Abstract
The relative frequency of serogroups of Neisseria meningitidis associated with meningococcal disease in Canada during the period January 1, 1999 to June 30, 2001 was examined. Of the 552 strains of N meningitidis collected from clinical specimens of normally sterile sites, 191 (34.6%), 276 (50.0%), 61 (11.1%) and 23 (4.2%) were identified by serological and molecular methods as serogroups B, C, Y and W135, respectively. About half (50.8%) of the serogroup Y isolates were isolated in the province of Ontario. The two most common serotypes found were 2c and 14. Most of the serogroup Y strains isolated from patients in Ontario were serotype 2c, while serotype 14 was the most common serotype associated with disease in the province of Quebec. The two most common serosubtypes found among the serogroup Y meningococci were P1.5 and P1.2,5. Laboratory findings, based on antigenic analysis, did not suggest that these serogroup Y strains arise by capsule switching from serogroups B and C strains. This study documented a higher incidence of finding serogroup Y meningococci in clinical specimens from patients in Ontario compared to the rest of Canada, and parallels the increase in serogroup Y meningococcal disease reported in some parts of the United States.
Key Words: Meningococcal disease, Neisseria meningitidis, Serogroups
Invasive meningococcal disease (IMD) is a notifiable communicable disease that is monitored by a national surveillance program coordinated by the Division of Disease Surveillance and the Division of Respiratory Diseases, Centre for Infectious Disease Prevention and Control, Health Canada. Starting in 1971 and with the help of provincial public health officials, Health Canada began to collect data on the serogroup information on IMD cases. Also, isolates of meningococci collected from patients are routinely sent to Health Canada's National Microbiology Laboratory (NML) in Winnipeg for further antigenic and genetic analyses.
IMD is a serious disease globally but the serogroups of meningococci causing diseases in various countries may vary in frequency. For example, serogroup A is a major cause of disease in Africa and China (1), while serogroups B and C meningococci are the most frequent cause of IMD in Western countries (2). In Canada, most IMD cases are caused by meningococci belonging to serogroups B, C, Y and W135. Serogroups B and C account for over 75% of the isolates collected from patients (3).
In the past decade, Neisseria meningitidis serogroup Y has emerged as a frequent cause of IMD in the United States (4,5). In view of these findings, it is important to monitor the incidence of serogroup Y disease. This report presents the frequency of isolation of serogroups of meningococci in normally sterile clinical specimens collected from patients (likely to be presented as IMD) in various parts of Canada and describes the distribution of serotypes and serosubtypes found among the serogroup Y isolates.
MATERIALS AND METHODS
Isolates of N meningitidis, submitted from provincial public health laboratories across Canada to the NML in Winnipeg, were confirmed by biochemical tests. Serogroups were identified by bacterial agglutination with rabbit anti-sera. In some instances, nonagglutinable or nonsero-groupable strains were tested by a molecular method for serogroup identification (6). Serotyping and serosubtyping were carried out using whole cell antigens and monoclonal antibodies in the enzyme-linked immunosorbent assay method (7). Serogroup Y strains were also tested with monoclonal antibody against the serotype 2c antigen using the same method. Statistical significance was tested by t test and Χ2 statistics (Epi Info6, Centers for Disease Control and Prevention, USA/World Health Organization, version 6, 1994).
RESULTS
Distribution of serogroups of N meningitidis in clinical specimens (of normally sterile sites) collected from patients in Canadian provinces
From January 1, 1999 to June 30, 2001, 552 strains of N meningitidis were received by the Central Nervous System Infection Division of NML in Winnipeg. Serogroups were identified for 543 isolates and nine were deemed nonserogroupable by bacterial agglutination test. These nine nonserogroupable isolates were further analyzed by a molecular method that detects sequence differences in the polysialyltransferase genes, which encode the enzymes required for assembly of the sialic acid-containing serogroup-specific B, C, Y and W135 capsules (8). This molecular method identified four isolates as serogroup B, one as serogroup C and two each as serogroups Y and W135. Thus, a combination of serological and molecular methods was used to identify the serogroup nature of all 552 isolates collected from clinical specimens of normally sterile sites.
Table 1 depicts the distribution of the serogroups of meningococci isolated from across Canada. The most frequently isolated organisms belonged to serogroup C (50.0%) followed by serogroup B (34.6%) and then serogroups Y (11.05%), W135 (4.17%) and 29e (0.18%). There was an increase in the number of isolates received in the first half of 2001 because of an increase in meningococcal disease activity (mostly due to serogroup C) in several provinces across the country (Dr Theresa Tam, Division of Respiratory Diseases, personal communication).
Table 1.
Distribution of serogroups of Neisseria meningitidis isolates collected from clinical specimens of normally sterile sites across Canada from January 1, 1999 to June 30, 2001
| Serogroups | ||||||
|---|---|---|---|---|---|---|
| Province | B | C | Y | W135 | 29e | Total |
| British Columbia | 30 | 28 | 7 | 2 | 0 | 67 |
| Alberta | 19* | 111 | 2 | 4 | 0 | 136 |
| Saskatchewan | 5 | 2 | 2 | 0 | 0 | 9 |
| Manitoba | 7 | 11 | 1 | 1 | 0 | 20 |
| Ontario | 57 | 77 | 31 | 15 | 0 | 180 |
| Quebec | 57 | 43 | 11 | 1 | 1 | 113 |
| Nova Scotia | 4 | 0 | 2 | 0 | 0 | 6 |
| New Brunswick | 12 | 1 | 4 | 0 | 0 | 17 |
| Newfoundland | 0 | 3 | 1 | 0 | 0 | 4 |
| Total | 191 | 276 | 61 | 23 | 1 | 552 |
One strain was isolated from a patient in Nunavut
Sixty-one serogroup Y isolates were obtained during the 2.5 years from the following clinical specimens: blood (53 strains), cerebrospinal fluid (six strains) and joint (two strains). These serogroup Y IMD cases involved 27 male and 31 female patients; there was no information on the sex of three subjects. Their age distribution is presented in Table 2. The median ages of the patients according to year were 55 years for 1999, 55 years for 2000 and 17 years for 2001 (P=0.017 by t test).
Table 2.
Age group distribution of patients with serogroup Y invasive meningococcal disease (IMD) by year from January 1, 1999 to June 30, 2001
| IMD cases by year | ||||
|---|---|---|---|---|
| Age group | 1999 | 2000 | 2001* | Total cases |
| Under 1 | 1 | 0 | 1 | 2 |
| 1-5 | 1 | 2 | 0 | 3 |
| 6-10 | 0 | 1 | 5 | 6 |
| 11-20 | 2 | 3 | 6 | 11 |
| 21-50 | 2 | 3 | 5 | 10 |
| +50 | 10 | 14 | 3 | 27 |
| Not known | 1 | 1 | 0 | 2 |
| Total | 17 | 24 | 20 | 61 |
| Median age (years) | 55 | 55 | 17 | 39.5 |
January 1 to June 30
About one-half of the serogroup Y isolates was collected from the Province of Ontario, where this serogroup accounted for 17% (31 of 180) of all the meningococcal strains isolated there. Serogroup Y meningococci was isolated 2.1 times more frequently in Ontario than in all other provinces combined (8% or 30 of 372) (P=0.002 by Χ2 statistic) and 1.6 times more frequently than in the country as a whole (11%, or 61 of 552).
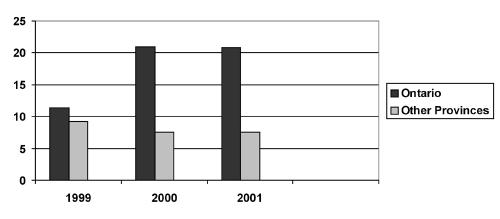
When analyzed by year, the frequency of isolation of serogroup Y meningococci compared with all serogroups of meningococci isolated was 17 of 167 (10.2%) for 1999, 24 of 206 (11.7%) for 2000 and 20 of 179 (11.2%) for the first half of 2001. The frequency of serogroup Y meningococcal isolates in Ontario in 1999, compared with that from all other provinces combined, was 11.4% and 9.2%, respectively. Frequencies for 2000 and 2001 were 21% and 20.8%, respectively for Ontario, and 7.6% for all the provinces combined in both years (Figure 1).
Figure 1.
Comparison of the frequencies of serogroup Y meningococci as a percentage of all meningococci isolated in Ontario and other provinces combined during the period of January 1, 1999 to June 30, 2001
Distribution of serotypes and serosubtypes among serogroup Y meningococcal disease isolates
The distribution of serotypes among serogroup Y isolates from some provinces is shown in Table 3. Thirty-three (54.1%) of the serogroup Y isolates were found to have the class 2 Por B major outer membrane protein bearing the serotype 2c determinant. Serotype 2c was the major type of serogroup Y meningococci isolated in Ontario (74% of the isolates). In contrast, serotype 2c had limited association (9.1% of isolates) with serogroup Y strains in Quebec.
Table 3.
Numbers of serotypes found in Group Y meningococci collected during January 1, 1999 to June 30, 2001 in the Canadian provinces
| Serotypes | |||||||
|---|---|---|---|---|---|---|---|
| Province | 1 | 2a | 2c | 14 | 15 | NT* | Total |
| British Columbia | 0 | 0 | 3 | 1 | 0 | 3 | 7 |
| Alberta | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| Saskatchewan | 0 | 0 | 0 | 1 | 0 | 1 | 2 |
| Manitoba | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Ontario | 1 | 0 | 23 | 6 | 0 | 1 | 31 |
| Quebec | 0 | 0 | 1 | 8 | 1 | 1 | 11 |
| New Brunswick | 0 | 0 | 3 | 0 | 0 | 1 | 4 |
| Nova Scotia | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Newfoundland | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Total | 1 | 1 | 33 | 18 | 1 | 7 | 61 |
Nonserotypeable
The second most common serotype was 14, which accounted for 29.5% of all serogroup Y isolates. This serotype was found on 72.7% of serogroup Y meningococci in the province of Quebec. Serotype 14 was also present (19.4% of isolates) in Ontario.
The incidence of serosubtypes in relation to serotypes is shown in Table 4. Serosubtype P1.5, either alone or in combination with P1.2, was found on 78.7% of the isolates. Serosubtype P1.2 was found on 39.3% of the isolates and always in combination with P1.5. Eleven (18.0%) strains failed to react with the serosubtype monoclonal antibodies. Overall, 46 (75.0%) strains were assigned a serotype and serosubtype, 12 (19.7%) strains were assigned either a serotype or a serosubtype, and 3 (5%) strains failed to react with any of the monoclonal antibodies.
Table 4.
Serotypes and serosubtypes of Canadian serogroup Y meningococcal disease isolates
| Serotype:serosubtype | Number of isolates |
|---|---|
| 2c:P1.5 | 16 |
| 2c:P1.2,5 | 16 |
| 2c:P1.-* | 1 |
| 14:P1.5 | 4 |
| 14:P1.2,5 | 7 |
| 14:P1.-* | 7 |
| NT†:P1.5 | 3 |
| NT:P1.2,5 | 1 |
| NT:P1.-* | 3 |
| 1:P1.16 | 1 |
| 2a:P1.5 | 1 |
| 15:P1.16 | 1 |
| Total | 61 |
Nonserosubtypeable
Nonserotypeable
DISCUSSION
This report concentrates mainly on the laboratory characterization of N meningitidis strains, particularly those belonging to serogroup Y, isolated from IMD cases in Canada between January 1, 1999 and June 30, 2001. Although it is unlikely that we had received meningococcal isolates from every case of IMD in Canada, the 552 strains received during this study period probably were the majority of the isolates involved, as well as a good representation of all case isolates. For example, of the 252 cases that were reported to the national surveillance program in 1997, 202 were identified with isolates received at the NML. Although there were some cases for which no isolates were received, NML also reported an additional 13 cases that were not reported by the provinces to the national surveillance program. These 13 cases were identified by the meningococcal strains submitted to our laboratory from individual patients' blood, cerebrospinal fluid or other normally sterile body sites. The number and percentage of IMD cases in 1998 not reported by the provinces but identified by NML based on organisms received were even higher and amounted to 19 cases out of a total of 174 (10.9%) (3). Although it is difficult to ascertain how representative this collection of 552 strains is, there is no indication to suggest that they are biased because certain serogroups or strains from certain regions are under-represented.
The distribution data of serogroups of meningococci in IMD cases in Canada as presented in this paper are based solely on the strains characterized in our laboratory using strains submitted to us from provincial public health laboratories across the country. Data on meningococcal serogroup distribution in Canada for previous years can be found in the literature (summarized in Table 5). However, direct comparison of the data presented in this paper with those in the literature may not be feasible because some of the previous data were presented as cases of IMD and may, therefore, differ from the figures based just on strains received at NML as explained above. Nevertheless, certain trends are apparent: first, serogroup A meningococci, which used to be responsible for about 5% of the IMD cases (9,10), are now rare, and in fact no group A strain was found in the past three years (3, present data); second, it was first recognized in the mid-1980s that serogroup C strains were increasingly being detected in clinical samples, and they continue to cause a significant percentage of IMD cases (11), and beginning in 2000 they were isolated more frequently than serogroup B strains; and third, serogroup Y meningococci, which used to be rarely isolated from IMD cases in the 1980s (9,10), are now causing about 10% of the cases. This increase is even more dramatic when the frequencies of isolation of serogroup Y strains are broken down by provinces or regions (Figure 1). For example, during the period January 1, 1999 to June 31, 2001, 136 meningococcal strains in total were received from Alberta that were isolated from normally sterile body sites of patients, and only two were identified as serogroup Y. In contrast, 31 of the 180 strains received from Ontario and 11 of the 113 strains from Quebec belonged to serogroup Y. Further epidemiological analysis is required to determine the onset of the increase in serogroup Y meningococcal disease in Canada.
Table 5.
Distribution of meningococcal serogroups in invasive meningococcal disease cases in Canada during selected periods from 1979 to 2001
| Percentage of cases by serogroup | |||||||
|---|---|---|---|---|---|---|---|
| Period of study | A | B | C | Y | W135 | Others | Reference |
| 1979-1982* | 5.0 | 45.0 | 12.0 | 2.0 | 13.0 | 23.0 | 9 |
| 1983-1987 | 4.8 | 51.6 | 25.8 | Unknown | 10.2 | 7.6† | 10 |
| 1995 | 1.0 | 48.0 | 38.0 | 9.0 | 3.0 | 1.0 | 11 |
| 1996 | 0 | 46.0 | 42.0 | 10.0 | 1.0 | 1.0 | 11 |
| 1997 | 0.5 | 47.9 | 31.8 | 14.7 | 3.7 | 1.4 | 3 |
| 1998 | 0 | 50.0 | 29.0 | 13.7 | 4.0 | 3.2 | 3 |
| 1999‡ | 0 | 47.9 | 35.9 | 10.2 | 5.4 | 0.6 | Present study |
| 2000‡ | 0 | 34.0 | 49.5 | 11.7 | 4.9 | 0 | Present study |
| 2001 (first 6 months)‡ | 0 | 22.9 | 63.7 | 11.2 | 2.2 | 0 | Present study |
Based on isolates obtained from all body sites
Including strains of serogroups Y and 29e, as well as strains that were rough, polyagglutinable and nongroupable
Based on isolates received at the National Microbiology Laboratory and obtained from normally sterile body sites only
This study did not collect clinical data on the serogroup Y IMD cases; however, it did collect data on the isolation sites of the clinical specimens, which could be used as a proxy for clinical presentation. In 53 (87%) cases isolates were from blood, which may indicate that the majority of patients presented with septicemia rather than meningitis. The overall median age of the patients was 37 years, but when the data were analyzed by year (Table 2), the median age (17 years) for 2001 was significantly lower than the median ages for 1999 and 2000 (55 years, P=0.017 by t test). A significant drop in the median age of patients with serogroup Y meningococcal disease, from 19 years to 11.5 years, was similarly observed in the Chicago area when data from 1995 were compared with data obtained between 1991 and 1994 (12). No explanation was given for the drop in the median age of patients with serogroup Y IMD in Chicago, nor do we know from our current study why patients with serogroup Y IMD in 2001 were younger than those recorded in 1999 and 2000. Determining whether this trend will continue would require further careful monitoring over the next few years. It is equally important to analyze retrospective epidemiological information on serogroup Y IMD cases over the past several years, as well as to compare epidemiological data of IMD cases caused by different serogroups over a period of time in order to understand whether there have been other changes in the patterns of the disease.
Antigenic analysis of the current collection of 61 serogroup Y strains suggests the existence of two separate types that have either the serotype antigen 2c (33 strains) or 14 (18 strains). Also of interest is their distribution: serotype 2c strains were found mainly in Ontario, while serotype 14 strains were found in both Quebec and Ontario. Serotype 2c antigen is rarely found in serogroup B or serogroup C meningococci in Canada (13-15) and the United States (16-18). Previous studies done in the 1980s (18-20) indicated that serotype 2c was restricted mainly to serogroup Y meningococci, but both serotypes 2a and 2c had been found in association with serogroup Y strains. Our studies indicate that over one-half of the Canadian serogroup Y meningococcal isolates collected during 1999 to 2001 were serotype 2c, while only one isolate was serotype 2a.
When the serotype and serosubtype antigens of the 61 group Y meningococci were compared with the antigens of serogroups B and C strains isolated during the same time period, the antigenic combinations of 2c:P1.5, 2c:P1.2,5, 14:P1.5 and 14:P1.2,5 were found only in serogroup Y and not in serogroups B and C organisms. Strains of serogroup B meningococci isolated from IMD cases in Canada are very diverse, with many different combinations of serotype and serosubtype antigens found on their cell surface (15). Also, clones of group B strains that are known to be hypervirulent and cause systemic disease in other parts of the world such as the ET-5, cluster A4 and lineage III strains all carry different combinations of serotype and serosubtype antigens such as 15:P1.7,16 (ET-5), 2b:P1.2 (cluster A4) and 4:P1.4 (lineage III) (21-23). Over 80% of serogroup C strains isolated from IMD cases in Canada during the past decade belonged to the hypervirulent clone of ET-15, which was first identified in Canada and has since spread widely throughout other parts of the world (24). Most of the earlier isolates of this clone of ET-15 group C meningococci were found to have the antigenic formula C:2a:P1.2,5 (14,25). Recently, a new variant with the serosubtype antigens of P1.1,7 was identified in many of the group C meningococci isolated in Quebec and Ontario (NML, unpublished data). Therefore, on the basis of the unique antigenic formulas of strains in the serogroups of meningococci, it does not appear that the serogroup Y meningococci arise from serogroups B or C strains by capsule switching. Our preliminary genetic data on the serogroup Y meningococci also suggest that they are not related to and, therefore, do not appear to arise from strains of serogroups B and C meningococci.
CONCLUSIONS
The methods of serogrouping, serotyping and serosubtyping continue to provide useful information for the surveillance of IMD in Canada. The finding that serotype 2c is frequently associated with group Y isolates warrants the use of the anti-2c monoclonal antibody in the routine typing of this group of meningococci in the future. Further studies are underway to examine the clonal nature of serogroup Y meningococci in Canada. Comparison of the characteristics of group Y meningococci collected from the United States and Canada may provide additional information on the epidemiology of this emerging serogroup of N meningitidis in both countries. Results from this study suggest a changing pattern (involving frequency and age group of affected patients) of IMD caused by one meningococcal serogroup. Such findings highlight the importance of continued surveillance of the disease to evaluate, plan and update our control programs against a disease that has been reported to behave like shifting sands (26).
Acknowledgments
The authors thank the directors and staff of the provincial and territorial public health laboratories for providing the strains for this study, and Health Canada's Genomics R & D Fund for financial support to carry out the molecular analysis. The expert technical assistance of Francoise Collins, Jan Stoltz and Dennis Law is also gratefully acknowledged.
References
- 1.Wang JF, Caugant DA, Li X, et al. Clonal and antigenic analysis of serogroup A Neisseria meningitidis with particular reference to epidemiological features of epidemic meningitis in the People's Republic of China. Infect Immun 1992;60:5267-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achtman M. Global epidemiology of meningococcal disease. In: Cartwright K, ed. Meningococcal Disease. Chichester: John Wiley & Sons, 1995:159-75. [Google Scholar]
- 3.Squires SG, Pelletier L, Mungai M, et al. Invasive meningococcal disease in Canada, 1 January 1997 to 31 December 1998. Can Commun Dis Rep 2000;26:177-82. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Serogroup Y meningococcal disease: Illinois, Connecticut, and selected areas, United States, 1989-1996. MMWR Morb Mortal Wkly Rep 1996;45:1010-3. [PubMed] [Google Scholar]
- 5.Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J Infect Dis 1999;180:1894-901. [DOI] [PubMed] [Google Scholar]
- 6.Tsang RSW, Law D, Squires SG. Two serologically non-groupable Neisseria meningitidis strains from clinical specimens identified by molecular method as serogroup B meningococci. Can Commun Dis Rep 2001;27:9-12. [PubMed] [Google Scholar]
- 7.Abdillahi H, Poolman JT. Whole-cell ELISA for typing of Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol Lett 1987;48:367-71. [PubMed] [Google Scholar]
- 8.Claus H, Vogel U, Muhlenhoff M, et al. Molecular divergence of the sia locus in different serogroups of Neisseria meningitidis expressing polysialic acid capsules. Mol Gen Genet 1997;257:28-34. [DOI] [PubMed] [Google Scholar]
- 9.Varughese P, Acres S. Meningococcal disease in Canada and serogroup distribution. Can Dis Wkly Rep 1983;9:177-80. [Google Scholar]
- 10.Varughese PV, Carter AO. Meningococcal disease in Canada. Surveillance summary to 1987. Can Dis Wkly Rep 1989;15:89-96. [PubMed] [Google Scholar]
- 11.Deeks S, Kertesz D, Ryan A, et al. Surveillance of invasive meningococcal disease in Canada, 1995-1996. Can Commun Dis Rep 1997;23:121-5. [PubMed] [Google Scholar]
- 12.Racoosin JA, Whitney CG, Conover, CS, et al. Serogroup Y meningococcal disease in Chicago, 1991-1997. JAMA 1998;280:2094-8. [DOI] [PubMed] [Google Scholar]
- 13.Ashton FE, Ryan A, Diena BB, et al. Serotypes among Neisseria meningitidis serogroups B and C strains isolated in Canada. Can J Microbiol 1980;26:1480-8. [DOI] [PubMed] [Google Scholar]
- 14.Ashton FE, Ryan JA, Borczyk A, et al. Emergency of a virulent clone of Neisseria meningitidis serotype 2a that is associated with meningococcal group C disease in Canada. J Clin Microbiol 1991;29:2489-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashton FE, Caugant DA. The panmictic nature of Neisseria meningitidis serogroup B during a period of endemic disease in Canada. Can J Microbiol 2001;47:283-9. [PubMed] [Google Scholar]
- 16.Jackson LA, Schuchat A, Reeves MW, et al. Serogroup C meningococcal outbreaks in the United States. JAMA 1995;273:383-9. [PubMed] [Google Scholar]
- 17.Tondella MLC, Popovic T, Rosenstein NE, et al. Distribution of Neisseria meningitidis serogroup B serosubtypes and serotypes circulating in the United States. J Clin Microbiol 2000;38:3323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caugant DA, Zollinger ZD, Mocca LF, et al. Genetic relationships and clonal population structure of serotype 2 strains of Neisseria meningitidis Infect Immun 1987;55:1503-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zollinger WD, Moran EE, Connelly H, et al. Monoclonal antibodies to serotype 2 and serotype 15 outer membrane proteins of Neisseria meningitidis and their use in serotyping. Infect Immun 1984;46:260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poolman JT, Hopman CTP, Zanen HC. Immunochemical characterization of Neisseria meningitidis serotype antigens by immunodiffusion and SDS-polyacrylamide gel electrophoresis immunoperoxidase techniques and the distribution of serotypes among cases and carriers. J Gen Microbiol 1980;116:465-73. [DOI] [PubMed] [Google Scholar]
- 21.Caugant DA, Froholm LO, Bovre K, et al. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci 1986;83:4927-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caugant DA, Bol P, Hoiby EA, et al. Clones of serogroup B Neisseria meningitidis causing systemic disease in the Netherlands, 1958 through 1986. J Infect Dis 1990;162:867-74. [DOI] [PubMed] [Google Scholar]
- 23.Martin DR, Walker SJ, Baker MG, et al. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J Infect Dis 1998;177:497-500. [DOI] [PubMed] [Google Scholar]
- 24.Jelfs J, Munro R, Ashton FE, et al. Genetic characterization of a new variant within the ET-37 complex of Neisseria meningitidis associated with outbreaks in various parts of the world. Epidemiol Infect 2000;125:285-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krizova P, Musilek M. Changing epidemiology of meningococcal invasive disease in the Czech Republic caused by new clone Neisseria meningitidis C:2a:P1.2 (P1.5), ET- 15/37. Cent Eur J Public Health 1995;3:189-94. [PubMed] [Google Scholar]
- 26.Shafran SD, Conly JM. The shifting sands of meningococcal disease. Can J Infect Dis 1999;10:109-12. [Google Scholar]