Abstract
BACKGROUND:
On September 30, 2000, staff at the Canadian Food Inspection Agency's Centre of Expertise for Rabies, located at the Animal Diseases Research Institute in Ottawa, Ontario, diagnosed rabies in a child from Quebec. This was the first case of rabies in a human in Canada in 15 years and in 36 years in the province of Quebec. After spending a week in intensive care in a Montreal hospital, the nine-year-old boy succumbed to this nearly always fatal disease. The boy had been exposed to a bat in late August 2000, while vacationing with his family in the Quebec countryside.
METHODS:
Antemortem specimens taken from the patient were sent to the Canadian Food Inspection Agency laboratory for rabies diagnosis. Samples included saliva, eye secretions, corneal impressions, cerebral spinal fluid and skin. Specimens were examined by direct immunofluorescence microscopy, and results were confirmed using molecular biological techniques. Virus strain identification was performed by both genetic methods and phenotypic analysis with monoclonal antibodies.
RESULTS:
Initial results from direct immunofluorescence staining indicated that rabies virus was present in the skin biopsy specimen but not in the corneal impressions. This diagnosis of rabies was confirmed by polymerase chain reaction product analysis from several of the submitted specimens. Virus isolation from postmortem samples was not possible because fresh brain tissue was not available. Rabies virus was isolated from saliva and was determined to be similar to a variant that circulates in populations of silver-haired bats.
INTERPRETATION:
Intravitam diagnosis of rabies in humans is very dependent on the samples submitted for diagnosis and the method used for testing. Upon first examination, only skin specimens were positive for rabies virus antigen; using polymerase chain reaction analysis, only saliva yielded positive results for rabies virus genome. Extensive testing and retesting of specimens submitted for rabies diagnosis in humans must be done to avoid false negative results.
Key Words: Bat rabies, Rabies
Rabies in humans is a disease rarely seen in Canada. The last reported case of human rabies in Canada occurred in 1985 in British Columbia, after a man was bitten by a bat (1), and it has been over 36 years since a human died of rabies in Quebec (2). Because human rabies is seen infrequently in Canada, physicians may not always suspect it when presented with encephalitides of unknown origin.
In Canada, rabies is a reportable disease that falls under federal jurisdiction. All diagnoses of rabies are made by the staff of the Canadian Food Inspection Agency's Centre of Expertise for Rabies at the Animal Diseases Research Institutes in Ottawa, Ontario and Lethbridge, Alberta. Diagnosis of rabies is a relatively straightforward procedure in animals, where only postmortem diagnosis on brain tissues is performed (3). Direct immunofluorescent (IF) microscopic examination of brain impressions is the only method used in Canada for rabies diagnosis using fresh animal tissues. The IF test is very sensitive with 100% specificity (3).
Diagnosis of rabies in humans by examination of biopsy specimens and bodily fluids is performed only at the laboratory in Ottawa. This type of diagnosis is much more complicated than rabies diagnosis in animals because brain material is not available for testing. Attending physicians who suspect that a patient may have rabies usually telephone the Centre of Expertise for Rabies in Ottawa for instructions on which samples to submit. Samples for submission usually include saliva, eye secretions, corneal impressions and a plug of highly enervated skin from the back of the neck. Cerebral spinal fluid (CSF) and serum samples may also be sent to the laboratory if they are available. Prediction of which specimens will be positive if a rabies virus infection is present is very difficult; therefore, physicians should always try to send all of the above listed specimens to aid in a rabies diagnosis.
In the present report, we describe the laboratory diagnosis of rabies in a nine-year-old boy from Quebec. A case report describing the clinical findings in this case was published previously (4,5). Briefly, a young boy vacationing with his parents in the Laurentian mountains of western Quebec was believed to have been exposed to a rabid bat on or about August 28, 2000. The patient was admitted to hospital on September 27, from which time his condition rapidly deteriorated. On September 29, a diagnosis of rabies was considered, and biopsy material was collected and submitted to the Canadian Food Inspection Agency for rabies diagnosis.
TISSUES AND METHODS
Human biopsy tissue is examined for the presence of rabies virus in a number of ways. In the present case, skin biopsy specimens were prepared for cryosectioning by freezing the tissue onto wooden blocks in optimum cutting temperature compound (Miles Laboratories Inc, USA) at -25°C. After freezing, approximately 8 µm sections were cut from the embedded tissue for examination using direct IF microscopy. Briefly, sections were picked up on slides and placed under an ultraviolet light source for 2 min for virus inactivation. Tissue sections were fixed in ice cold acetone at -20°C for 20 min before immunostaining with a fluorescein isothiocyanate (FITC)-labelled goat antirabies virus antibody (prepared in house) diluted in PBS containing Evans blue as a counterstain. Following a 35 min incubation period at 37°C, the slides were washed in PBS and were examined with an epifluorescence microscope (Leica Canada Inc). As a positive control for this test, skin from a known rabies-positive fox was used.
Corneal impressions, made on glass microscope slides by the attending physician, were fixed and stained as described above for the skin sections. Positive and negative mouse brain impressions were used as controls.
Saliva samples, eye washings, CSF and skin biopsy were used in attempts to isolate rabies virus by the Rabies Tissue Culture Inoculation Test (RTCIT), a method recommended by the World Health Organization (6). Dilutions of these samples, made in minimal essential media (Life Technologies Inc, Canada), were inoculated onto mouse neuroblastoma (MNA) cells (BioWhitaker, USA) in 96-well cell culture plates (Nunc, Life Technologies Inc). All cultures were incubated at 36.5°C in the presence of 5% carbon dioxide for four days, after which the cells were fixed with ice cold 80% acetone. The same FITC-labelled goat antirabies virus antibody used for direct IF immunostaining was used to identify foci of rabies virus in infected cell cultures. To provide a contrast when observing cell cultures, all material was counterstained with Evans blue.
For the purpose of antigenic typing, samples of the skin biopsy and two saliva specimens were used to inoculate MNA cells in suspension in 25 cm2 cell culture flasks (VWR Canlab, Canada) and 96-well Terasaki plates (VWR Canlab, Canada) to monitor the infections. Specimens were passaged blindly three times to amplify low levels of rabies virus. When the presence of virus was detected in monitor plates, infected cells were plated onto 96-well Terasaki plates and incubated at 36.5°C for 24 h before being fixed with ice cold 70% acetone. Each of 406 different monoclonal antibodies (Mabs), as cell culture supernatants, were applied to individual wells and incubated for 1 h at 37°C. After thorough washing with Tris-buffered saline with 0.5% Tween 20 (Sigma-Aldrich, Canada) (TTBS), appropriately diluted goat antimouse FITC conjugate (Organon Teknica Co, USA) was applied to each well and incubated at 37°C for 1 h. The plates were again washed with TTBS and were counterstained with Evans blue. Mab staining patterns were determined by observation under an inverted epifluorescence microscope.
An alternative method used for virus amplification is the intercranial injection of any suspect samples into weanling mice. Tissue suspensions of the skin biopsy and the saliva were prepared in minimal essential media, and 50 µL were inoculated into the brains of three-week-old ND4 Swiss Webster mice (Harlan Sprague Dawley, USA). Mice were observed for clinical signs of rabies and euthanized with carbon dioxide when they showed signs of the disease. After 30 days, mice not showing clinical signs of rabies were also euthanized.
For genetic analyses, total RNA was purified from aliquots of the specimens obtained as follows: CSF (150 µL), eye secretions (200 µL) and saliva (450 µL divided into two duplicate samples). All samples were processed using TRIzol LS according to the manufacturer's directions (Gibco BRL, USA). Skin biopsy material (0.1 g) was finely chopped and processed similarly using TRIzol LS as directed. Precipitated RNA was dissolved in 20 to 40 µL of diethyl pyrocarbonate-treated water and stored at -80°C. Amplification of the complete nucleoprotein (N) gene coding region of the rabies virus (1485 base pair [bp] product) was performed by reverse transcriptase polymerase chain reaction (RT-PCR) using primers RabN1/RabN5 and reaction conditions as detailed previously (7), and was followed by visualization of the amplicons by agarose gel electrophoresis. In many cases, a second round of PCR was performed using internal primers RabNfor/RabNrev (7) to generate a 750 bp nested fragment of the N-terminal region of the gene.
For sequence analysis, amplicons were purified with the Wizard PCR preps DNA purification system (Promega, USA) and were subjected to manual sequencing using the fmol DNA cycle sequencing system (Promega) with 32P-labelled primers. A 384 bp nucleotide sequence, corresponding to bases 287 to 670 of the Pasteur virus reference strain of rabies virus (8), was aligned, using CLUSTALX1.8 (9), with corresponding sequences of viruses representative of the strains circulating in Canada. Phylogenetic analysis of this alignment was performed using the PHYLIP3.5 package of programs (University of Washington, USA).
RESULTS
The results of all the tests performed for diagnosing rabies in the present case are summarized in Table 1. Under direct IF examination, rabies antigen was detected only in sections of the skin biopsy material; corneal impressions were all negative. Live virus was not detected in the skin biopsy, eye washings or saliva when the RTCIT method was used. Virus was, however, isolated from the first saliva sample after amplification through two passages in MNA cells. Attempts at virus isolation from the skin, eye washings and CSF samples were unsuccessful. Rabies virus was also isolated from one mouse that was injected with a portion of the first saliva submission.
TABLE 1.
Tests used and results obtained for antemortem diagnosis of rabies in an 11-year-old boy
| Skin biopsy | Corneal Impressions | Eye secretions | Saliva sample 1 | Saliva sample 2 | CSF | |
|---|---|---|---|---|---|---|
| Direct immunofluorescence microscopy | P | N | ND | ND | ND | ND |
| RTCIT | N | ND | N | N | ND | N |
| Virus isolation | N | ND | ND | P | N | ND |
| RT-PCR, Nucleoprotein gene | N | ND | N | P | P | N |
| Nested RT-PCR, Nucleoprotein gene | P | ND | P | P | P | N |
| Date received in the laboratory | Sept 30/00 | Sept 30/00 | Sept 30/00 | Sept 30/00 | Oct 15/00 | Sept 30/00 |
CSF Cerebral spinal fluid; N Negative for rabies virus; ND Not done; Oct October; P Positive for rabies virus; Sept September; RTCIT Rabies Tissue Culture Inoculation Test; RT-PCR Reverse transcriptase-polymerase chain reaction
Phenotypic strain identification was achieved with virus that was isolated from saliva in cell culture. Immunostaining was done with Mabs generated from murine spleen cell donors immunized with a variety of different lyssavirus antigens. For the characterization of the isolate, 368 anti-N and 38 antiphosphoprotein Mabs were used. The reaction pattern with these 406 Mabs is identical to the patterns observed with isolates made from silver-haired bats (Lasionycteris noctivagans) in Canada. The database used for rabies virus strain identifications contains Mab reaction patterns of numerous isolates of 12 different Canadian bat rabies virus variants and 50 distinct lyssavirus strains from around the world. Though a large number of the Mabs used at the Centre of Expertise for Rabies are specific for a particular lyssavirus species (serotype, genotype), none of them react exclusively with a single virus variant ('strain'). All variants, however, as characterized by phylogenetic analysis of genome sequences, display unique patterns of reactivity with panels of selected Mabs.
The diagnosis of rabies based on the IF test was confirmed by molecular biological methods. By RT-PCR analysis, several specimens from the patient revealed the presence of rabies virus-specific gene sequences. A positive signal was detected in saliva samples after a single round of PCR, while nested PCR was required to detect viral genome in the eye secretions and skin biopsy material. No viral gene sequence was detectable in the CSF, even after nested PCR.
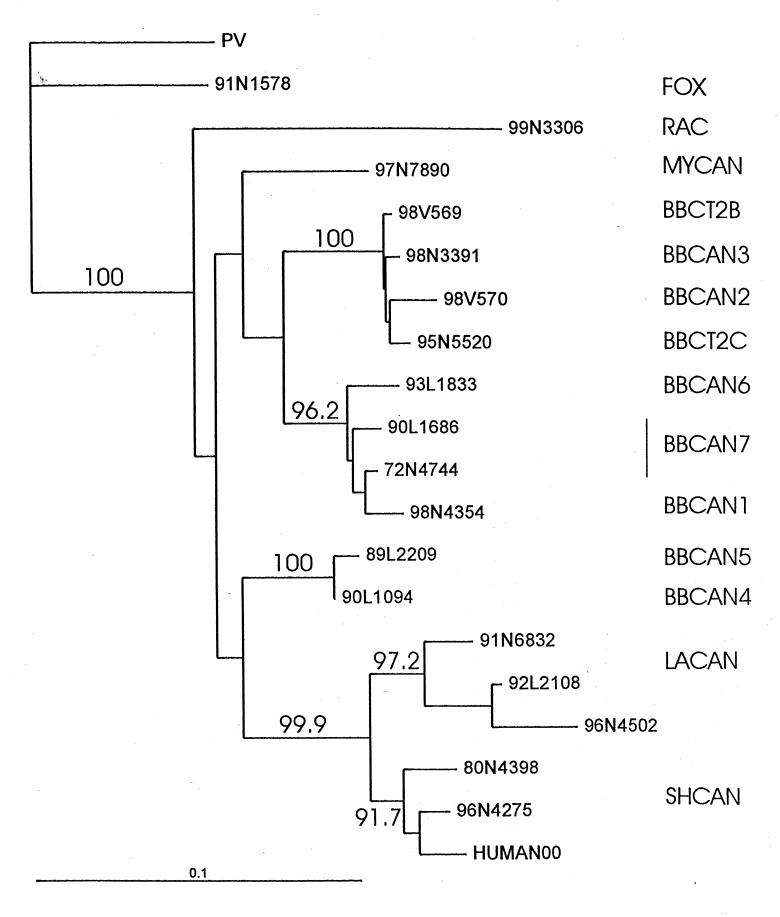
Consensus nucleotide sequencing of the amplicons generated from saliva and skin over a 384 bp window in the 5' terminal region of the viral N gene yielded identical sequences. These data were employed in a phylogenetic analysis to compare the human clinical isolate with rabies viruses representative of the strains circulating in terrestrial (fox, raccoon) hosts within eastern Canada and strains specific to several species of bats throughout North America. The results of this analysis, presented as a phylogenetic tree in (Figure 1), strongly support the conclusion that the human subject was infected with a rabies virus very closely related to those known to circulate in silver-haired bats (bootstrap value of 91.7 % for this association).
Figure 1.
Phylogenetic tree comparing the recent Canadian human isolate with representative North American rabies viruses. A 384 base pair sequence of a segment of the rabies virus nucleoprotein gene from 19 selected rabies viruses, including the Pasteur virus reference strain as an outgroup, were aligned with the sequence of the human isolate (HUMAN00) and used for phylogenetic analysis by the PHYLIP3.5 package (University of Washington, USA) using the neighbor joining algorithm with bootstrap resampling (1000 replicates). Isolates are numbered according to year (two digits), laboratory code (L for Lethbridge and N for Nepean) and the submission number (four digits). Specimens indicated by V were generously provided by the state of Connecticut. Numbers within the tree are the bootstrap values (expressed as a percentage) indicating the number of times that the isolates to the right of the branch grouped together within this consensus tree. To the right of the tree, the antigenic grouping of each of these branches is indicated as follows: terrestrial strains - FOX Arctic fox (Ontario); RAC Mid-Atlantic raccoon (Ontario); insectivorous bat strains - MYCAN From Myotis species; BBCAN and BBCT Big brown bat (Eptesicus fuscus) variants (1 to 7, and 2B and 2C) of Canada and Connecticut; LACAN From Lasiurus cinereus and Lasiurus borealis bats; SHCAN From silver-haired bats (Lasionycteris noctivagans). The horizontal line at the bottom left of the figure indicates the line length corresponding to a nucleotide distance value of 0.1
DISCUSSION
Rabies has a unique pathogenesis that is marked by virus dissemination within nerve fibres rather than by blood and lymph. Rapid expansion of the infection occurs within the central nervous system (CNS) after a variable, long incubation period, followed by virus excretion in saliva toward the end of the incubation period. The clinical outcome of rabies virus infection is almost invariably fatal.
Antemortem laboratory diagnosis of rabies is often difficult. During the incubation period, rabies virus replicates at such low levels that it is difficult to detect and usually does not induce measurable immune responses. Infectious virus, antigen and specific immune responses become detectable only after the virus has reached the CNS and has invaded the peripheral nervous system through centrifugal spread. Virus spread is usually concurrent with clinical symptoms; however, there is significant temporal variability in the appearance of manifestations that are detectable by laboratory tests. The utility of various methods for intravitam diagnosis of human rabies has been reported previously (10). Application of the RT-PCR technique to both saliva and CSF material collected from nine rabies-infected patients over a period of time after onset of symptoms indicated overall positivity rates of 0.3 (11 of 37) for saliva and 0.09 (two of 22) for CSF. There was no obvious pattern with respect to the period after onset of symptoms that virus was detectable in saliva. However, the most sensitive indicator for rabies infection was the direct IF assay on skin biopsy material with a positivity rate of 0.86 (six of seven). RT-PCR was not performed on skin biopsy material in that study (10). Our findings are in accord with those of Crepin et al (10), with successful diagnosis of the present case being achieved with both skin biopsy tissue (direct IF, RT-PCR) and saliva (RT-PCR); the presence of virus was not found in CSF.
In view of the dissimilarity of individual cases, we recommend that saliva, tears (eye wash fluid), impression smears of cornea, but most importantly, a skin biopsy be submitted for the detection of viral antigen and/or genome, and the isolation of infectious virus. All of these tissues may eventually test positive during the final period of the disease. CSF usually remains negative when examined for virus or viral genome; however, the examination of serum and CSF for rabies-specific antibodies can be useful, particularly in cases of prolonged clinical illness. Antibody levels generally remain below the threshold of detection during the incubation period, but usually rise sharply with the more widespread antigen distribution during the symptomatic phase of the disease. Rabies antibodies resulting from vaccination or passive administration are restricted to serum, while their appearance in CSF is indicative of the presence of rabies virus antigen in the CNS or a breach in the blood-brain barrier. It should be noted that the standard test for the diagnosis of rabies in humans, as recommended by the World Health Organization, is the postmortem examination of the impression smears of brain tissue with FITC-labelled antirabies virus immunoglobulin.
The identification of the silver-haired bat variant as being responsible for this human case continues a trend that has been observed over several years in the United States (11). From 1990 to October 31, 1999, 27 human rabies cases, of which 22 were indigenously acquired, were reported in the United States. Of the 22 indigenous cases, 20 were found to have been infected with bat strains, and 15 of these were identified as the variant known to circulate in silver-haired and eastern pipistrelle bat (Pipistrellus subflavus) populations. A similar, if not identical, variant circulates in silver-haired bats in Canada; this variant also causes significant numbers of rabies cases in other bat species, especially little brown bats (Myotis lucifugus) (12). In light of these reports, it is recommended by both Canadian and American health officials that postexposure treatment for rabies be initiated immediately for persons who were exposed to a bat and who cannot be certain of whether they were bitten (13,14).
References
- 1.McLean AE, Noble MA, Black WA, et al. A human case of rabies-British Columbia. Can Dis Wkly Rep 1985;11:213-4. [Google Scholar]
- 2.Tabel H, Corner AH, Webster WA, Casey GA. History and epidemiology of rabies in Canada. Can Vet J 1974;15:271-81. [PMC free article] [PubMed] [Google Scholar]
- 3.Dean DJ, Abelseth MK, Atanasiu P. The fluorescent antibody test. In: Meslin F-X, Kaplan MM, Koprowski H, eds. Laboratory Techniques in Rabies, 4th edn. Geneva: World Health Organization, 1996:88-95. [Google Scholar]
- 4.Turgeon N, Tucci M, Deshaies D, et al. [Human rabies in Montreal, Quebec - October, 2000.] Can Commun Dis Rep 2000;26:209-10. [PubMed] [Google Scholar]
- 5.Turgeon N, Tucci M, Teitelbaum J, et al. Public health dispatch: Human rabies - Quebec, Canada, 2000. MMWR Morb Mortal Wkly Rep 2000;49:1115-6. [PubMed] [Google Scholar]
- 6.Webster WA, Casey GA. Virus isolation in neuroblastoma cell culture. In: Meslin F-X, Kaplan MM, Koprowski H, eds. Laboratory Techniques in Rabies, 4th edn. Geneva: World Health Organization, 1996:96-104. [Google Scholar]
- 7.Nadin-Davis SA. Polymerase chain reaction protocols for rabies virus discrimination. J Virol Methods 1998;75:1-8. [DOI] [PubMed] [Google Scholar]
- 8.Tordo N, Poch O, Ermine A, Keith G, Rougeon F. Walking along the rabies genome: Is the large G-L intergenic region a remnant gene? Proc Natl Acad Sci U S A 1986;83:3914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson JD, GibsonTJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997;24:4876-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crepin P, Audry L, Rotivel Y, Gacoin A, Caroff C, Bourhy H. Intravitam diagnosis of human rabies by PCR using saliva and cerebrospinal fluid. J Clin Microbiol 1998;36:1117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krebs JW, Smith JS, Rupprecht CE, Childs JE. Rabies surveillance in the United States during 1998. J Am Vet Med Assoc 1999;215:1786-98. [PubMed] [Google Scholar]
- 12.Nadin-Davis SA, Huang W, Armstrong J, et al. Antigenic and genetic divergence of rabies viruses from bat species indigenous to Canada. Virus Res 2001;74:139-56. [DOI] [PubMed] [Google Scholar]
- 13.Health Protection Branch, Laboratory Centre for Disease Control. Canadian Immunization Guide, 5th edn. Ottawa: Canadian Medical Association, 1998:149-56. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Human rabies prevention - United States, 1999. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1999;48(RR-1):1-21. [PubMed] [Google Scholar]