Abstract
BACKGROUND:
A multinational randomized controlled trial has shown a trend toward early discharge of patients taking oral linezolid versus intravenous vancomycin (IV) in the treatment of methicillinresistant Staphylococcus aureus (MRSA) infections. Infection treatments resulting in shorter hospitalization durations are associated with cost savings from the hospital perspective.
OBJECTIVE:
To determine whether similar economic advantages are associated with oral linezolid, the costs and consequences of linezolid use following vancomycin IV versus the existing practice in the treatment of infections caused by MRSA were compared.
METHODS:
The charts of all patients admitted to one of three tertiary care teaching hospitals between January 1, 1997 and August 31, 2000 and treated with vancomycin IV for an active MRSA infection (skin and soft tissue only) were reviewed. Based on the vancomycin IV chart review data set and a simulated linezolid data set, the clinical consequences and the associated costs of MRSA treatment with vancomycin IV, and oral and IV forms of linezolid were quantified and compared within the framework of a cost-consequence analysis.
RESULTS:
Patients treated with oral and IV forms of linezolid compared with the existing practice had a shorter length of stay and required fewer home IV care services, which resulted in a cost savings of $750 (2001 values) to the Canadian health care perspective.
CONCLUSIONS:
The estimated cost savings associated with linezolid use not only offset the higher acquisition cost of the anti-infective, but may be substantial to health care systems across Canada, especially as the incidence of MRSA continues to rise.
Key Words: Consequences, Costs, Linezolid, Methicillin-resistant Staphylococcus aureus, MRSA, Vancomycin
Antibiotic resistance is widely acknowledged to be a serious global threat to the treatment of infectious diseases. The morbidity, mortality and economic burden of infections caused by drug-resistant organisms, for which there are limited therapeutic options, pose an increasing burden to health care systems worldwide. In a recent report by the World Health Organization (1), it was estimated that as many as 60% of hospitalized infections in the industrialized world are caused by drug-resistant microbes.
One of the most frequently encountered antibiotic-resistant organisms is methicillin-resistant Staphylococcus aureus (MRSA), an organism known to be associated with considerable morbidity and mortality in infected patients (2,3). In the late 1960s and 1970s, the prevalence of MRSA was less than 5% in most hospital settings worldwide, but increased in the 1990s to as high as 40% in several hospitals in the United States and Europe (4,5). Although the emergence of MRSA in Canada has been slower than in the United States, with the first isolate not being reported until 1981 (6), the pathogen is now commonly encountered in Canadian health care facilities (7). The Canadian Nosocomial Infection Surveillance Program (CNISP) has reported an increase in MRSA incidence from 1% (a mean of 0.46 cases per 1000 admissions) in 1995 to approximately 8% (5.3 cases per 1000 admissions) in 2000 (8). Although MRSA has primarily been recognized as a hospital-acquired pathogen, the first report (7) of community-acquired MRSA in Canada occurred in 1990, and additional cases have since been reported (9-12).
As the incidence of MRSA continues to rise, so will its economic impact. Treatment of infections caused by antimicrobialresistant pathogens is costly due to the need for prolonged hospitalizations requiring isolation or barrier precautions, the need for expensive therapeutic agents, and increased laboratory use for extensive surveillance or screening (13). A study conducted at the Vancouver General Hospital and Health Sciences Centre (14) found that the costs attributable to MRSA were approximately $8,400 per case in 1999. Similar results were found at a Toronto hospital, where the total attributable cost to treat MRSA infections between 1996 and 1998 was $14,360 per patient, and the cost for isolation and management of colonized patients was $1,363 (13). Considering the costs of managing MRSA-infected and -colonized patients, and for screening of high-risk patients, the investigators projected the total costs associated with MRSA in Canadian hospitals to be at least $42 to $59 million per annum (13).
Linezolid, the first of a novel class of oxazolidinone antibiotics, represents a new option for the treatment of MRSA infections. Linezolid has in vitro and in vivo activity against Gram-positive organisms (15) and antibacterial activity against staphylococci similar to that of vancomycin, the usual treatment option for MRSA infections (16). Clinical trials comparing linezolid and vancomycin demonstrated that the two antibiotics have similar clinical efficacy in the treatment of patients with hospital-acquired pneumonia and infections caused by methicillin-resistant Staphylococcus species (17,18). However, a major advantage of linezolid over vancomycin is a 100% bioavailable oral formulation which showed a trend toward earlier discharge of patients (18) versus intravenous (IV) vancomycin in a multinational randomized clinical trial. Infection treatments resulting in shorter hospitalization durations are associated with cost savings (19-22) from the hospital perspective. To determine whether similar economic advantages are associated with linezolid, we undertook this study to compare the costs and consequences of using linezolid following vancomycin IV versus the existing practice in the treatment of infections caused by MRSA.
METHODS
The study involved a multistage process that included a chart review to determine current MRSA treatment patterns, a simulation exercise to predict the outcomes of linezolid use, and a cost analysis to examine resources associated with each treatment group for use in a cost-consequence analysis.
Chart review of MRSA treatment patterns
Details regarding the methodology and results of the chart review have been previously reported (23). In summary, the charts of all patients admitted to one of three tertiary care teaching hospitals (Vancouver Hospital and Health Sciences Centre, Vancouver, British Columbia; The University Health Network, Toronto, Ontario; and Hôpital Maisonneuve-Rosemont, Montreal, Quebec) between January 1, 1997 and August 31, 2000 and treated with vancomycin IV for an active MRSA infection were reviewed. MRSA infections were limited to those of the skin and soft tissue (SSTI).
For each eligible patient, data collection began on the first day of treatment for the infection and continued until the infection was successfully treated or until the patient died, whichever occurred first. Parameters collected included basic demographics, length of hospitalization, antimicrobial treatment use, rate of switch therapy and use of home IV care services. In addition, based on the common guidelines for switch therapy used at the participating sites (which generally consider patients' ability to tolerate oral or nasogastric nutrition and the level of improvement of the infection [24,25]), the study coordinator determined a date on which each patient met all criteria for switch therapy. For patients who met all criteria, it was determined whether the patient was switched to an oral antimicrobial and, if not; a reason was provided explaining why the patient was not switched. Treatment patterns and resource use associated with MRSA infections for the entire vancomycin-treated group were determined.
Trial simulation
Because the chart review data were collected before linezolid became available in Canada, rigorous steps were taken to simulate a data set for patients eligible for treatment with linezolid to predict the resource outcomes of linezolid use. Linezolid IV was restricted to second-line use in patients intolerant to vancomycin IV (eg, patients who switched from vancomycin IV to another anti-infective due to vancomycin hypersensitivity, or hepatic or renal impairment), while the oral form of linezolid was limited to use in step-down therapy following treatment with vancomycin IV. Given these definitions for linezolid use, patients enrolled in the chart review were divided based on their tolerance or intolerance to vancomycin IV, and the patient-level data for each group were subjected to a series of treatment and early discharge algorithms to develop a data set for a linezolid-treated group.
For patients intolerant to vancomycin IV, the following assumptions were used to predict their new treatment sequences:
Patients were switched from vancomycin IV to linezolid IV on the date of their first dose adjustment due to vancomycin IV intolerance;
Patients on linezolid IV were eligible for a switch to oral linezolid on the date that the criteria for switch therapy were met;
All patients remained on linezolid for the duration of the original treatment course assuming the recommended dosing (600 mg every 12 h);
The same level of home IV care received by a patient discharged on vancomycin IV was assumed if the patient was discharged on linezolid IV; and
Patients discharged on oral linezolid would not require home IV care services.
Patients tolerant to vancomycin IV were subjected to a similar algorithm to determine which patients were eligible for a switch to oral linezolid and the date on which the switch would occur. Each patient's treatment sequence was determined based on the following assumptions:
All patients who switched to an oral therapy were eligible to receive oral linezolid on the date they switched to that therapy or on the date they met the criteria for switch therapy, whichever occurred first;
All patients who did not switch from vancomycin IV to an oral therapy were eligible to be switched to oral linezolid on the date the switch therapy criteria were met and if their reason for not switching did not preclude a switch (eg, 'no suitable oral anti-infective was available' or 'a suitable oral anti-infective was available, but was not prescribed');
Patients who switched remained on oral linezolid (600 mg every 12 h) for the duration of the original treatment course; and
Home IV care services were not required for patients discharged on oral linezolid.
Following the identification of patients eligible to receive linezolid and the assignment of new treatment dates, the next step of the trial simulation was to determine if patients switched to oral linezolid were eligible for earlier discharge and, if so, the date on which they would have been discharged. To receive an earlier discharge date, patients must have been switched to oral linezolid, must not have died during their hospitalization, and must have originally completed their IV anti-infective treatment component either postdischarge or within two days before their original discharge date. The final assumption was based on the hypothesis that patients who were discharged during or shortly after the completion of their IV anti-infective treatment remained in hospital only to receive their IV therapy (versus treatment for comorbidities) and, therefore, would have been discharged earlier if an oral agent had been prescribed. The cut-off point of two days or less was determined based on an analysis of the average length of stay following the completion of vancomycin IV therapy until discharge in patients in the original data set who switched to an oral agent.
Each patient who qualified for early discharge was assigned a new discharge date of two days following the date of switch from vancomycin IV to oral linezolid. A period of two days was determined to be a reasonable and conservative length of time for clinical observation of a patient who switched to an oral medication before discharge. Because each of these patients met the clinical criteria for switch therapy, their level of medical stability had been established. In the event that the addition of a two-day observation period extended the patient's stay beyond their original discharge date, the original discharge date was assumed.
Cost-consequence analysis
Based on the vancomycin IV chart review data set and the simulated linezolid data set the clinical consequences and the associated costs of MRSA treatment with vancomycin IV and linezolid were quantified and compared within the framework of a costconsequence analysis. Consequences were analyzed by treatment group and statistical methods were used to test for differences between groups. Consequences considered in the analysis included the average length of hospitalization (using Kaplan-Meier survival analysis and a median test for differences using the Wilcoxon rank sum test) from the first day of vancomycin IV therapy until death or discharge, the per cent of patients discharged from hospital by day 7, 14, 21, 28 and 56 (χ2 test with continuity correction for differences), and the mean days of IV or oral therapy (Student's t test for differences).
Costing of resource utilization was conducted from the provincial health care perspective for Ontario, from which costing data were most accessible to the investigators. In Ontario, health insurance coverage is universal for a common set of health care services, and is funded and administered by the Ministry of Health and Long-term Care (MOHLTC). The analysis considered the direct costs of MRSA treatment between the first day of vancomycin IV treatment until the infection was successfully treated or death. Within this time frame, costs were assigned to days spent in hospital while receiving anti-infective treatment, professional fees within the hospital setting and home care visits specifically for the administration of IV antimicrobials. In addition, the costs of anti-infectives reimbursed by the MOHLTC were considered, including all anti-infective treatments received in hospital, all IV anti-infectives received on an outpatient basis, and reimbursable oral agents received out of hospital by patients eligible for coverage by the provincial drug benefits program.
Unit prices of resources were obtained from the most recently available sources for Ontario and were assumed to be reflective of 2000/2001 Canadian dollars. The price of vancomycin IV prescribed in and out of hospital was $15.00/1 g (26) and $57.89/1 g (MOHLTC, 2001, personal communication), respectively. Because hospital pricing of linezolid IV and oral was not available, the out of hospital prices of $95.51/600 mg and $70.64/600 mg, respectively, were used. Other anti-infectives received in hospital were priced according to a Toronto hospital formulary (26). Those not listed on the hospital formulary were assigned prices from the Ontario Drug Benefit (ODB) Formulary (27). Anti-infectives prescribed out of hospital were priced according to the best available price for drugs listed on the ODB Formulary. A 10% markup and appropriate pharmacy dispensing fees were added (27) to the cost of these medications.
The daily cost of hospitalization was derived from a recently reported study of MRSA costs from a Canadian perspective (13). Although the average hospitalization cost reported was $1,026 per day, consideration of only patients with SSTIs resulted in a cost of $417 per day. Because this cost was fully allocated and included the cost of antimicrobials, 4% of this cost (representing the proportion of the total per diem represented by antimicrobials [13]) was removed, allowing for the inclusion of patient-specific study medication costs without double-counting the cost of this resource. The resultant hospitalization per diem used in the present study was $401. Physician fees for consultations with an internal medicine physician were added to the hospitalization cost according to the Ontario Schedule of Benefits for Physician Services (28). Home IV care visits were valued using a fully allocated cost of $121 (29).
The costs of tests, procedures, and drug preparation and administration were not considered as separate resource items, because their costs were included in the fully allocated average cost of hospitalization. Total costs were determined on a per patient basis by assigning unit costs to resources consumed, and comparisons between the two treatment groups were made using the Student's t test.
Sensitivity analyses
The sensitivity of the results of the cost-consequence analysis was explored by varying several key assumptions in one-way sensitivity analyses. The first sensitivity analysis explored the uncertainty in criteria used in the trial simulation to identify patients eligible for a switch to oral linezolid. In the base case analysis, patients who met the criteria for switch therapy were switched to oral linezolid if their reason for not switching included either 'no suitable oral anti-infective was available (n=36)' or 'a suitable oral anti-infective was available, but was not prescribed (n=7)'. As a less aggressive estimate of switch therapy eligibility, patients who cited the latter reason for not switching were excluded from consideration for a switch to oral linezolid. A second sensitivity analysis explored the impact of the early discharge algorithm. As the most conservative estimate, all patients were considered ineligible for earlier discharge; hence, all original discharge dates were used.
Several costing assumptions were also tested in one-way sensitivity analyses. First, because hospitalization was the major cost driver in the analysis and the cost estimate was based on a relatively small sample size, the per diem was increased to $1,002, the value reported in a previous study (13) (excluding the cost of anti-infectives) for all types of MRSA infections. Next, although the hospital anti-infective costs were obtained from a reliable and recent source (26), it is probable that anti-infective costs vary by institution due to contract negotiations with suppliers. Therefore, the cost of all anti-infectives received by patients in hospital were increased and then decreased by 20%.
RESULTS
In total the charts of 89 patients treated with vancomycin IV for an MRSA infection were reviewed for the study (23). The demographic and clinical characteristics of patients are shown in Table 1. The mean age was 62 ±15.7 years and 70.8% of the patients were male. A very large proportion of patients underwent surgery in the six-month period before the MRSA infection and the most common type of SSTI reported was an infected surgical site incision. The treatment sequences and treatment termination locations of patients in the original vancomycin IV group are described in the top panel of (Figure 1).
TABLE 1.
Patient characteristics (n=89)
| Mean age in years | 62.16 (range 16-89) |
| Males, % | 70.8 |
| Surgery in previous six months before infection, % | 82.0 |
| Deaths during hospitalization, % | 18.0 |
| Type of methicillin-resistant Staphylococcus aureus infection, % | |
| Infected surgical incision | 62.9 |
| Infected skin ulcer | 12.4 |
| Skin abscess | 10.1 |
| Infected diabetic foot ulcer | 4.5 |
| Cellulitis | 3.4 |
| Other | 6.7 |
Figure 1.
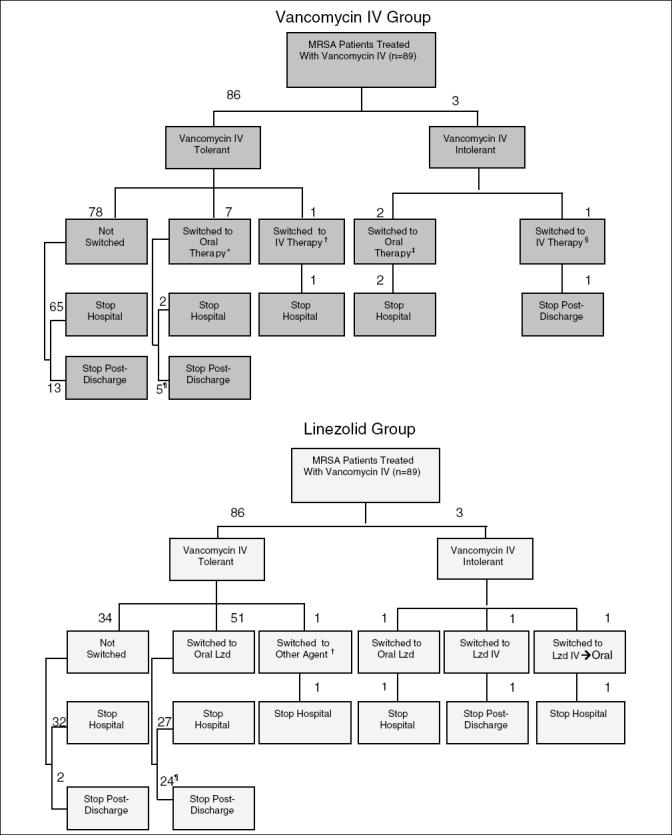
Comparison of treatment sequences and termination locations for patients in the vancomycin intravenous (IV) and linezolid (Lzd) groups. *Therapies included amoxicillin-clavulanic acid, doxycycline, ciprofloxacin, ciprofloxacin plus clindamycin, clindamycin, metronidazole, trimethoprimsulfamethoxazole; †Cefazolin; ‡Ciprofloxacin, metronidazole; §Imipenem; ¶One patient was discharged on vancomycin IV, but switched to oral therapy postdischarge. MRSA Methicillin-resistant Staphylococcus aureus
Following the execution of the treatment and early discharge algorithms on the vancomycin IV group, the predicted treatment sequences of patients in the linezolid group are shown in the bottom panel of Figure 1. As noted in the schematic, 54 of 89 patients (61%) qualified for treatment with linezolid. Compared with the vancomycin IV group, where seven tolerant patients switched to an oral anti-infective, the simulation exercise resulted in 51 patients switching to an oral therapy (eg, oral linezolid). In addition, eight extra patients in the linezolid group were discharged to complete their treatment out of hospital, while a total of 22 patients became eligible for earlier discharge.
The clinical consequences of each treatment are shown in Table 2. The median length of stay was 16 days for patients in the linezolid group compared with 17 days for those in the vancomycin IV group. There were also differences noted in the proportion of patients discharged by day 7, 14, 21 and 28. Approximately 9%, 8%, 5% and 1% more patients in the linezolid group were discharged by these time periods, respectively. Compared with the vancomycin IV group, the mean number of IV treatment days decreased over seven days (P=0.01), because both the increased number of patients switching to an oral agent and the assignment of earlier switch therapy dates to five patients who had originally switched to an oral anti-infective. Because the algorithms applied to the linezolid group did not affect the duration of therapy, it can be noted that the total number of anti-infective treatment days are the same in each group (values differ slightly due to rounding).
TABLE 2.
Base case treatment consequences
| End point | Linezolid group | Vancomycin IV group |
|---|---|---|
| Length of stay (days) | ||
| Median† (95% CI) | 16.0 (11-21) | 17.0 (15-25) |
| Mean† (SE) | 21.4 (1.8) | 23.4 (1.7) |
| % discharged by: | ||
| Day 7 | 29.2 | 20.2 |
| Day 14 | 42.7 | 34.8 |
| Day 21 | 60.7 | 56.2 |
| Day 28 | 67.4 | 66.3 |
| Day 56 | 86.5 | 86.5 |
| Duration of treatment (days) | ||
| IV only | ||
| Mean (SE) | 12.3 (1.9)* | 19.7 (2.1)* |
| Median | 7.0** | 14.0** |
| IV and oral | ||
| Mean (SE) | 21.1 (2.2) | 20.8 (2.2) |
| Median | 15.5 | 16.0 |
Estimated using Kaplan-Meier survival function
P=0.01;
P=0.0002. IV Intravenous
(Table 3) displays the total cost to the health care perspective associated with each treatment group, disaggregated by cost component with totals provided for hospital and nonhospital costs. The total cost of MRSA treatment was approximately $750 per patient higher in the vancomycin IV group (eg, $8,444/patient versus $7,693/patient). Because hospitalization represented over 80% of the total cost of MRSA treatment, the shorter length of stay of one day for patients in the linezolid group was primarily responsible for this difference, although the lower costs of outpatient IV anti-infectives and home care were also important factors. It is important to note that, although not apparent from the results in Table 3, the discharge of patients on oral linezolid versus vancomycin IV resulted in a shift of some of the costs (approximately $360) of outpatient oral anti-infectives from the MOHLTC to patients not receiving provincial formulary benefits; it was not possible to estimate the proportions of this cost that would be covered by private insurance versus out-of-pocket by patients. However, even after considering this additional cost to non- ODB patients, the total cost of MRSA treatment remained lower in the linezolid group.
TABLE 3.
Base case analysis of per patient methicillin-resistant Staphylococcus aureus infection-related costs
| Cost component | Linezolid group† | Vancomycin IV group† |
|---|---|---|
| Hospital costs | ||
| Hospitalization | $6,191 ($812) | $6,979 ($806) |
| Professional fees | $325 ($23) | $358 ($23) |
| Anti-infectives | $984 ($144) | $509 ($62)* |
| Total hospital cost | $7,499 ($940) | $7,846 ($888) |
| Nonhospital costs | ||
| Intravenous anti-infectives | $74 ($44) | $370 ($105) |
| Oral anti-infectives | $378 ($149) | 0** |
| Home IV care services | $12 ($9) | $227 ($89) |
| Total nonhospital cost | 464 ($154) | $597 ($173) |
| Total cost to MOHLTC | $7,693 ($937) | $8,444 ($907) |
Costs expressed as cost per patient (SE);
P=0.005;
P=0.04. IV Intravenous; MOHLTC Ministry of Health and Long-term Care
The cost results from the perspective of the MOHLTC of the various one-way sensitivity analyses have been summarized in Table 4. It can be noted that the trend observed in the total base case costs reversed in only one scenario (eg, the elimination of early discharge eligibility), making the cost of MRSA treatment in the vancomycin IV group less costly from the health care perspective. The analysis was also sensitive to the cost of hospitalization which, when raised to approximately 2.5 times the base case, increased the cost savings associated with linezolid to over $1,100 per patient.
TABLE 4.
Sensitivity analysis results
| Linezolid group* | Vancomycin IV Group* | |||||
|---|---|---|---|---|---|---|
| Sensititvity analysis | Hospital cost | Nonhospital cost | Total cost | Hospital cost | Nonhospital cost | Total cost |
| Conservative switch therapy eligibility for linezolid group | $7,694 | $442 | $8,136 | $7,846 | $597 | $8,444 |
| No early discharge eligibility for linezolid patients | $8,462 | $316 | $8,778 | $7,846 | $597 | $8,444 |
| Increased hospitalization cost ($1,002/day) | $17,326 | $442 | $17,768 | $18,324 | $597 | $18,921 |
| Decreased hospital anti-infective costs (-20%) | $7,505 | $442 | $7,947 | $7,744 | $597 | $8,342 |
| Increased hospital anti-infective costs (20%) | $7,882 | $442 | $8,325 | $7,948 | $597 | $8,545 |
Costs expressed as cost per patient. IV Intravenous
DISCUSSION
The present study has compiled information obtained from a retrospective observational study and a trial simulation exercise to estimate the costs and consequences of linezolid use in the treatment of MRSA infections from a Canadian perspective. Our basic hypothesis was that even though the acquisition cost of linezolid is higher than vancomycin IV, the benefits of the availability of an oral form of linezolid would result in cost reductions that may, in part, offset its higher cost. The costs and consequences that we have explored in our analysis included those relevant to both the hospital and nonhospital settings because the treatment of MRSA occurs both in and out of hospital.
Our analysis of the consequences of treatment with linezolid versus vancomycin IV revealed trends toward the earlier discharge of patients who switched to oral linezolid. Although the differences observed in our base case analysis were not statistically significant, the median length of stay for patients in the linezolid group was one day shorter than for patients in the vancomycin IV group. More notably, patients in the linezolid-treated group tended to be discharged from hospital earlier, with differences in the cumulative discharge rates of 9.0%, 7.9%, 4.5% and 1.1% at weeks 1 through 4, respectively. This trend in discharge rates suggests that, from an economic perspective, the patients who will benefit most from oral linezolid treatment are those whose primary reason for being hospitalized is the serious infection as opposed to other comorbid illnesses that may preclude an earlier discharge.
It is interesting to note that the trends observed in the current study are very similar to those found in a recently conducted multinational randomized control trial (18) comparing vancomycin IV and linezolid in patients with MRS species infections, although the magnitude of the effects are somewhat different (see (Table 5) [18] versus Table 2). In the randomized control trial, the median length of stay for patients in the linezolid arm was five days shorter than for patients randomized to vancomycin IV. This difference suggests that our criteria for early discharge may have been conservative. Alternatively, this variation may also be reflective of differences in real-world treatment patterns in Canada, a hypothesis that is supported by the observation that the median lengths of stay were three to seven days shorter in the randomized clinical trial than in our analysis.
TABLE 5.
Validation of consequences against a randomized clinical trial (18)
| End point | Linezolid† (n=122) | Vancomycin IV† (n=108) |
|---|---|---|
| Length of stay (days) | ||
| Median‡ (95% CI) | 9 (8-16)* | 14 (12-18)* |
| Mean‡ (SE) | 17.2 (1.4) | 19.4 (1.5) |
| % discharged by | ||
| Day 7 | 36.1** | 16.7** |
| Day 14 | 54.1 | 46.3 |
| Day 21 | 62.3 | 64.8 |
| Day 28 | 75.4 | 75.9 |
| Day 56 | 87.7 | 89.8 |
| Duration of treatment (days) | ||
| Intravenous only | ||
| Mean | 5.8*** | 12.6*** |
| Median | 4.0*** | 11.0*** |
| Intravenous and oral | ||
| Mean | 14.2 | 12.6 |
| Median | 14.0 | 11.0 |
Results shown are for the skin and soft tissue subgroup;
Estimated using Kaplan-Meier survival function.
P=0.052;
P=0.001;
P=0.0001. IV Intravenous
The cost results of our base case analysis found that the decreased length of stay observed in the linezolid group contributed to cost savings of approximately $750 per patient. Although this difference between treatment groups was not statistically significant, it nevertheless may be important. When this result is extrapolated to all patients with an MRSA SSTI across Ontario (30), the cost savings to the MOHLTC may be in excess of $1.1 million per year. Several previous studies (22,31-33) also demonstrated that early switch strategies have resulted in reduced lengths of stay and that shorter hospital durations (ranging from 1 to 2.4 fewer days) translated into cost savings (19,20-22). In only one sensitivity analysis, the elimination of early discharge eligibility for linezolid patients, did the results of the analysis favour vancomycin IV over linezolid. However, because the benefits of switch therapy in terms of decreasing length of stay have been well established, it is highly unlikely that patients treated with oral linezolid would be ineligible for earlier discharge.
Because of the retrospective nature of our study, it was not possible to collect data on the consequences of each treatment related to patient satisfaction and quality of life. However, the benefits of oral therapy have been well documented in Canadian hospitals (24,25,34,35), and include important factors such as reduced risk of intravenous-related adverse events and improved patient mobility. In addition, the resultant ability to discharge patients earlier allows patients to complete treatment in more comfortable home surroundings and, perhaps more importantly, reduces exposure time to other nosocomial pathogens - the significance of which is highlighted when recollecting the gravity of the recent severe acute respiratory syndrome outbreak in Canadian hospitals.
We recognize the limitations of our study, including the fact that the data set for the linezolid group were simulated based on observational data collected on vancomycin IV-treated patients. Furthermore, we acknowledge that the early discharge benefits observed for patients treated with linezolid rely on several key assumptions involving both the switch therapy and discharge algorithms. An important assumption of the switch therapy algorithm was the specification that patients who met the eligibility criteria for switch therapy were switched to oral therapy on the same day that the clinical criteria were met. For similar benefits to be realized in actual clinical practice, continuing efforts by all health care providers involved in patient care, including hospital pharmacists, nurses and physicians, will be required to ensure that switching occurs in a similar timely manner. Increased familiarity with switch therapy guidelines and the clinical and economic benefits associated with the practice, in addition to heightened awareness of the various oral pharmaceutical options available will help to ensure such efficiencies.
In the absence of actual data collected in an uncontrolled environment for Canadian patients treated with linezolid, we believe our study provides a reasonable estimate of the costs and consequences associated with linezolid use in the treatment of MRSA infections in Canada. Our data were subjected to a set of comprehensive algorithms that were based on 'real-life' clinical experience and conservative assumptions were used throughout the study to limit the introduction of bias into our results, with the impact of several of our assumptions tested in sensitivity analyses. Further research will be required following the introduction of linezolid to confirm these findings.
Acknowledgments
Funding for this study was provided by Pharmacia Canada, Mississauga (now Pfizer Canada Inc, Montreal). The authors wish to thank the project advisors, Dr Grant Stiver (Vancouver Hospital and Health Sciences Centre, Vancouver, British Columbia), Dr Karl Weiss (Hôpital Maisonneuve-Rosemont, Montreal, Quebec), Dr Walter Schlech III (Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia) and Dr Tom Louie (Foothills Medical Centre, Calgary, Alberta) for their contributions to the study design and chart review execution. We would also like to thank Mr Bob Lan (Innovus Research Inc, Burlington, Ontario) for programming all cost analyses.
References
- 1.World Health Organization. Overcoming Antimicrobial Resistance World Health Report on Infectious Diseases 2000 <www.who.int/infectious-disease-report/2000/> (Version current at July 1, 2004).
- 2.Romero-Vivas J, Rubio M, Fernandez C, Picazo JJ. Mortality associated with nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus.Clin Infect Dis 1995;21:1417-23. [DOI] [PubMed] [Google Scholar]
- 3.Holmberg SD, Solomon SL, Blake PA. Health and economic impacts of antimicrobial resistance.Rev Infect Dis 1987;9:1065-78. [DOI] [PubMed] [Google Scholar]
- 4.Panlilio AL, Culver DH, Gaynes RP, et al. Methicillin-resistant Staphylococcus aureus in US hospitals 1975-1991.Infect Control Hosp Epidemiol 1992;13:582-6. [DOI] [PubMed] [Google Scholar]
- 5.Voss A, Milatovic D, Wallrauch-Schwarz C, Rosdahl VT, Braveny I. Methicillin-resistant Staphylococcus aureus in Europe.Eur J Clin Microbiol Infect Dis 1994;13:50-5. [DOI] [PubMed] [Google Scholar]
- 6.Low DE, Garcia M, Callery S. Methicillin-resistant Staphylococcus aureus - Ontario.Can Dis Wkly Rep 1981;7:249-50. [Google Scholar]
- 7.Conly J, Johnston BL. Antibiotic resistance in Canada at the dawn of the new millennium - a model for the developed world?Can J Infect Dis 2000;11:232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conly J. Antimicrobial resistance in Canada.CMAJ 2002;167:885-91. [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor G, Kirkland T, Kowalewska-Grochowska K, Wang Y. A multistrain cluster of methicillin-resistant Staphylococcus aureus based in a native community.Can J Infect Dis 1990;1:121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Embil J, Ramotar K, Romance R, et al. Methicillin-resistant Staphylococcus aureus in tertiary care institutions on the Canadian prairies 1990-1992.Infect Control Hosp Epidemiol 1994;15:646-51 [DOI] [PubMed] [Google Scholar]
- 11.Berlet G, Richards RS, Roth JH. Clenched-fist injury complicated by methicillin-resistant Staphylococcus aureus.Can J Surg 1997;40:313-4. [PMC free article] [PubMed] [Google Scholar]
- 12.Gardam MA. Is methicillin-resistant Staphylococcus aureus an emerging community pathogen? A review of the literature.Can J Infect Dis 2000;11:202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim T, Oh P, Simor AE. The economic impact of methicillin-resistant Staphylococcus aureus in Canadian hospitals.Infect Control Hosp Epidemiol 2001;22:99-104. [DOI] [PubMed] [Google Scholar]
- 14.Bryce EA, Kerschbaumer V. The cost of doing business - managing MRSA and VRE. 4th Decennial International Conference on Nosocomial and Healthcare-associated Infections.Infect Control Hosp Epidemiol 2000;21:119(Abst SM5-05) [Google Scholar]
- 15.Ford CW, Hamel JC, Stapert D, et al. Oxazolidinones: New antibacterial agents.Trends Microbiol 1997;5:196-200. [DOI] [PubMed] [Google Scholar]
- 16.Devlin HR. Bacteria for the nineties.Ostomy Wound Manage 1998;44:32-40. [Google Scholar]
- 17.Rubinstein E, Cammarata SK, Iliphant TH, Wunderink RG. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: A randomized, double-blind, multicenter study.Clin Infect Dis 2001;32:402-12. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Willke RJ, Pinto LA, et al. Comparison of length of hospital stay for patients with known or suspected methicillin-resistant Staphylococcus species infections treated with linezolid or vancomycin: A randomized, multicenter trial.Pharmacotherapy 2001;21:263-74. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich ES, Joseph U, Vogel F, et al. Cost-effectiveness of ceftriaxone 1 g vs second-generation cephalosporins in the treatment of pneumonia in general medical wards in Germany.Infection 1999;27:148-54. [DOI] [PubMed] [Google Scholar]
- 20.Rittenhouse BE, Stinnett AA, Dulisse B, et al. An economic evaluation of levofloxacin versus cefuroxime axetil in the outpatient treatment of adults with community-acquired pneumonia.Am J Manag Care 2000;6:381-9 [PubMed] [Google Scholar]
- 21.Dresser LD, Niederman MS, Paladino JA. Cost-effectiveness of gatifloxacin versus ceftriaxone with a macrolide for the treatment of community-acquired pneumonia.Chest 2001;119:1439-48. [DOI] [PubMed] [Google Scholar]
- 22.Omidvari K, de Boisblanc BP, Karam G, Nelson S, Haponik E, Summer W. Early transition to oral antibiotic therapy for community-acquired pneumonia: Duration of therapy, clinical outcomes and cost analysis.Respir Med 1998;92:1032-9. [DOI] [PubMed] [Google Scholar]
- 23.Conly JM, Stiver GH, Weiss KA, Becker DL, Rosner AJ, Miller E. A retrospective analysis of practice patterns in the treatment of methicillin-resistant Staphylococcus aureus skin and soft tissue infections at three Canadian tertiary care centers.Can J Infect Dis 2003;14:315-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jewesson P. Cost-effectiveness and value of an IV switch.Pharmacoeconomics 1994;5(Suppl 2):20-6. [DOI] [PubMed] [Google Scholar]
- 25.Zamin MT, Monique MP, Conly JM. Development of an intravenous-to-oral route conversion program for antimicrobial therapy at a Canadian tertiary care health facility.Ann Pharmacother 1997;31:564-70. [DOI] [PubMed] [Google Scholar]
- 26.Sunnybrook and Women's College Health Sciences Centre.Formulary and Drug Use Guidelines.Toronto, 1997. [Google Scholar]
- 27.Ontario Ministry of Health and Long-term Care. Ontario Drug Benefit Formulary,Comparative Drug Index No. 37. 2001.
- 28.Ontario Schedule of Benefits.Physician Services under the Health Insurance Act. 2000.
- 29.Hamilton-Wentworth District Health Council.Inventory of Longterm Care Services. 2000.
- 30.Fleming CA, Green K, Richardson H, et al. Evolution of antimicrobial resistance in nosocomial gram positive pathogens in Ontario, Canada. Conjoint Meeting of CACMID, CHICA, and CIDS. 2001:52. (Abst).(Version current at July 23, 2004). [Google Scholar]
- 31.Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin.JAMA 2000;283:749-55. [DOI] [PubMed] [Google Scholar]
- 32.Hendrickson JR, North DS. Pharmacoeconomic benefit of antibiotic step-down therapy: Converting patients from intravenous ceftriaxone to oral cefpodoxime proxetil.Ann Pharmacother 1995;29:561-5. [DOI] [PubMed] [Google Scholar]
- 33.Siegal RE, Halpern NA, Almonoff PL, Lee A, Cashin R, Greene JG. A prospective randomized study of inpatient iv. antibiotics for community-acquired pneumonia. The optimal duration of therapy.Chest 1996;110:965-71. [DOI] [PubMed] [Google Scholar]
- 34.Frighetto L, Nickoloff D, Martinusen SM, Mamdani FS, Jewesson PJ. Intravenous-to-oral stepdown program: Four years of experience in a large teaching hospital.Ann Pharmacother 1992;26:1447-51. [DOI] [PubMed] [Google Scholar]
- 35.Paladino JA. Pharmacoeconomic comparison of sequential IV/oral ciprofloxacin versus ceftazidime in the treatment of nosocomial pneumonia.Can J Hosp Pharm 1995;48:276-83 [PubMed] [Google Scholar]


