Abstract
The present article describes the laboratory diagnosis of Neisseria gonorrhoeae by culturing of the organism from different types of clinical specimens followed by confirmatory tests. The success of culture methods requires good quality collection and transport of clinical specimens. The present guide describes the media requirements and cultural conditions for N gonorrhoeae growth and the characteristics for a presumptive identification of N gonorrhoeae. Confirmatory tests include biochemical tests, chromogenic enzyme substrate tests, immunoassays and nucleic acid methods. Nucleic acid detection methods include either amplification-based methods or nonamplification tests, and are increasingly used in clinical laboratories where a viable culture is not possible to obtain. Nucleic acid methods can also be used to detect the presence of low numbers in a specimen. Nucleic acid detection methods need confirmation with another amplification method or gene target. Controls must be included to ensure true positive and negative results, and to rule out nucleic acid contamination. Monitoring of antimicrobial susceptibilities of N gonorrhoeae is important to investigate treatment failure and to evaluate the efficacy of currently recommended therapies. Many methods for the characterization of N gonorrhoeae require cultures. The useful typing methods for determining strain relatedness include auxotyping, serotyping, plasmid profile analysis, DNA sequencing of the porB gene and pulsed-field gel electrophoresis. Quality assurance programs for diagnostic testing and antimicrobial susceptibility testing is reviewed.
Key Words: Clinical laboratory, diagnosis, Neisseria gonorrhoeae
Neisseria gonorrhoeae is an obligate human pathogen and is the etiological agent of gonorrhea. Syndromes include cervicitis in women, and urethritis, pharyngitis and proctitis in both sexes. If untreated, women may experience severe sequelae of pelvic inflammatory disease, chronic pelvic pain, ectopic pregnancy and tubal infertility, while men may develop epididymitis, prostatitis and urethral stricture (1). Occasionally, some individuals may develop disseminated infections with systemic complications, while others may have asymptomatic infections and transmit gonococci unknowingly. Oropharyngeal and anorectal gonococcal infections may be acquired by persons practicing receptive oral or anal intercourse or by contamination from cervical secretions. Occasionally, adults may present with conjunctivitis. Newborns exposed to infected secretions during birth may develop ocular infections (ophthalmia neonatorum) but the incidence of this is low in Canada, with only six reported cases in 1998 (2) due to routine eye prophylaxis (1). When handling clinical specimens or N gonorrhoeae cultures, biosafety precautions should be taken, including the use of protective goggles to protect the eyes from splashed infectious material and inadvertent touching with contaminated hands.
The incidence of gonococcal infections in Canada peaked in 1985 with an infection rate of 157 cases/100,000 population and declined to 17.6 cases/100,000 population in 1999. The drastic decrease in infection rates in Canada was partly due to improved public health interventions (1), including the use of single dose antibiotic therapies, enhanced contact tracing and surveillance, improved diagnostic methods and implementation of educational programs. Between 1997 and 2001, the national rate of reported gonorrhea cases increased by 53% among men and 33% among women (3).
To ensure that the recommended therapies are efficacious, an ongoing surveillance program has been in place since the 1970s to monitor the prevalence and emergence of antimicrobial-resistant strains in different parts of Canada. The antibiotic-resistant strains are further characterized by typing methods and compared with international surveillance data. The geographical and temporal trends have been used to develop or improve treatment guidelines.
Specimen Choice, Collection And Transport
Specimen choice and collection
The specimen choice and collection method (Table 1) depends on the testing technique used in a laboratory and the age, sex and sexual orientation of the patient (4). Specimens should be collected with Dacron or rayon swabs because calcium alginate may be toxic to gonococci. Fatty acids inhibit the growth of gonococci; therefore, cotton swabs that do not list acceptable manufacturer specifications should not be used (5). To minimize the inhibitory effects of unknown substances in the specimen, the swabs should be inoculated directly onto growth medium or placed in swab transport medium immediately after sampling (3).
TABLE 1.
Methods of collection of clinical specimens for the laboratory diagnosis of gonorrhea
| Specimen type | Method of collection |
|---|---|
| Urethral | Express urethral exudates when patients have discharge. If there is no discharge, compress the meatus vertically to open the distal urethra and insert a thin, water-moistened swab (calcium alginate or Dacron) with flexible wire slowly (3 cm to 4 cm in males or 1 cm to 2 cm in females), rotate slowly and withdraw gently. |
| Urine | Ask patients to collect only the first 10 mL to 15 mL of urine. Patients should not have voided for at least 2 h before specimen collection to increase the chance of detecting the organism. |
| Cervical | Insert a speculum into the vagina to view the cervix. Insert a swab 1 cm to 3 cm into the endocervical canal and rotate for 10 s to 30 s to allow absorption of exudates. |
| Vaginal | Collect pooled vaginal secretions, if present. Vaginal wash specimens are most preferred and acceptable to prepubertal girls. If not possible, rub a sterile cotton swab against the posterior vaginal wall and allow the swab to absorb the specimen. |
| Rectal | Specimens may be obtained blindly or, preferably, through an anoscope. Insert a swab 2 cm to 3 cm into the anal canal. Avoiding fecal material, rotate to sample crypts just inside the anal ring; allow the swab to absorb specimen for 10 s. |
| Oropharyngeal | Rub sterile swabs over the posterior pharynx and tonsillar crypts, or obtain nasopharyngeal aspirate from infants. |
| Conjunctiva | Any exudate or pus present in the eye should be carefully removed with a sterile swab. A second swab moistened with saline should be used to rub the affected conjunctiva. This swab should be broken off into a vial of transport medium. |
| Sterile body fluids | Clean skin puncture site with iodine (1% to 2%, or 10% solution of povidone-iodine [1% free iodine]). If tincture of iodine is used, remove with 70% ethanol to avoid burn. Perform percutaneous aspiration for pleural, pericardial, peritoneal or synovial fluids. Use nonheparinized collection if possible. |
Urethral specimens:
Discharge from the meatus is preferred for the detection of N gonorrhoeae. If there is no meatal exudate in postpubertal patients, an intraurethral swab can be used for the detection of gonococci. To increase the chance of detecting the organisms, swab samples should be collected from patients who have not voided for at least 2 h. The swabs are suitable for smear preparation, culturing on appropriate media or for transport to other laboratories.
Urine:
Urine is one of the specimen types suitable for nucleic acid tests for diagnosing N gonorrhoeae infections in males and females. Leak-proof containers should be provided to patients for the collection of urine specimens.
Cervical and vaginal specimens:
Cervical specimens should be taken from young and adult females. Gonococcal infections in prepubertal girls involve the vagina and not the cervix; therefore, vaginal specimens should be collected from this age group. When the patient presents with a urethral discharge, additional urethral or meatal specimens should be taken because the sensitivity of detecting gonococci from cervical specimens is lower than that from urethral or meatal specimens. Similar to urethral swabs, cervical and vaginal swabs can be used to prepare smears, to inoculate culture media directly, for nucleic acid-based tests or for submission in transport media to distant laboratories.
In cases of suspected coinfections of N gonorrhoeae and Chlamydia trachomatis, the cervical specimen for N gonorrhoeae detection should be taken before the specimen for C trachomatis, because N gonorrhoeae is present in the mucus from the endocervix and C trachomatis is present in the cervical epithelial cells.
Oropharyngeal and rectal specimens:
Oropharyngeal and rectal specimens should be processed only for culture because the performance of nonculture methods is not well established for these specimen types.
Conjunctiva:
The purulent discharge in gonococcal ophthalmia neonatorum and conjunctivitis in adults should be used for diagnosis.
Body fluids:
When patients have symptoms of systemic or disseminated infections, blood and fluid from arthritic joints are suitable samples for culturing.
Transport of specimens
Methods of transportation vary with the specimen and the type of test being done, but in all instances when commercially available transport systems are used, the instructions provided by the manufacturer should be followed.
Ideally, the specimens should be inoculated onto culture medium immediately after collection to preserve the viability of gonococci for isolation. If the inoculated media are being transported to a local laboratory, the plates should be held at room temperature for no more than 5 h in a CO2-enriched atmosphere using a candle jar or a commercial CO2-generating system. If long-distance shipping is required, the specimens should be inoculated onto media contained in a CO2-generating system, incubated for 18 h to 24 h and have visible growth on the plate before shipping.
CO2-generating systems such as JEMBEC (Becton, Dickinson and Company, USA) (5) and InTray GC (BioMed Diagnostics, USA) preserve the viability of the organisms for longer periods of time than non-nutritive systems.
The specimens should be packaged properly to avoid exposure to extreme temperatures and to meet the standards specified in the Transportation of Dangerous Goods Act and Regulations (6).
Transport of cultures
Several transport systems are suitable for transporting cultures to laboratories for susceptibility testing.
Frozen cultures for transport:
Cells from a 24 h agar plate are suspended in brain heart infusion broth with 20% glycerol and placed in a screw cap cryovial. The suspension can be frozen in a dry ice bath, a -70°C freezer or liquid nitrogen. The frozen cultures should be shipped on dry ice in accordance with the instructions for the Transportation of Dangerous Goods Act and Regulations.
Nonfrozen transport media:
Transport swab systems with or without charcoal (eg, StarSwab, Starplex Scientific, Ontario; PROBACT Transport Swab System, Med-Ox Diagnostics Inc, Ontario; Copan Transport Swab, Oxoid, Ontario) are suitable for sustaining viable gonococci for 6 h to 12 h at room temperature. A heavy inoculum is required for overnight transportation of pure cultures within Canada.
The JEMBEC plate contains Martin-Lewis agar in a rectangular polystyrene plate with an inner well that holds a CO2-generating tablet to provide a modified atmosphere for the cultures.
InTray GC medium with preincubation.
Chocolate agar slants with preincubation.
Diagnostic Tests
Microscopy
A direct smear for Gram staining may be performed as soon as the swab specimen is collected from the urethra, cervix, vagina or rectum. The swab should be rolled gently onto the slide to preserve cellular morphology and over an area less than 1 cm2 (1). Gram staining kits are commercially available. Under oil immersion (1000x magnification), urethral smears from symptomatic males usually contain intracellular Gram-negative kidney-shaped diplococci in polymorphonuclear leukocytes, the presence of which is required for the presumptive diagnosis of gonorrhea (5). The presence of extracellular Gram-negative diplococci is an equivocal finding that must be confirmed by culture (1,5) or nucleic acid test. The endocervical smears from females or rectal specimens are more difficult to interpret due to the presence of other Gram-negative coccobacilli, including Moraxella osloensis, Moraxella phenylpyruvica, Kingella denitrificans and Acinetobacter species (7). The sensitivity and specificity for urethral smears is 90% to 95%, respectively, versus 50% to 70% sensitivity and over 90% specificity for endocervical smears (1,8). However, the reliability of microscopic tests depends on the quality of the specimen and the experience of the microscopist (1). Based on a recent study (9), Gram-strained urethral smears would not be cost-effective in places with a low prevalence of gonorrhea.
Culture
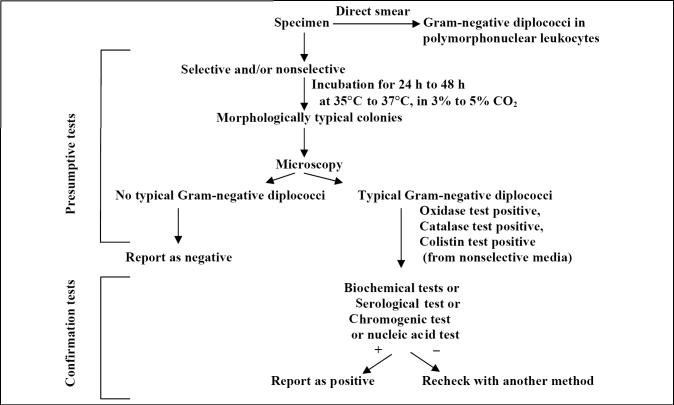
The current preferred laboratory method for the diagnosis of N gonorrhoeae infections is the isolation and identification of the agent (Figure 1). Culturing isolates is important for antimicrobial susceptibility testing, surveillance purposes, detecting treatment failure and characterizing outbreaks.
Figure 1.
Algorithm for culture and identification of Neisseria gonorrhoeae
Media and cultural conditions for isolation:
The primary specimens should be inoculated onto nonselective chocolate agar and selective agar containing antimicrobial agents that inhibit the growth of commensal bacteria and fungi. The antibacterial agents in modified Thayer-Martin, Martin Lewis and New York City medium are vancomycin, colistin, trimethoprim lactate and the antifungal agents nystatin and anisomycin or amphotericin B (5). Some fastidious strains, such as the arginine-, hypoxanthine- and uracil-requiring strains, are more susceptible to the concentrations of vancomycin or trimethoprim used in the selective media (5). Isolates that are inhibited by supplements in selective media should be grown on media with lower concentrations of antibiotic. Isolates that are atypical, such as vancomycin-susceptible strains, should be forwarded to reference laboratories to confirm their identification. Therefore, a quality assessment program that periodically compares isolation rates on selective and nonselective media is desirable.
The inoculated plates should be incubated at 35°C to 37°C in a moist atmosphere enriched with CO2 (3% to 7%) (5). An 18 h to 24 h culture should be used as the inoculum for additional tests. Plates should not be incubated for longer than 48 h because most old cultures would not survive storage conditions. Autolysis may occur during prolonged incubation, and growth from agar plates becomes difficult to suspend in solutions. Isolates should be subcultured at least once on nonselective medium after initial isolation before being used in a diagnostic test that requires pure culture or heavy inoculum.
Long-term storage:
N gonorrhoeae cultures should be stored long term in a -80°C freezer, in liquid nitrogen (-196°C) or by lyophilization. An 18 h to 24 h culture should be used as the material for freezing or lyophilization. To prepare a frozen culture, cells should be suspended in brain heart infusion broth with 20% glycerol. To prepare lyophilized cultures, cells are suspended in 2% skim milk and distributed into lyophilization vials in small aliquots. Once the cultures are lyophilized, one vial should be tested to ensure the culture is pure and viable. Lyophilized cultures are stored at 4°C or room temperature. To ensure viability over the long term, cultures - reference cultures in particular - should be subcultured every five years. Reference cultures that are frequently used as controls should be stored as multiple aliquots to avoid freezing and thawing.
Presumptive identification of N gonorrhoeae:
The presumptive identification of N gonorrhoeae rests on the isolation of an oxidase-positive, Gram-negative diplococcus recovered from urogenital sites that grows on selective media (5) (Figure 1). The Gram stain of a smear of urethral exudates or endocervical secretions shows typical Gram-negative intracellular diplococci. The oxidase test uses the tetramethyl derivative of the oxidase reagent (1% aqueous solution of N, N, N, N-tetramethyl-1, 4-phenylenediamine) that is commercially available (BACTIDROP Oxidase, Remel Inc, USA) or prepared in-house. To perform the test, a drop of reagent is applied to filter paper or the tip of a cotton swab. Culture is then applied to the filter paper or cotton swab tip using a platinum or plastic loop, wooden applicator stick or toothpick. A dark-purple colour change within 10 s indicates a positive sample (5). The catalase test (3% hydrogen peroxide) or superoxol (30% hydrogen peroxide) are other rapid tests used in the presumptive identification of N gonorrhoeae. A drop of the reagent is placed in the centre of a clean glass slide and the suspect colony is picked with a loop and emulsified in the reagent. N gonorrhoeae will produce a positive reaction with bubbling within 1 s to 2 s. Weak bubbling or bubbling after 3 s indicates a negative reaction (5) (Table 2). The reagents are tested daily against reference oxidase-positive and -negative cultures to ensure quality.
TABLE 2.
Supplemental tests that permit differentiation between Neisseria gonorrhoeae and related species
| Acid from | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | Glucose | Maltose | Sucrose | Fructose | Lactose | Nitrate reduction | Polysaccharide from sucrose | Superoxol |
| Neisseria gonorrhoeae | +* | –† | – | – | – | – | – | Strong (4+) positive ‘explosive |
| Neisseria meningitidis | + | + | – | – | – | – | – | Weak (2+) positive |
| Kingella denitrificans | + | – | – | – | – | + | – | – |
| Neisseria cinerea | – | – | – | – | – | – | – | Weak (2+) positive |
| Neisseria subflava biovar subflava | + | + | – | – | – | – | – | Weak (2+) positive |
| Neisseria subflava biovar flava | + | + | – | + | – | – | – | Weak (2+) positive |
| Neisseria subflava biovar perflava | + | + | + | + | – | – | + | Weak (2+) positive |
| Neisseria sicca | + | + | + | + | – | – | + | Weak (2+) positive |
| Neisseria mucosa | + | + | + | + | – | + | + | Weak (2+) positive |
| Neisseria flavescens | – | – | – | – | – | – | + | Weak (2+) positive |
+ most strains positive;
– most strains negative. Data from reference 5
Confirmatory identification tests:
Several methods are available to confirm the identification of N gonorrhoeae, including biochemical testing, serological testing, colourimetric testing and nucleic acid methods. More than one system may be required to confirm identification.
Biochemical tests:
N gonorrhoeae can be differentiated from other Neisseria species, Moraxella species, Kingella species and other commensals based on its ability to grow on appropriate selective and nonselective media, produce acid from glucose, maltose, lactose, sucrose and fructose, reduce nitrate, produce polysaccharide from sucrose and exhibit DNase production (Table 2). The ability to ferment different sugars is traditionally determined using conventional cystine trypticase agar medium (CTA) containing 1% sugar. This method is based on fermentative species and is not sensitive enough to detect acid from oxidative species. In addition, differentiating N gonorrhoeae and Neisseria cinerea is difficult using CTA sugars. As a result, this test is no longer preferred for the detection of acid production from carbohydrates. This method has been replaced by more rapid commercially available methods in some laboratories because the preparation of the necessary media is tedious and labour intensive, and quality control of the purity of sugars is required. The rapid carbohydrate test is a nongrowth-dependent method for the detection of acid production from carbohydrates by Neisseria species. The Rapid Identification Method-Neisseria (Remel Inc, USA) is a commercially available rapid acid production test that compares well with the conventional method, but may also not differentiate between N gonorrhoeae and N cinerea (10). The rapid nongrowth tests have a specificity of 99% to 100% and are more sensitive than CTA sugars. Some of the commercial tests include not only acid production tests, but also tests for other biochemical characteristics (such as enzyme production), including DNase and nitrate reduction. All tests must include appropriate control strains.
Chromogenic enzyme substrate :
These tests are based on the presence of preformed chromogenic enzyme in the culture and, thus, require a heavy inoculum of the organism grown on selective medium to permit rapid speciation of isolates. The enzymes that are detected by these systems include beta-galactosidase, gamma-glutamylaminopeptidase and prolyl-hydroxyprolyl aminopeptidase. Many of these methods are commercially available (Gonochek II, EY Laboratories, USA; Gonochek II, TCS Biosciences Ltd, UK; Identicult-Neisseria, Adams Scientific, USA; BactiCard Neisseria, Remel Inc, USA) (10). Nongonococcal Neisseria isolates that may be isolated on gonococcal-selective media may be misidentified as N gonorrhoeae if additional tests are not performed (5).
None of the culture confirmation tests for the identification of N gonorrhoeae are 100% sensitive and specific, and tests based on different principles may be required to resolve equivocal results (11).
Serological tests:
Fluorescent antibody test: The fluorescein isothiocyanate-labelled monoclonal antibody in the MicroTrak N gonorrhoeae Culture Confirmation Test (Trinity Biotech Plc, Ireland) reacts specifically with N gonorrhoeae. The antibody binds specifically to both proteins IA and IB (porins, or outer membrane proteins). When viewed under a fluorescence microscope, cultures positive for N gonorrhoeae show apple-green fluorescent staining of the kidney-shaped diplococci. Although this test has been reported to be 100% specific for N gonorrhoeae (11), nonspecific Fc binding to unrelated organisms such as Staphylococcus aureus has been observed. Therefore, only Gram-negative, oxidase-positive diplococci should be tested. Because test sensitivity is 99% (11), an alternative confirmation method should be used when this test gives an unexpected negative result.
Coagglutination tests: Coagglutination tests can be performed on primary culture and, therefore, confirmed results can be obtained one day earlier than tests that require subculturing from primary culture plates. Some of these tests do not require fresh or viable cells. The cell suspensions are boiled and combined with monoclonal antibodies that detect heat-stable epitopes on the PorI outer membrane protein and detection reagent. Coagglutination with test reagents indicates a positive result.
Both false-positive results (cross-reactions with other Neisseria species such as Neisseria meningitidis, Neisseria lactamica, N cinerea and K denitrificans) and false-negative results have been reported for the coagglutination tests (10,12-14). Therefore, another test should be performed if the following conditions occur: the isolates suspected of being gonococci do not react with appropriate reagents or give equivocal results; positive results are obtained on isolates from extragenital sites in low-risk patients; and finally, positive results are obtained on isolates from children.
There are two commercially available coagglutination tests: the Phadebact GC OMNI (Karo Bio Diagnostics AB, Sweden) and GonoGen (New Horizons Diagnostics, USA). Meritec GC (Meridian Diagnostics, USA) has not been available since 1995.
Colourimetric test: GonoGen II (New Horizons Diagnostics, USA) does not require the heat treatment step of the coagglutination test. This test eliminates the reading of agglutination; instead, the mixture of antigen-antibody complex is passed through a filtration unit. The appearance of a red dot on the filter indicates a positive reaction. Like GonoGen, this test does not require a pure or viable culture.
Nucleic acid detection with and without amplification (nonculture diagnostic method)
Molecular detection methods allow for a more rapid and specific diagnosis of pathogens that are fastidious or cannot be cultured. Nucleic acid methods developed for sexually transmitted pathogens are rapid, highly sensitive and specific for the detection of these organisms in clinical specimens. These methods permit the use of specimens that are unsuitable for culture, such as urine and vaginal swabs that can be obtained from patients without discomfort (15-17). Single detection systems or dual detection tests for C trachomatis and N gonorrhoeae are now commercially available. Some Canadian laboratories are using nucleic acid techniques concurrently with culture for the diagnosis of gonorrhea (18).
Nucleic acid methods are suitable for detecting N gonorrhoeae in specimens that may not contain viable organisms due to long transportation time or exposure to extreme temperature conditions. Unlike the experience with C trachomatis, the performance of nucleic acid methods for the detection of N gonorrhoeae has not been appreciably better than that of a proficient specimen transport and culture system (5,19-21). DNA amplification tests may not be suitable for test-of-cure because gonococcal DNA may be present for weeks after successful treatment of an infection (5). However, the Pace 2 (Gen-Probe, USA) has been reported to be a suitable test-of-cure assay for gonorrhea as early as six days after the treatment of genital infections (22).
Highly sensitive amplification methods may present cross-contamination problems and, thus, strict precautions are required to eliminate amplicon carryover (23). Proper design of work areas and operational precautions or use of an automated enclosed system may reduce the risks of false-positive results due to cross-contamination. Primers for the amplification of N gonorrhoeae DNA may also crossreact with DNA from other nonpathogenic species; therefore, confirmatory tests are required to eliminate false-positive results (21,24). This is more important when these tests are used in low prevalence populations. Problems with reproducibility may also arise due to inhibitors of amplification in the specimens (23). A comparison of the performance of three nucleic acid methods and culture has been reported (25).
Results obtained using probe or nucleic acid amplification methods are currently inadmissible as evidence in medicolegal cases (5).
Nucleic acid methods for confirmation of culture
A DNA probe system (AccuProbe, Gen-Probe, USA) has been developed for culture confirmation (5). The AccuProbe system uses a chemiluminescent-labelled, single-stranded DNA probe that is complementary to the ribosomal RNA (rRNA) of N gonorrhoeae. The DNA/RNA hybrid from a positive culture is detected using a luminometer. In comparison with rapid acid production and coagglutination tests, a DNA probe test is more specific and sensitive (5,19).
Specific primers for polymerase chain reaction (PCR)-based detection methods have also been described in the literature (8,24). These methods are the most sensitive, although specificity depends on the choice of primers. The use of primers to target the cryptic plasmid is not recommended in areas where there is a high prevalence of proline-, citrulline- and uracil-requiring strains, which are typically plasmidless. Commercial kits are available but are more expensive when used as confirmation tests.
Amplification methods
PCR:
The specificity and sensitivity of PCR reactions depend on the primers used (24) and the presence of inhibitory substances in the specimens (26). At present, fully automated tests (COBAS AMPLICOR CT/NG, Roche Diagnostic Systems, USA) and semi-automated tests (AMPLICOR CT/NG, Roche Diagnostic Systems, USA) are commercially available in Canada (26). The target sequence for the detection of N gonorrhoeae is the DNA methyl-transferase gene. To detect the presence of inhibitors in samples, internal control target DNA is available for coamplification in each reaction. The sensitivity and specificity of PCR methods have been evaluated and reported in the literature (20,25,27). Dacron swabs from the Amplicor PCR collection kit (Roche Diagnostic Systems, USA) should be used to collect intravaginal or cervical samples. The swab can be transported in wet or dry tubes (17,25).
A multiplex PCR method for the co-amplification of C trachomatis and N gonorrhoeae has also been described (28). The sensitivity of this test was 92.3% when compared with the culture method for detecting N gonorrhoeae in urethral specimens (8). A real-time PCR assay has the advantage of reducing the detection time of regular PCR procedures (29). The start-up cost of setting up the real-time PCR may be prohibitive, and routine laboratories may need to refer specimens to reference laboratories. A multiplex qualitative real-time PCR assay that detects C trachomatis and N gonorrhoeae and contains an internal control has been developed (30).
Some samples may be inhibitory to the PCR. These inhibitory effects may be removed or overcome by one of the following: use of a DNA purification procedure for the sample (some purification kits are available commercially [Qiagen, Ontario]); dilution of urine samples (23); or, repeating the amplification using another urine sample (23).
Ligase chain reaction:
The ligase chain reaction (LCR) (LCx Uriprobe, Abbott Laboratories, USA) uses probes that target the opa and pil genes for the detection of N gonorrhoeae in endocervical, urine and vaginal specimens. The amplification steps include an elevated temperature cycle for the denaturation of target DNA to a single strand, a lower temperature cycle for annealing of adjacent probes to targets, gap-filling with DNA polymerase in the presence of deoxyguanosine triphosphate, and ligation of probes with ligase. The ligated amplicons are captured and detected by a fluorescence immunoassay. The sensitivity and specificity of LCR for detecting N gonorrhoeae in different specimens have been shown to be 97.3% to 100% and 97.8% to 100%, respectively (8). As of 2003, the Abbott LCx Uriprobe was off the market in Canada.
Strand displacement amplification system:
The Strand Displacement Amplification (SDA) system is an isothermal amplification system. A semi-automated system using the SDA system is available commercially (BD ProbTec ET system, Becton, Dickinson and Company, USA) for the simultaneous detection of C trachomatis and N gonorrhoeae. The BDProbTec ET system is a 1 h assay that can process a higher number of specimens than the COBAS AMPLICOR system within an 8 h day (31). Therefore, high-throughput laboratories may view the SDA system as more efficient than PCR systems (31).
Nucleic acid sequence-based amplification:
Nucleic acid sequence-based amplification (NASBA) is an isothermal nucleic acid amplification system that does not require a thermal cycler. The NucliSens Basic kit (Organon Teknika, Boxtel, The Netherlands) provides an RNA amplification and detection platform. The kit contains reagents for RNA extraction and amplification, and a ruthenium-labelled probe for electrochemiluminescent detection of amplicons. This system has been evaluated for the detection of 16S rRNA of N gonorrhoeae in genital tract specimens (32). In comparison with culture methods for 216 swab specimens, the NASBA using the NucliSens Basic kit was 97.9% sensitive and 98.7% specific (32).
Nonamplification tests
The Pace 2NG (Gen-Probe, USA) test uses a single nucleic acid probe to detect the target rRNA of N gonorrhoeae in endocervical specimens, while the Pace 2C (Gen-Probe, USA) test uses combination probes to detect both C trachomatis and N gonorrhoeae in a single assay. A positive Pace 2C result requires further testing using the individual tests Pace 2NG and Pace 2Ct to differentiate C trachomatis and/or N gonorrhoeae. The Pace 2NG test detects nucleic acid in patient specimens in 2 h. Only swabs and transport media available from the Pace collection kit should be used to collect and transport specimens to laboratories. The specimens are stable for transport at room temperature.
The performance of this test is highly specific when it is used to detect N gonorrhoeae in urogenital and endocervical specimens, but is less specific with pharyngeal and rectal specimens from high-risk patients (5). The performance of the Pace 2C was found to be similar to Pace 2NG for detecting N gonorrhoeae in endocervical specimens (33). The sensitivity, specificity and positive and negative predictive values for Pace 2C were found to be 96.3%, 98.8%, 92.9% and 99.4%, respectively (33).
The Hybrid Capture II (Digene Corporation, USA) test is a combination DNA probe test that uses signal amplification to detect both C trachomatis and N gonorrhoeae in a single specimen. The proprietary endocervical brush appeared to be better than Dacron swabs for collecting specimens (34).
Table 3 summarizes all of the nucleic acid detection tests including DNA probe tests and direct probe and amplified probe detection.
TABLE 3.
Nucleic acid detection tests (including DNA probe tests, direct probe and amplified probe detection)
| Test | Description | Sensitivity/specificity* | |
|---|---|---|---|
| DNA probe test for culture confirmation | AccuProbe Neisseria gonorrhoeae culture confirmation test (Gen-Probe, USA) | Detection of species-specific ribosomal RNA (rRNA) sequences using a chemiluminescent acridinium ester-labelled, single-stranded gonococcal rRNA | 100% sensitive,100% specific (194 strains tested) (51) |
| Detection of N gonorrhoeae directly from clinical specimens types (urine, endocervical swab [women], urethral swabs [men]; some kits can use vaginal swab) | |||
| Direct probe | Gen-Probe PACE2 PACE2C (Gen-Probe, USA) | Detection of a specific sequence of Chlamydia trachomatis or N gonorrhoeae rRNA using a chemiluminescent acridinium ester-labelled DNA probe | 96.3% sensitive, 98.8% specific (33) |
| Hybrid Capture II assay (Digene Corporation, USA) | RNA hybridization probes specific for genomic DNA and cryptic plasmid DNA sequences of C trachomatis and N gonorrhoeae | 93% sensitive (94 specimens tested) 98.5% specific 1263 specimens tested) (27) | |
| Nucleic acid amplification tests | Roche AMPLICOR (Roche Diagnostic Systems, USA) | Detection of a 201 base pair sequence in the cytosine methyltransferase gene | 96% specific, 100% sensitive (618 samples) (52) |
| Becton Dickinson BD ProbeTec (BD Biosciences, USA) | Detection of a DNA sequence in the multicopy pilin gene-inverting protein homologue | 100% sensitive and specific in a limited sampling of 57 specimens (27) | |
| NucliSens Basic (Organon Teknika, The Netherlands) | Detection of 16S rRNA of N gonorrhoeae using RNA amplification | 97.9% sensitive, 98.7% specific (216 specimens tested) (32) | |
| Abbott LCx (Abbott Laboratories, USA) No longer available in Canada | Detection of a 48 base pair sequence in the opa genes of N gonorrhoeae | 97.3% to 100% sensitive, 97.8% to 100% specific (8) | |
Most studies use culture identification as the gold standard to establish the sensitivity and specificity values
Confirmation of nucleic acid methods
Nucleic acid detection methods should be confirmed using an alternative test or target gene; for example, real-time PCR-based assays targeting the cppB gene of the 4.2 kb cryptic plasmid of N gonorrhoeae (35) and the porA pseudogene (36) have been developed.
Serological tests
There is no specific and sensitive serological test available to detect recent gonococcal infections through the demonstration of N gonorrhoeae-specific antibodies or antigens in patients' sera.
Typing of N gonorrhoeae
Strain differentiation has been used in epidemiological and medicolegal cases, outbreak investigations, and to monitor the distribution of antimicrobial-resistant strains and track the transmission of specific strains in a population. In Canada, most of the strain typing is performed in provincial public health and reference laboratories or by the Bacteriology and Enteric Diseases Program (National Microbiology Laboratory, Winnipeg, Manitoba). The typing methods developed for strain differentiation include auxotyping (A), serotyping (S), plasmid profile analysis (P), porB sequencing, opa typing, pulsed-field gel electrophoresis (PFGE), ribotyping, amplified ribosomal-DNA restriction analysis, arbitrarily primed PCR and fluorescent amplified fragment length polymorphism analysis (5,37-39). Molecular subtyping methods together with epidemiological data collection can be used to study disease transmission patterns within a population and construct accurate sexual networks (18). Currently, A, S and P are the most common typing methods used for such studies. A is based on the nutrient growth requirements of strains, and S requires the use of monoclonal antibodies directed to protein I epitopes. Both of these procedures are easy to perform and do not require special equipment. P will differentiate types of beta-lactamase plasmids and plasmid-mediated tetracycline resistance (40). Conjugative plasmids and other plasmids add discriminatory power (37).
The discriminatory power of the A, S and P typing to differentiate strains for surveillance of antimicrobial-resistant N gonorrhoeae has been demonstrated (37). Plasmid analysis has limited value, being used mainly to identify strains carrying plasmid-mediated resistance. A in combination with S provides higher levels of discrimination. Used together, these three methods produce adequate discriminatory indices for national surveillance. One of the shortcomings of S is that some strains are nontypable. Another problem is that, since 1996, commercially produced monoclonal S reagents have not been available. The remaining S reagents in national reference laboratories in different parts of the world are being used for outbreak investigations and medicolegal cases.
To resolve these serotyping problems, DNA sequencing of the porB gene encoding the serotyping antigen, protein I or the porin, can be used as an alternative method (41,42). DNA sequencing has the advantage over conventional serotyping because all strains are typable.
Opa-typing based on restriction fragment length polymorphism of PCR amplicons of the opa locus has been demonstrated to be a highly discriminatory subtyping method for the investigation of outbreaks and mapping of sexual networks (41). One of the disadvantages of this method is the lack of interlaboratory standardization of banding patterns (and the resulting difficulty for interlaboratory comparison of data).
Similar to the opa-typing system, PFGE, ribotyping and amplified ribosomal-DNA restriction analysis are difficult to standardize for interlaboratory comparison. PFGE has high discriminatory power for differentiating N gonorrhoeae strains, but the other molecular methods are not as discriminatory (38). One of the disadvantages of PFGE is that some PFGE banding patterns have large molecular size fragments that do not resolve well. Fluorescent amplified fragment length polymorphism analysis has discriminatory power approaching that of opa-typing (39). This method produces fragments that can be sized accurately using internal standards (39).
Antimicrobial Susceptibility Testing
When cultures are available, susceptibility testing should be performed on N gonorrhoeae in the laboratory or sent to a reference laboratory where such tests are available; these results are useful for investigating treatment failure and monitoring the efficacy of currently recommended therapies.
Monitoring geographical and temporal trends of antimicrobial susceptibilities provides information useful in the development of treatment guidelines and interventions for the control of infections. In Canada, an antibiotic would not be used to treat gonorrhea if the prevalence of resistance to that antibiotic exceeded 3.0% (43). Canadian policy is more stringent than policies in the United States (US), UK and other countries that have adopted the World Health Organization's (WHO) recommendation of a 5.0% cutoff (44). Antibiograms are sometimes used as one of the epidemiological markers for laboratory surveillance of bacterial pathogens. Effective public health interventions would reduce the emergence and transmission of antimicrobial-resistant strains.
Antibiotic panel
Antibiotics recommended for treating gonorrhea in Canada include ciprofloxacin, spectinomycin and third-generation cephalosporins such as ceftriaxone and cefixime. All of these antibiotics should be included in a susceptibility testing panel. Although penicillin and tetracycline are not currently being used in Canada, resistance to these antibiotics should be monitored because they are still used in other parts of the world and because the worldwide prevalence of resistance to these two antibiotics remains high. Doxycycline and azithromycin or erythromycin, currently used for treating C trachomatis coinfections, may impose selective pressure on N gonorrhoeae, thus causing the emergence of resistant strains (1). Although azithromycin is not used for treating gonorrhea in Canada, this antibiotic is used in some countries, and resistant strains have been described in the literature (45).
Materials and methods
There are different published methods and different basal media for susceptibility testing of N gonorrhoeae; therefore, interlaboratory comparison of data collected in different parts of the world or from different time periods can be difficult. In Canada and the US, the agar dilution method described in the National Committee for Clinical Laboratory Standards (NCCLS) guideline is used to determine the susceptibilities of N gonorrhoeae for the national antimicrobial surveillance program (46).
The media used for the determination of minimum inhibitory concentrations (MICs) may vary between laboratories. In Canada, all laboratories use GC medium base (Difco, USA or BBL, Becton Dickinson, USA), but the growth supplement may differ among laboratories. Either the recommended supplement in NCCLS or modified Kellogg's growth supplement (0.05 g ferric nitrate, 1.0 g cocarboxylase, 5.0 g glutamine and 200.0 g glucose per litre of H2O) should be used (46,47).
Detection of beta-lactamase:
The detection of beta-lactamase to identify penicillinase-producing N gonorrhoeae strains (46) should be done on all isolates using one of the following: the liquid nitrocefin test, Cefinase disks (Becton Dickinson, Canada) or DrySlide Nitrocefin (Difco, USA).
Interpretation of MICs
The antibiotic panel included in the NCCLS guideline does not include the macrolides (46). Therefore, there are no recommended breakpoints available from NCCLS for the interpretation of the MICs of macrolides. The observed potency of macrolides are affected by pH and the CO2 concentration used in the incubation of inoculated plates. In Canadian laboratories, the inoculated agar dilution plates are incubated in an atmosphere containing 5% CO2, and the MICs are interpreted using published data (45,48). Table 4 shows the MICs of reference strains to different antibiotics. Reference cultures can be requested from the National Microbiology Laboratory. Strain ATCC 49226 is available for purchase from the American Type Culture Collection (USA).
TABLE 4.
Minimal inhibitory concentration (MIC) ranges (mg/L) of Neisseria gonorrhoeae reference strains *†
| Antibiotic | WHO B* | WHO C* | WHO F* | ATCC 49226† |
|---|---|---|---|---|
| Penicillin | 0.032–0.125 | 0.25–1.0 | 0.008–0.032 | 0.25–1.0 |
| Spectinomycin | 16.0–32.0 | 16.0–32.0 | 16.0–32.0 | 8.0–32.0 |
| Tetracycline | 0.125–0.25 | 0.5–1.0 | 0.25–0.5 | 0.25–1.0 |
| Erythromycin | 0.063–0.125 | 0.5–1.0 | 0.5–1.0 | 1.0–2.0 |
| Ceftriaxone | 0.002–0.008 | 0.008–0.032 | 0.00025–0.001 | 0.004–0.016 |
| Ciprofloxacin | 0.002–0.008 | 0.002–0.008 | 0.002–0.008 | 0.001–0.008 |
| Cefixime | 0.004–0.016 | 0.008–0.032 | 0.0005–0.002 | 0.004–0.032 |
| Azithromycin | 0.032–0.063 | 0.063–0.125 | 0.125–0.25 | 0.5–1.0* |
*MICs for the World Health Organization (WHO) strains were determined using GC medium base (Difco, USA) supplemented with 1% Kellogg's defined supplement. Ranges established by the National Microbiology Laboratory in Winnipeg, Manitoba. †Acceptable ranges of MICs as per National Committee for Clinical Laboratory Standards document M100-S13 (M7-A6), January 2003 (45)
There are published data for the use of the E-test for antimicrobial susceptibility testing of N gonorrhoeae (49,50). However, there are no well-established breakpoints for the E-test results. The NCCLS agar dilution breakpoints must be evaluated before they can be used for the interpretation of E-test results.
Proficiency and Quality Assurance
At present, some laboratories may perform both culture and nucleic acid-based tests. This is because some laboratories serve remote sites that cannot successfully transport specimens containing viable organisms. Proficiency in culture methods must be maintained when monitoring antimicrobial resistance and validating molecular methods, and for outbreak analysis and medicolegal case investigations.
Diagnostic tests
An ongoing quality control program should be implemented to ensure that media, reagents, equipment and laboratory personnel do not adversely influence diagnostic testing. Guidelines on quality control for culture methods have been described in the literature (11). Some gonococci are susceptible to trimethoprim or vancomycin at concentrations that are used in some of the selective media (eg, strains that require arginine, uracil and hypoxanthine for growth) (5). A high ratio of Gram stain-positive:culture-negative results may indicate the presence of inhibitory effects in the culture media being used (11). Periodic assessment of the adequacy of isolation of the more fastidious strains can be achieved by comparing isolation rates on selective and nonselective isolation media. When there is a need to use a candle jar for the incubation of the isolation plates, unscented candles should be used because the vapour from fragrant candles may be toxic to some N gonorrhoeae strains.
At present, quality assurance programs for nucleic acid testing are more difficult to implement because properly designed, standardized material is not available. Also, there is no national interlaboratory standardization program in place for nucleic acid tests.
Diagnostic laboratories should use only commercial kits that are licensed by Health Canada for processing clinical specimens. The manufacturer's instructions should be followed, and a kit should not be used with unlicensed specimen types.
N gonorrhoeae is naturally competent, and lateral gene transfer may alter the sequences of specific targets. Periodic comparison of nucleic acid methods with cultural methods or alternative systems could detect the occurrence of false-positive or false-negative results. When using in-house tests to investigate suspected false-positive results, careful test optimization is required to achieve the highest accuracy. The problem is that standardized reference material is often lacking. A panel of controls comprised of low copy samples, high copy samples, DNA without the target sequence and a control without DNA should be included in each test.
Internal standard DNA added to the amplification reactions will detect the presence of inhibitors in the sample. Some commercial kits, such as the Roche Amplicor and the Becton Dickinson BD ProbeTec, include the internal standard DNA.
Thermal cyclers should be checked regularly or according to the manufacturer's instructions to ensure that all the wells provide uniform amplification conditions. Also, the positive controls should be randomly positioned through the block. The frequency of monitoring of equipment will depend on the quality assurance policies and throughput of the individual laboratory. The work environment should be monitored regularly for contamination by PCR amplicons. Any sudden increase in the number of positive results should initiate a quality assessment audit to rule out contamination problems in the environment or the process. The use of an enclosed automated system will minimize errors due to contamination. All dispensing apparatus for delivering reagents should be calibrated regularly.
Antimicrobial susceptibility testing
Appropriate control strains are needed to perform quality control tests of antibiotic media. The designated strain N gonorrhoeae ATCC 49226 should be included when testing each batch of antibiotics (46). It is also desirable to include a panel of strains with well-defined MIC values representative of susceptible, intermediate and resistant phenotypes (Table 4). Well-characterized antimicrobial resistance mechanisms including beta-lactamase production, tet(M)-mediated resistance and mutations of chromosomal loci (eg, overexpression of mtr efflux pumps, gyrA and parC), should be represented among the control strains.
When results obtained with control strains are out of the acceptable ranges, then the incubator, the ingredients of the media and the suspending solution for preparation of the inoculum should be checked as sources of error.
Antibiotic solutions:
The preparation of stock solutions should take into account the potency of each lot of antibiotics. It is important not to use antibiotics after their expiry date because the potency of antibiotics may drop during storage. Some antibiotics, such as tetracycline, are photosensitive and should be stored in the dark.
Media:
There is variation between different lots of GC basal media. The result may be inadequate growth of N gonorrhoeae or the production of unacceptable MIC values with control strains. Plates without antibiotics should be evaluated for growth of fastidious strains such as arginine-, hypoxanthine-and uracil-requiring strains and proline-, citrulline- and uracil-requiring auxotypes. The water used to prepare media must not contain impurities that may interact with the antibiotics.
Growth supplements:
Commercially available growth supplements may have lot-to-lot variation and cause fluctuation of the MIC values. They should be included in the quality control program.
Internal and external quality assurance programs:
It is not unusual that some laboratories experience turnover of staff or require staff to rotate in different work units. Therefore, it is important that an internal proficiency program is implemented to ensure competency and consistency in interpreting and reporting susceptibility data.
It has been recognized that global surveillance data are also important for proactive prevention interventions. To ensure that the interlaboratory data are comparable, the Bacteriology and Enteric Diseases Program provides a national proficiency program to evaluate the performance of susceptibility testing in different laboratories. Three panels of strains are distributed each year to participating laboratories. In addition, the National Microbiology Laboratory standardizes the GC basal media in Canada for all participating laboratories. The National Microbiology Laboratory also participates in the proficiency program offered by the Centers for Disease Control and Prevention (Atlanta, Georgia, USA) to the Gonococcal Infections Surveillance Program network in the US.
To provide additional reference cultures, The International Collaboration on Gonococci group, coordinated by the WHO and Centers for Disease Control and Prevention, is currently assembling a panel of reference cultures including strains susceptible, intermediate and resistant to ciprofloxacin, azithromycin and other antibiotics.
CONCLUSIONS
At present, culture is the preferred laboratory test. However, if the specimens require long transportation times or have been exposed to extreme temperatures, culture is less sensitive than the nucleic acid methods.
The nucleic acid tests, especially the semi-automated and automated systems, are less labour intensive than cultural methods and allow high throughput processing of clinical specimens. The processing time for nucleic acid methods is shorter than for cultural methods. There are several disadvantages when using nucleic acid methods: the requirement to monitor the working environment for amplicon contamination, which could cause false-positive results; results cannot be validated using cultural methods; no cultures are available for susceptibility testing; and finally, they are not suitable for test-of-cure, outbreak investigation and medicolegal cases. Identification of N gonorrhoeae requires confirmation using two different methods involving different principles (or different gene targets in nucleic acid detection methods), especially in medicolegal cases (5).
The laboratory diagnostic methods for N gonorrhoeae are constantly evolving, and standard operating procedures in laboratories should be reviewed regularly and updated as necessary. The standard operating procedures must also include proper quality control tests. Ongoing proficiency testing should be conducted to ensure consistency in performance of laboratory procedures.
Laboratory testing is not only for diagnosing and treating patients but also for screening in the asymptomatic population. Such activity is important for the control of disease transmission and to determine prevalence and incidence for epidemiological purposes.
References
- 1.LCDC Expert Working Group on Canadian Guidelines for Sexually Transmitted Diseases. Canadian STD Guidelines. Ottawa: Health Canada, 1998:57-58,140-9. [Google Scholar]
- 2.Health Canada. <http://www.hc-sc.gc.ca/pphb-dgspsp/publicat/ ccdr-rmtc/98pdf/nds.pdf> (Version current at November 22, 2004).
- 3.Hansen L, Wong T, Perrin M. Gonorrhoea resurgence in Canada. Int J STD AIDS 2003;14:727-31. [DOI] [PubMed] [Google Scholar]
- 4.Whittington W, Ison, C, Thompson S. Gonorrhea. In: Morse SA, Moreland AA, Holmes KK, eds. Atlas of Sexually Transmitted Diseases and AIDS, 2nd edn. London: Mosby-Wolfe, 1996:99-117. [Google Scholar]
- 5.Janda WJ, Knapp JS. Neisseria and Moraxella catarrhalis In: Murray PR, Baron EJ, Pfaller MA, Jorgensen JH, Yolken RH, eds. Manual of Clinical Microbiology, 8th edn. Washington: American Society Microbiology, 2003:585-608. [Google Scholar]
- 6.Transport Canada. <http://www.tc.gc.ca/acts-regulations/ GENERAL/T/tdg/menu.htm> (Version current at December 2, 2004)
- 7.Weyant RS, Moss CW, Weaver RE, et al. Section 3 - Description of Species. In: Identification of Unusual Pathogenic Gram-Negative Aerobic and Facultatively Anaerobic Bacteria, 2nd edn. Baltimore: Williams and Wilkins, 1996:223-563. [Google Scholar]
- 8.Gaydos CA, Quinn TC. Neisseria gonorrhoeae: Detection and typing by probe hybridization, LCR and PCR. In: Peeling R, Sparling PF, eds. Sexually Transmitted Diseases: Methods and Protocols. New Jersey: Humana Press, 1999:15-27. [DOI] [PubMed] [Google Scholar]
- 9.Ghanem M, Radcliffe K, Allan P. The role of urethral samples in the diagnosis of gonorrhoea in women. Int J STD AIDS 2004;15:45-7. [DOI] [PubMed] [Google Scholar]
- 10.Dillon JR, Carballo M, Pauzé M. Evaluation of eight methods for identification of pathogenic Neisseria species: Neisseria-Kwik, RIM-N, Gonobio-Test, Minitek, Gonochek II, GonoGen, Phadebact Monoclonal GC OMNI Test, and Syva MicroTrak Test. J Clin Microbiol 1988;26:493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehret JM, Judson FN. Gonorrhea. In: Wentworth BB, ed. Laboratory Methods for the Diagnosis of Sexually Transmitted Disease, 2nd edn. Washington: American Public Health Association, 1991:53-94. [Google Scholar]
- 12.Dolter J, Bryant L, Janda JM. Evaluation of five rapid systems for the identification of Neisseria gonorrhoeae Diagn Microbiol Infect Dis 1990;13:265-7. [DOI] [PubMed] [Google Scholar]
- 13.Janda WM, Wilcoski LM, Mandel LK, Ruther P, Stevens JM. Comparison of monoclonal antibody-based methods and a ribosomal ribonucleic acid probe test for Neisseria gonorrhoeae culture confirmation. Eur J Clin Microbiol Infect Dis 1993;12:177-84. [DOI] [PubMed] [Google Scholar]
- 14.Kellogg JA, Orwig LK. Comparison of GonoGen, GonoGen II, and MicroTrak direct fluorescent-antibody test with carbohydrate fermentation for confirmation of culture isolates of Neisseria gonorrhoeae J Clin Microbiol 1995;33:474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stary A. Correct samples for diagnostic tests in sexually transmitted diseases: Which sample for which test? FEMS Immun Med Microbiol 1999;24:455-9. [DOI] [PubMed] [Google Scholar]
- 16.Smith K, Harrington K, Wingood G, Oh MK, Hook EW 3rd, DiClemente RJ. Self-obtained vaginal swabs for diagnosis of treatable sexually transmitted diseases in adolescent girls. Arch Pediatr Adolesc Med 2001;155:676-9. [DOI] [PubMed] [Google Scholar]
- 17.Gaydos CA, Crotchfelt KA, Shah N, et al. Evaluation of dry and wet transported intravaginal swabs in detection of Chlamydia trachomatis and Neisseria gonorrhoeae infections in female soldiers by PCR. Clin Microbiol 2002;40:758-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peeling RW, Ng LK. Sexually transmitted diseases: Impact of molecular laboratory diagnostic methods on disease control and prevention. Can J Infect Dis 1999;10:387-90. [Google Scholar]
- 19.Chapin-Robertson K. Use of molecular diagnostics in sexually transmitted diseases: Critical assessment. Diagn Microbiol Infect Dis 1993;16:173-84. [DOI] [PubMed] [Google Scholar]
- 20.Hammerschlag MR. Use of nucleic acid amplification tests in investigating child sexual abuse. Sex Transm Infect 2001;77:153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Doornum GJ, Schouls LM, Pijl A, Cairo I, Buimer M, Bruisten S. Comparison between the LCx Probe system and the COBAS AMPLICOR system for detection of Chlamydia trachomatis and Neisseria gonorrhoeae infections in patients attending a clinic for treatment of sexually transmitted diseases in Amsterdam, the Netherlands. J Clin Microbiol 2001;39:829-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanks JW, Scott CT, Butler CE, Wells DW. Evaluation of a DNA probe assay (Gen-Probe PACE 2) as the test of cure for Neisseria gonorrhoeae genital infections. J Pediatr 1994;125:161-2. [DOI] [PubMed] [Google Scholar]
- 23.Gronowski AM, Copper S, Baorto D, Murray PR. Reproducibility problems with the Abbott Laboratories LCx assay for Chlamydia trachomatis and Neisseria gonorrhoeae J Clin Microbiol 2000;38:2416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrell DJ. Evaluation of AMPLICOR Neisseria gonorrhoeae PCR using cppB nested PCR and 16S rRNA PCR. J Clin Microbiol 1999;37:386-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Dyck E, Ieven M, Pattyn S, Van Damme L, Laga M. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by enzyme immunoassay, culture, and three nucleic acid amplification tests. J Clin Microbiol 2001;39:1751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin DH, Cammarata C, Van Der Pol B, et al. Multicenter evaluation of AMPLICOR and automated COBAS AMPLICOR CT/NG tests for Neisseria gonorrhoeae J Clin Microbiol 2000;38:3544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morse SA. New tests for bacterial sexually transmitted diseases. Curr Opin Infect Dis 2001;14:45-51. [DOI] [PubMed] [Google Scholar]
- 28.Mahony JB, Song X, Chong S, Faught M, Salonga T, Kapala J. Evaluation of the NucliSens Basic Kit for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in genital tract specimens using nucleic acid sequence-based amplification of 16S rRNA. J Clin Microbiol 2001;39:1429-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiley DM, LeCornec GM, Mackay IM, Siebert DJ, Sloots TP. A real-time PCR assay for the detection of Neisseria gonorrhoeae by LightCycler. Diagn Microbiol Infect Dis 2002;42:85-9. [DOI] [PubMed] [Google Scholar]
- 30.Abravaya K, Huff J, Marshall R, et al. Molecular beacons as diagnostic tools: Technology and applications. Clin Chem Lab Med 2003;41:468-74. [DOI] [PubMed] [Google Scholar]
- 31.Chan EL, Brandt K, Olienus K, Antonishyn N, Horsman GB. Performance characteristics of the Becton Dickinson ProbeTec system for direct detection of Chlamydia trachomatis and Neisseria gonorrhoeae in male and female urine specimens in comparison with the Roche Cobas system. Arch Pathol Lab Med 2000;124:1649-52. [DOI] [PubMed] [Google Scholar]
- 32.Mahony JB, Song X, Chong S, Faught M, Salonga T, Kapala J. Evaluation of the NucliSens Basic Kit for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in genital tract specimens using nucleic acid sequence-based amplification of 16S rRNA. J Clin Microbiol 2001;39:1429-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwen PC, Walker RA, Warren KL, Kelly DM, Hinrichs SH, Linder J. Evaluation of nucleic acid-based test (PACE 2C) for simultaneous detection of Chlamydia trachomatis and Neisseria gonorrhoeae in endocervical specimens. J Clin Microbiol 1995;33:2587-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schachter J, Hook EW 3rd, McCormack WM, et al. Ability of the Digene Hybrid Capture II test to identify Chlamydia trachomatis and Neisseria gonorrhoeae in cervical specimens. J Clin Microbiol 1999;37:3668-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabrizi SN, Chen S, Cohenford MA, et al. Evaluation of real time polymerase chain reaction assays for confirmation of Neisseria gonorrhoeae in clinical samples tested positive in the Roche Cobas Amplicor assay. Sex Transm Infect 2004;80:68-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiley DM, Buda PJ, Bayliss J, Cover L, Bates J, Sloots TP. A new confirmatory Neisseria gonorrhoeae real-time PCR assay targeting the porA pseudogene. Eur J Clin Microbiol Infect Dis 2004;23:705-10. [DOI] [PubMed] [Google Scholar]
- 37.Dillon JA, Rahman M, Yeung KH. Discriminatory power of typing schemes based on Simpson's index of diversity for Neisseria gonorrhoeae J Clin Microbiol 1993;31:2831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Looveren M, Ison CA, Ieven M, et al. Evaluation of the discriminatory power of typing methods for Neisseria gonorrhoeae J Clin Microbiol 1999;37:2183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer HM, Arnold C. Genotyping Neisseria gonorrhoeae using fluorescent amplified fragment length polymorphism analysis. J Clin Microbiol 2001;39:2325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dillon J-A, Nasim A, Nestmann ER. Recombinant DNA methodology. New York: John Wiley & Sons Inc, 1985.
- 41.Viscidi RP, Demma JC, Gu J, Zenilman J. Comparison of sequencing of the por gene and typing of the opa gene for discrimination of Neisseria gonorrhoeae strains from sexual contacts. J Clin Microbiol 2000;38:4430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKnew DL, Lynn F, Zenilman JM, Bash MC. Porin variation among clinical isolates of Neisseria gonorrhoeae over a 10-year period, as determined by Por variable region typing. J Infect Dis 2003;187:1213-22. [DOI] [PubMed] [Google Scholar]
- 43.Sarwal S, Wong T, Sevigny C, Ng LK. Increasing incidence of ciprofloxacin-resistant Neisseria gonorrhoeae infection in Canada. CMAJ 2003;168:872-3. [PMC free article] [PubMed] [Google Scholar]
- 44.Tapsall J. Antimicrobial resistance in Neisseria gonorrhoeae World Health Organization, Geneva. 2001 WHO/CDS/CSR/DRS. [Google Scholar]
- 45.Tapsall JW, Shultz TR, Limnios EA, Donovan B, Lum G, Mulhall BP. Failure of azithromycin therapy in gonorrhea and discorrelation with laboratory test parameters. Sex Transm Dis 1998;25:505-8. [DOI] [PubMed] [Google Scholar]
- 46.National Committee for Clinical Laboratory Standards. Performance Standard for Antimicrobial Susceptibility Testing: Thirteenth informational supplement M100-S13. Wayne, Pennsylvania: National Committee for Clinical Laboratory Standards, 2003;23:43 [Google Scholar]
- 47.Kellogg DS Jr, Peacock WL Jr, Deacon WE, Brown L, Pirkle DI. Neisseria gonorrhoeae I. Virulence genetically linked to clonal variation. J Bacteriol 1963;85:1274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehret JM, Nims LJ, Judson FN. A clinical isolate of Neisseria gonorrhoeae with in vitro resistance to erythromycin and decreased susceptibility to azithromycin. Sex Transm Dis 1996;23:270-2. [DOI] [PubMed] [Google Scholar]
- 49.Yasin RM, Suan KA, Meng CY. Comparison of E-test with agar dilution methods in testing susceptibility of N gonorrhoeae to azithromycin. Sex Transm Dis 1997;24:257-60. [DOI] [PubMed] [Google Scholar]
- 50.Daly CC, Hoffman I, Hobbs M, et al. Development of an antimicrobial susceptibility surveillance system for Neisseria gonorrhoeae in Malawi: Comparison of methods. J Clin Microbiol 1997;35:2985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janda WM, Wilcoski LM, Mandel KL, Ruther P, Stevens JM. Comparison of monoclonal antibody methods and a ribosomal ribonucleic acid probe test for Neisseria gonorrhoeae culture confirmation. Eur J Clin Microbiol Infect Dis 1993;12:177-84. [DOI] [PubMed] [Google Scholar]
- 52.Livengood CH 3rd, Wrenn JW. Evaluation of COBAS AMPLICOR (Roche): Accuracy in detection of Chlamydia trachomatis and Neisseria gonorrhoeae by coamplification of endocervical specimens. J Clin Microbiol 2001;39:2928-32. [DOI] [PMC free article] [PubMed] [Google Scholar]