Abstract
Bacteremia due to Mycobacterium neoaurum, a rapidly growing mycobacterium, is described in a diabetic woman on hemodialysis. This is the first reported case of M neoaurum bacteremia in Canada. The organism initially grew on standard BacT/Alert SA aerobic blood cultures, and was subsequently positively identified using 16S rRNA sequence analysis. The present case serves to reinforce the need for a high index of clinical suspicion of infections caused by unusual microorganisms in the context of an immunocompromised host
Key Words: 16S rRNA sequencing, Hemodialysis infection, Mycobacterium neoaurum
The present report describes a case of Mycobacterium neoaurum bacteremia and arteriovenous shunt infection in a hemodialysis patient. M neoaurum, a member of the Mycobacterium parafortuitum complex, belongs to the group of rapidly growing environmental mycobacteria that are responsible for a broad spectrum of illnesses, including surgical wound and catheter infections, and disseminated cutaneous and pulmonary diseases (1). To our knowledge, this is the first case of infection due to M neoaurum reported in the Canadian literature.
CASE PRESENTATION
A 40-year-old woman with type 2 diabetes mellitus, end stage renal disease and hepatitis C presented with a two-month history of intermittent fevers, chills and malaise, and a weight loss of 6 kg. Additional complaints included episodes of pleuritic left-sided chest pain associated with shortness of breath and a dry cough. She had been receiving hemodialysis for one year, and for the previous nine months was dialyzed via a left arm fistula that included a 5 cm long, 6 mm diameter piece of interpositioned polytetra-fluoroethylene graft. Initial physical examination did not reveal a focus of infection. Investigations that had been obtained after one month of symptoms included a normal chest x-ray and two peripheral blood cultures drawn on May 3 and 8, 2001 on routine media (BacT/Alert SA aerobic medium using BacT/Alert (bio Merieux, France) Classic blood culture continuous monitoring system with no growth. On June 2, she was reassessed and repeat blood cultures were obtained from a peripheral site and from the fistula. These were incubated in BacT/Alert SA aerobic medium and both became positive after seven days. The initial Gram stain of the culture medium was negative, but the acridine orange stain revealed numerous rods. Subsequent Kinyoun stain confirmed the presence of acid-fast bacilli (AFB).
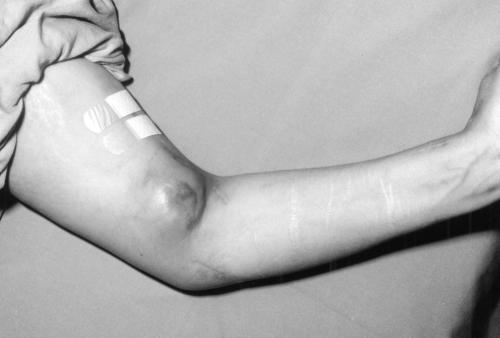
The patient was admitted to hospital on June 9, 2001 following the report of the positive blood culture. She had ongoing complaints of intermittent fever and shortness of breath, but was afebrile on admission and physical examination again showed no localizing findings. The peripheral leukocyte count was 11.2x109 with 89% neutrophils. A repeat chest x-ray was unremarkable. Antimicrobial agents were held pending susceptibility testing of the organism. On June 11, the patient developed left arm tenderness and enlargement of the fistula (Figure 1). An ultrasound of her arteriovenous fistula revealed a 4x2x2 cm aneurysm. On June 14, she was taken to the operating room for removal of the synthetic material and repair of the fistula. The graft material was submitted for AFB cultures on June 15 and became positive for AFB on July 6.
Figure 1.
The arteriovenous fistula aneurysm in the antecubital fossa of the patient's left arm
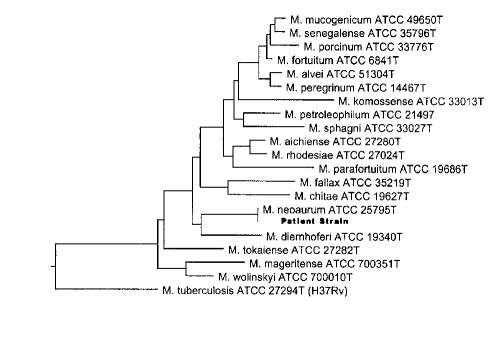
The organism was subcultured onto blood agar, chocolate agar, Lowenstein-Jensen media, Middlebrook 7H10 agar and 7H9-bovine albumin liquid media. Sequencing of the 16S rRNA gene for the identification of the organism (2) and standard biochemical tests for the characterization of mycobacteria were performed (3). Sensitivity testing of the organism was determined using E-test methodology. The organism grew at 25°C to 42°C and showed a bright orange pigment in both light and dark conditions. Relevant biochemical tests included a negative three-day and positive 14-day arylsulfatase test; positive nitrate reductase, urease, Tween 80 hydrolysis and iron uptake; tolerance to 5% sodium chloride; negative niacin; and no growth on MacConkey agar. Heat stable catalase activity was weak. Acid was produced from fructose, mannitol and inositol, but not sorbitol. While these biochemical test results did not conclusively identify our organism as M neoaurum, they were consistent with that identification. Partial 16S rRNA gene sequencing of this isolate from the initial blood cultures on June 12 and the tissue graft on July 12 displayed 100% identity to the type strain of M neoaurum ATCC 25795T sequenced at the National Reference Centre for Mycobacteriology (Figure 2). Standard methods were used for DNA extraction, 16S rRNA amplification and sequencing (3). E-test susceptibilities on June 12 revealed that the organism was sensitive to ciprofloxacin, doxycycline, rifampin and imipenem, and was resistant to isoniazid, ethambutol and clarithromycin.
Figure 2.
Phylogenetic tree based on the 5' region of the 16S rRNA gene (Escherichia coli bp 54-510) of the patient strain and its closest relatives. Sequences of all reference strains were determined at the National Reference Centre for Mycobacteriology, Health Canada. This tree was constructed using the Clustal method algorithm in the Megalign component of the Lasergene program version 4.01 (DNASTAR, Inc, USA). The tree was rooted using Mycobacterium tuberculosis, a slow-grower, as the out-group sequence. T Type strain
The patient was started on twice daily oral ciprofloxacin 250 mg and twice daily oral doxycycline 100 mg on June 13. A transthoracic echocardiogram, performed on June 12, showed no evidence of cardiac vegetations. She remained afebrile in hospital and was discharged home six days after starting antibiotics. She received a further three-week course of the same antibiotics following discharge. Physical examination after the completion of the course of antibiotics revealed no focal findings, and there was no recurrence of symptoms. Repeat blood cultures for AFB were negative on August 1. The patient has remained well following completion of the course of antimicrobial therapy.
DISCUSSION
Molecular-based methodologies are increasingly taking place in diagnostic laboratories. In mycobacteriology, species identification based on conventional biochemical tests alone are time consuming (from four to eight weeks) and are quite often inaccurate or nonconfirmatory. The use of commercial DNA probes for the detection of the most commonly isolated species of mycobacteria, which include Mycobacterium tuberculosis complex, Mycobacterium avium complex, Mycobacterium kansasii and Mycobacterium gordonae, has become routine in most mycobacteriology laboratories, because results are available in a few hours. However, there are more than 90 established mycobacterial species to date (4). Therefore, more advanced methods are necessary to accurately identify species. Some of the more widely used methods performed in reference laboratories for the identification of nontuberculous mycobacteria (NTM) species include 16S rRNA gene sequencing, polymerase chain reaction-restriction fragment length polymorphism of the hsp65 gene, and high performance liquid chromatography (HPLC). As a member of the M parafortuitum complex, M neoaurum is one of the rapidly growing scotochromogenic mycobacteria and is difficult to conclusively identify based on biochemical tests alone. Consequently, the 16S rRNA sequence analysis has been the most commonly reported method for the identification of this particular mycobacterium species. In the present report, definitive identification involved 16S rRNA analysis with subsequent sequence comparison with two quality controlled databases; the National Reference Centre for Mycobacteriology database (5) and the Ribosomal Differentiation of Microorganisms database (6).
A review of M neoaurum human infections reveals only seven reported cases in the English literature (Table 1) (7-12). Observations among all reports include the clinical presentation consisting of nonspecific symptoms. The patients ranged in age from nine to 62 years, three of the cases were women, and four of the seven patients were immunocompromised. Five patients had catheters (four intravascular, one peritoneal) in place, and, in the course of treatment, all but one of the catheters were removed. Six of the seven patients received antibiotic therapy, and all seven patients were cured. The characteristics of these cases suggest a low pathogenicity and only moderate morbidity with a M neoaurum infection. The presentation of disease in our patient was in keeping with the other reported cases. Our patient presented with weight loss, fevers and chills, with no localizing signs or symptoms on initial physical examination. The sensitivity profile of this strain was also consistent with those reported previously (7). The patient was treated using a combination of surgical and pharmacological therapy. Given the paucity of cases reported in the literature, there are no specific treatment guidelines for M neoaurum. However, both the American Thoracic Society and the National Committee for Clinical Laboratory Standards (NCCLS) recommend susceptibility testing of clinically significant isolates of rapidly growing mycobacteria, and subsequent treatment with the appropriate antimicrobial agent (13-15). There exists a lack of standardization of methodology for the performance of antimycobacterial susceptibility of most NTM species (16), and, therefore, large multicentre trials are required to establish good correlation between laboratory findings and patient outcomes. Select well-established NTM species have consistently shown predictable antimicrobial susceptibility patterns, while newly characterized or rare species lack such patterns, largely due to a lack of abundance of isolates. There have been successful cases reported with monotherapy for the treatment of the rapidly growing mycobacterium, but in many cases, dual therapy is required. In addition, guidelines suggest that the removal of foreign bodies (eg, catheters) is important for clinical recovery (13). While surgery alone may have resulted in a cure, we used a combination of surgical and pharmacological approaches to eliminate bacteremia and to resolve the graft infection.
Table 1.
Characteristics of Mycobacterium neoaurum infections
| Case | Reference | Year | Country | Age | Sex | Underlying medical disease | Immuno-compromised | Catheter | Reason for catheter | Total WCC/L | Antibiotics | Removal of catheter | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 1994 | Australia | 17 | M | ALL | Yes | Yes | BMT | 0.1x109 | Ticarcillin, clavulanate, tobramycin | Yes | Cure |
| 2 | 8 | 1988 | Australia | 53 | F | Cystoadeno-carcinoma | Yes | Yes | TPN | N/A | Cefoxitin, gentamicin | No | Cure |
| 3 | 9 | 2000 | USA | 54 | M | DM, hyper-tension, renal failure | Yes | Yes (peritoneal) | CAPD | 3.62x109 | Cefoxitin, ethambutol, rifampin, clarithromycin | Yes | Cure |
| 4 | 10 | 2000 | China | 9 | F | ALL | Yes | Yes | hemotherapy | 1.9x109 | Ceftazidime, amikacin | Yes | Cure |
| 5 | 11 | 1999 | USA | 46 | M | Primary pulmonary hypertension | No | Yes | Prostacycline infusion | 4.7x109 | None | Yes | Cure |
| 6 | 11 | N/S | USA | N/S | M | Intravenous drug user | No | No | N/A | N/S | Piperacillin, gentamicin | N/A | Cure |
| 7 | 12 | 2000 | Italy | 62 | F | Recurrent UTI | No | No | N/A | N/A | Vancomycin, streptomycin | N/A | Cure |
| 8 | Present article | 2001 | Canada | 40 | F | DM, renal failure | Yes | Yes | Hemodialysis | 11.2x109 | Ciprofloxacin, doxycycline | Yes | Cure |
ALL Acute lymphocytic leukemia; BMT Bone marrow transplant; CAPD Continuous ambulatory peritoneal dialysis; DM Diabetes mellitus; F Female; M Male; N/A Not available; NS Not stated; TPN Total parenteral nutrition; UTI Urinary tract infection; WCC/L White cell count per litre
The original source of the organism in our patient is not known. However, we speculate that transient bacteremia from a break in the skin or the gastrointestinal tract may have led to 'seeding' of the graft. This hypothesis is consistent with her clinical presentation and the ongoing bacteremia, suggestive of an endovascular infection.
M neoaurum is an environmental organism that was first isolated from soil in 1972 and has since been isolated from multiple other environmental sites, including dust and water (17). Many environmental species of mycobacterium have been described to cause infections in immunocompromised as well as immunocompetent hosts. In the context of immunocompromised hosts, it is important to consider atypical mycobacterial species as potential infecting organisms, noting that infection must be differentiated from colonization. If infection with a clinically significant mycobacterial species is suspected (eg, isolates from blood, sterile body fluids, tissues or multiple isolates from sputum) (15), accurate identification using 16S rRNA sequencing or HPLC by a reference laboratory should be performed. In addition, as recommended in the NCCLS guidelines (3,14,15), specific antimicrobial agents and concentrations should be tested against rapidly growing mycobacterium. This information is essential to guide optimal therapy.
Acknowledgments
The authors acknowledge Christine Turenne for her invaluable contribution to this paper.
References
- 1.Wallace RJ. Recent changes in taxonomy and disease manifestations of the rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis 1994;13:953-60. [DOI] [PubMed] [Google Scholar]
- 2.Kirschner P, Meier A, Böttger EC. Genotypic identification and detection of mycobacteria - facing novel and uncultured pathogens. In: Diagnostic Molecular Microbiology: Principles and Applications. Washington, DC: American Society for Microbiology, 1993:173-90. [Google Scholar]
- 3.Kent PT, Kubica GP. Public Health Mycobacteriology: A Guide for the Level III Laboratory. Atlanta: United States Department of Health and Human Services, Centers for Disease Control, 1985.
- 4.Euzéby JP. List of bacterial names with standing in nomenclature - genus Mycobacterium <www.bacterio.cict.fr/m/mycobacterium.html> Version current at January 8, 2002.
- 5.Turenne CY, Tschetter L, Wolfe J, Kabani A. Necessity of quality-controlled 16S rRNA gene sequence databases: Identifying nontuberculous Mycobacterium species. J Clin Microbiol 2001;39:3637-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmsen DJ, Rothgänger C, Singer C, Albert J, Frosch M. Intuitive hypertext-based molecular identification of micro-organisms. Lancet 1999;353:291. [DOI] [PubMed] [Google Scholar]
- 7.Holland DJ, Chen SC, Chew WW, Gilbert GL. Mycobacterium neoaurum infection of a Hickman Catheter in an immunosuppressed patient. Clin Infect Dis 1994;18:1002-3. [DOI] [PubMed] [Google Scholar]
- 8.Davison MB, McCormack JG, Blacklock ZM, Dawson DJ, Tilse MH, Crimmins FB. Bactermia caused by Mycobacterium neoaurum J Clin Microbiol 1988;26:762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNally CF, Mangino JE. Mycobacterium neoaurum: a case report and review of the literature. Infect Dis Clin Prac 2000;9:273-5. [Google Scholar]
- 10.Woo PC, Tsoi H, Leung K, et al. Identification of Mycobacterium neoaurum isolated from a neutropenic patient with catheter related bacteremia by 16S rRNA sequencing. J Clin Microbiol 2000;38:3515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George SL, Schlesinger LS. Mycobacterium neoaurum - an unusual cause of infection of vascular catheters: case report and review. Clin Infect Dis 1999,28:682-3. [DOI] [PubMed] [Google Scholar]
- 12.Zanetti S, Faedda S, Fadda R, et al. Isolation and identification of Mycobacterium neoaurum from a patient with a urinary tract infection. New Microbiologica 2001;24:189-92. [PubMed] [Google Scholar]
- 13.Wallace RJ, Glassroth J, Griffith DE, Olivier KN, Cook JL, Gordin F. American Thoracic Society - diagnosis and treatment of disease caused by non-tuberculous mycobacteria. Am J Respir Crit Care Med 1997,156:S1-25. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Antimycobacterial susceptibility testing for mycobacteria, Nocardia, and other aerobic actinomycetes, 2nd edn. Tentative standard M24-T2. Wayne: National Committee for Clinical Laboratory Standards, 2000. [PubMed]
- 15.Woods GL. Susceptibility testing for Mycobacteria Clin Infect Dis 2000,31:1209-15. [DOI] [PubMed] [Google Scholar]
- 16.Salfinger M. Susceptibility testing for nontuberculous Mycobacteria: Should it be performed? Clin Microbiol News 1997,19:68-71. [Google Scholar]
- 17.Tsukamura M. A new species of rapidly growing, scotochromogenic mycobacteria, Mycobacterium neoaurum Med Biol (Tokyo), 1972;85:229-33. [Google Scholar]