosinophilia (personal communication, AP Ball).
Discontinuations
With the advent of modern drug development, the solicitations and subsequent reporting of adverse events has seemingly revealed major problems with new agents in comparison with older members of the same class. Consequently, it may be a more valid assessment of a drug's tolerability if discontinuation of therapy is compared. Unfortunately, few companies report the specific reasons for drug discontinuation but merely report the cumulative rates. Some of the known discontinuation rates are: levofloxacin 3.7%; moxifloxacin 3.3%; gatifloxacin 2.9% (48); and gemifloxacin 3.2% (Table 5) (31-33).
TABLE 5.
Cumulative drug discontinuation rates for some new fluoroquinolones
| Quinolone | Drug discontinuation rate | Various comparators (%) | References | |
|---|---|---|---|---|
| Levofloxacin | 3.7 | Not given | PI | |
| Moxifloxacin | 3.3 | 3.2 | 30 | |
| Gatifloxacin | 2.9 | Not given | 31 | |
| Gemifloxacin | 3.2 | 2.2 | 32 |
PI Product information
Postmarketing Surveillance
Class effects of the fluoroquinolones continue to be observed; these include upper GI effects, CNS disorders, tendonitis and phototoxicity. Recent studies have highlighted QTc prolongation as another probable class effect (22), although the clinical significance of these findings is still uncertain. Within the past year, other effects have been reported which may be class phenomena, eg, anaphylaxis, hepatic reactions (trovafloxacin) and severe cutaneous rashes (gemifloxacin) (49). However, as diligent surveillance of newly marketed compounds continues, millions of patients who have been treated with fluoroquinolones should provide the database to confirm the class nature, or not, of the new events. Current postmarketing surveillance data of over ten million moxifloxacin- (43), personal communication, C Reiter) and over three million gatifloxacin-treated patients (personal communication, S Nicholson) have not shown any notable adverse events, including the typical or predicted class effects (Table 6).
TABLE 6.
Comparison of adverse drug reactions associated with some approved fluoroquinolones (%)
| Reaction | Levofloxacin | Gatifloxacin | Moxifloxacin | ||||
|---|---|---|---|---|---|---|---|
| Reference | PI | 50* | 51 | 31 | 30* | 51 | 52 |
| No patients | NR | 2252 | 1655 | 15,625 | 6170 | 6500 | 18,409 |
| Nausea | 7.1 | 9 | 2.8 | 4 | 8 | 0.3 | 5.7 |
| Diarrhea | 5.6 | 4 | 1.7 | 1.4 | 6 | 0.9 | 2.4 |
| Headache | 6.4 | 4 | 0.5 | 0.9 | 2 | 0.1 | <2 |
| Vomiting | 2.2 | 2 | 0.6 | 0.7 | 2 | 0.2 | <1 |
| Vaginitis | 1.6 | 5 | <1 | <1 | <1 | NI | <1 |
| Dizziness | 2.9 | 3 | 1.3 | NR | 3 | 0.3 | 2.3 |
| Taste perversion | 1.0 | 2 | 0.8 | 0.5 | <1 | NR | <1 |
| Discontinuations | 3.4 | 3.2 | NR | 3.1 | NR | NR | NR |
Clinical trial programs. PI Package Insert trial data; NI No incidence; NR Not reported
Summary
The use of fluoroquinolones has advanced the treatment of genitourinary, various nosocomial and, most recently, community respiratory infections through greater antimicrobial coverage. Generally, these drugs are well tolerated and have proven to be safe and efficacious antimicrobial agents. Fluoroquinolones continue to be contraindicated for pregnant women; however, a growing body of evidence suggests that these drugs are safe in children. The concern now is not with toxicity in the paediatric age group, but with the rapid emergence of resistance to this class of compounds should these agents be widely used in children.
Adverse effects associated with several substituents that have been added to the quinolone pharmacore have been identified. The structure of the quinolones with increased adverse events and/or toxicities differs from that of the established agents with proven safety. While molecular structure of a drug may predict its potential for certain adverse effects, the safety of any new drug is established by careful postmarketing surveillance. Newer fluoroquinolones with improved activity against Gram-positive pathogens (such as gatifloxacin) and anaerobes (such as moxifloxacin) will be used to treat respiratory tract infection, and the older, established drugs with maintained Gram-negative activity (such as ciprofloxacin) may remain the fluoroquinolone of choice for the treatment of urinary tract infection. Further understanding of the structure-related side effects of these drugs may improve the predictability of adverse events of new medications.
References
- 1.Hooper DC. New uses for new and old quinolones and the challenges of resistance. Clin Infect Dis 2000;30:243-54. [DOI] [PubMed] [Google Scholar]
- 2.Lesher GY, Froelich ED, Gruet MD, Bailey JH, Brundage RP. 1,8 naphthyridine derivatives: a new class of chemotherapeutic agents. J Med Pharm Chem 1962;5:1063-8. [DOI] [PubMed] [Google Scholar]
- 3.Domagala JM. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother 1994;33:685-706. [DOI] [PubMed] [Google Scholar]
- 4.Naber KG. Fluoroquinolones in urinary tract infections, proper and improper use. Drugs 1996;52(Suppl 2):27-33. [DOI] [PubMed] [Google Scholar]
- 5.Echols RM, Heyd A, Tosiello R, Busch W, Schacht P. Efficacy and safety of ciprofloxacin for chronic bacterial prostatitis. Infect Med 1995;12:283-9. [Google Scholar]
- 6.Cooper B, Lawlor M. Pneumococcal bacteremia during ciprofloxacin therapy for pneumococcal pneumonia. Am J Med 1989;87(Suppl 5A):225-7. [DOI] [PubMed] [Google Scholar]
- 7.Gordon JJ, Kaufmann CA. Superinfection with Streptococcus pneumoniae during ciprofloxacin therapy. Am J Med 1990;89:383-4. [DOI] [PubMed] [Google Scholar]
- 8.Blum MD, Graham DJ, McCloskey CA. Temafloxacin syndrome: A review of 95 cases. Clin Infect Dis 1994;18:946-50. [DOI] [PubMed] [Google Scholar]
- 9.Pfizer relabels antibiotic Trovan for serious infections. Doctors Guide (e-mail edition), August 30, 1999. <http://www.pslgroup.com/dg/106B6.htm>
- 10.GlaxoWellcome voluntarily withdraws Raxar (grepafloxacin). Press Release, October 26, 1999. Available at <http://www.fda.gov/medwatch/safety/1999/raxar.html> Version current at January 14, 2002
- 11.Ball P, Mandell L, Niki Y, Tillotson G. Comparative tolerability of the newer fluoroquinolone antibacterials. Drug Saf 1999,21:407-21. [DOI] [PubMed] [Google Scholar]
- 12.Childs S. Safety of the fluoroquinolone antibiotics; focus on the molecular structure. Infect Urol 2000;13:3-10. [Google Scholar]
- 13.Wadworth AN, Goa KL. Lomefloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1991;42:1018-60. [DOI] [PubMed] [Google Scholar]
- 14.Tokura Y, Iwamoto Y, Mizutani K, Takigawa M. Sparfloxacin phototoxicity: potential photoaugmentation by ultraviolet A and B sources. Arch Dermatol Res 1996;288:45-50. [DOI] [PubMed] [Google Scholar]
- 15.Tack KJ, Eisemann I, Zervos M. Clinafloxacin for the treatment of serious infections caused by multiply-resistant pathogens. 6th International Symposium on New Quinalones. Denver: November 14-16, 1998. (Abst 178) [Google Scholar]
- 16.Paton J, Reeves DS. Clinical features and management of adverse events of quinolone antibacterials. Drug Saf 1991;6:8-27. [DOI] [PubMed] [Google Scholar]
- 17.Echols RM, Oliver MK. Ciprofloxacin safety relative to temafloxacin and lomefloxacin. 18th International Congress on Chemotherapy. Stockholm, June 18-21, 1993;349-50. (Abst 27) [Google Scholar]
- 18.Finch RG. The withdrawal of temafloxacin; Are there implications for other quinolones? Drug Saf 1993;8:9-11. [DOI] [PubMed] [Google Scholar]
- 19.Lietmann PS. Fluoroquinolone toxicities: An update. Drugs 1995;49(Suppl 2):159-63. [DOI] [PubMed] [Google Scholar]
- 20.Trovafloxacin (Trovan) package insert. New York: Pfizer, 1998. [Google Scholar]
- 21.Chen JL, MacLean JA. Trovafloxacin associated eosinophilic hepatitis. N Engl J Med 2000;342:359-60. [DOI] [PubMed] [Google Scholar]
- 22.Ball P. Quinolone-induced QT interval prolongation: A not-so-unexpected class effect. J Antimicrob Chemother 2000;45:557-9. [DOI] [PubMed] [Google Scholar]
- 23.Tillotson GS. Quinolones: structure-activity relationships and future predictions. J Med Micro 1996;44:320-4. [DOI] [PubMed] [Google Scholar]
- 24.Hori S, Shimada J, Saito A. Comparison of the inhibitory effects of new quinolones on gamma-aminobutyric-acid receptor binding in the presence of anti-inflammatory drugs. Rev Infect Dis 1989;(Suppl 5):s1397-8. [Google Scholar]
- 25.Brighty KE, Gootz td. The chemistry and biological profile of trovafloxacin. J Antimicrob Chemother 1997;39(Suppl B):1-4. [DOI] [PubMed] [Google Scholar]
- 26.Pestova E, Beyer R, Cianciotto NP, Noskin GA, Peterson LR. Contribution of topoisomerase IV and DNA gyrase in Streptococcus pneumoniae for resistance to novel fluoroquinolones. Antimicrob Agent Chemother 1999;43:2000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryskier A,Chantot JF. Classification and structure-activity relationships of fluoroquinolones. Drugs 1995;49(Suppl 2):16-28. [DOI] [PubMed] [Google Scholar]
- 28.Ball P, Tillotson GS. Tolerability of fluoroquinolone antibiotics: past, present and future. Drug Saf 1995;13:343-58. [DOI] [PubMed] [Google Scholar]
- 29.Jangknecht R, Hesker YA. Development in quinolones. Bacteriology, pharmacokinetics and initial clinical experience of several investigational quinolone derivatives. Pharm Weekbl Sci 1989;11:33-43. [DOI] [PubMed] [Google Scholar]
- 30.Baz MN, Janetti W, Villaneuva, et al. The efficacy and tolerability of moxifloxacin compared to trovafloxacin in the treatment of acute sinusitis. Todays Therapeutic Trends. 1999;17:303-19. [Google Scholar]
- 31.Kubin R, Reiter C. Safety update of moxifloxacin: A current review of clinical trials and post-marketing observational studies. 40th Interscience Congress on Antimicrobial Agents and Chemotherapy. Toronto, September 17 to 20, 2000. (Abst 820) [Google Scholar]
- 32.Von Seggern K, Russo R, Wikler MA. A novel approach to postmarketing surveillance: the Tequin experience study. 40th Interscience Congress on Antimicrobial Agents and Chemotherapy. Toronto, September 17 to 20, 2000. (Abst 2216) [Google Scholar]
- 33.Henkel TJ, McKay D, Young C. Safety of gemifloxacin in adult patients with respiratory and urinary infections. 3rd European Congress of Chemotherapy. Madrid, May 7 to 10, 2000. (Abst M130) [Google Scholar]
- 34.Gould JW, Mercurio MG, Imlets CA. Cutaneous photosensitivity diseases induced by exogenous agents. J Am Acad Dermatol 1995;33:551-71. [DOI] [PubMed] [Google Scholar]
- 35.Mann I, Murphy J, Ferguson J. Fluoroquinolone photoxicity: A comparison of moxifloxacin and lomefloxacin in normal volunteers. J Antimicrob Chemother 1999;43(Suppl B):77-82. [DOI] [PubMed] [Google Scholar]
- 36.Makinen M, Forbes PD, Stenback F. Quinolone antibacterials: A new class of photochemical carcinogens. J Photochem Photobiol B 1997;37:182-7. [DOI] [PubMed] [Google Scholar]
- 37.Dupont H, Tinsit JF, Souweine B, Gachot B, Wolff M, Regnier B. Torsade de Pointes related to sparfloxacin. Eur J Clin Micro Infect Dis 1996;15:350-1. [DOI] [PubMed] [Google Scholar]
- 38.Samaha FF. QTc interval prolongation and polymorphic ventricular tachycardia in association with levofloxacin. Am J Med 1999,107:528-9. [DOI] [PubMed] [Google Scholar]
- 39.FDA Report. Bayer Avelox cardiac safety studies recommended by FDA committee. Pink Sheet-Pres. Pharma Biotech 1999;61:4-5. [Google Scholar]
- 40.Iannini PB, Kramer H, Circiumaru I, Byazrova E, Doddamani S. QTc prolongation associated with levofloxacin. 40th Interscience Congress on Antimicrobial Agents and Chemotherapy. Toronto, September 17 to 20, 2000. (Abst 821) [Google Scholar]
- 41.Tequin(gatifloxacin) package insert. Princeton: Bristol-Myers Squibb, 1999.
- 42.Bird N, Lewis A, Montague T, Bygate E, Dixon R. Assessment of the effect of gemifloxacin on QTc interval in healthy volunteers. 40th Interscience Congress on Antimicrobial Agents and Chemotherapy. Toronto, September 17 to 20, 2000. (Abst 821) [Google Scholar]
- 43.Iannini PB, Kubin R, Reiter C, Tillotson G. Reassuring safety profile of moxifloxacin. Clin Infect Dis 2001;32:1112-4. [DOI] [PubMed] [Google Scholar]
- 44.Khan MF, Hayem G. Tendon and fluoroquinolones, unresolved issues. Rev Rheum 1997;64:437-9. [PubMed] [Google Scholar]
- 45.Decoq G, Moriniere P, Dufour I. [Is hemolysis a risk factor for tendinopathies due to fluoroquinolones?] Therapie 1997;52:613-4. [PubMed] [Google Scholar]
- 46.Marti HP, Stoller R, Frey FJ. Fluoroquinolones as a cause of tendon disorders in patients with renal failure/renal transplant. Br J Rheumatol 1998;37:343-4. [DOI] [PubMed] [Google Scholar]
- 47.Shakibaei M, Pfister K, Schwabe R, Vormann J, Stahlmann R. Ultrastructure of Achilles tendon of rats treated with ofloxacin and fed a normal or magnesium deficient diet. Antimicrob Agent Chemother 2000;44:261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breen J, Skuba K, Grasela D. Safety and tolerability of gatifloxacin, an advanced-generation, 8-methoxy fluoroquinolone. J Respir Dis 1999;20(Suppl S):70-6. [Google Scholar]
- 49.Smith Kline Beecham receives non-approval letter from FDA on Factive (gemifloxacin). <http://www.sb.com> December 7, 2000.
- 50.Jones RN, Pfaller MA, Wikler M, Nicholson S and the TeqCES participant group. Clinical efficacy and safety of gatifloxacin in S. pneumoniae community-acquired pneumonia: initial report from TeqCES. 40th Interscience Congress on Antimicrobial Agents and Chemotherapy. Toronto, September 17 to 20, 2000. (Abst 81753) [Google Scholar]
- 51.Miravitles M, Ros F, Cedos A, et al. Evaluation of efficacy and safety of moxifloxacin in acute exacerbations of chronic bronchitis: A Spanish community perspective. Int J Clin Prac. (In press) [PubMed] [Google Scholar]
- 52.Whitehouse A, Brar J, Kowalsky S, Perroncel R, Haverstock D, Church D. The Avelox Clinical Experience Study, a post-marketing observational study. 1st International Conference on Resistant Gram-Positive Infections. San Antonio, December 3 to 5, 2000. (Abst I-13) [Google Scholar]