Abstract
Francisella philomiragia is a rare and opportunistic pathogen capable of producing invasive infection in patients with compromised neutrophil function and in patients that have survived a near-drowning. A case of F philomiragia adenitis and lung nodules, refractory to cephalosporin therapy, is reported in a 10-year-old boy with chronic granulomatous disease following a facial abrasion from a saltwater crab. To the authors' knowledge, this is the first Canadian clinical isolate to be reported. Genus and species identification was confirmed via 16S ribosomal RNA sequence analysis. A literature review revealed three groups at risk of F philomiragia infection: young patients with chronic granulomatous disease; adults with hematogenous malignancy; and near-drowning patients. Pneumonia, fever without an apparent source and sepsis are the main clinical presentations. Invasive procedures may be required to isolate this organism and ensure appropriate antimicrobial therapy. Limited awareness of F philomiragia has led to delayed identification, patient death and misidentification as Francisella tularensis - a biosafety level three pathogen and potential bioterrorism agent.
Key Words: Chronic granulomatous disease, Francisella philomiragia
The present article describes the first case of Francisella philomiragia infection reported in Canada, which manifested as adenitis and pulmonary infection in a patient with chronic granulomatous disease (CGD). As a member of the Francisella genus, F philomiragia is a strictly aerobic, Gram-negative coccobacillus. Unlike its more virulent relative Francisella tularensis, however, F philomiragia is an opportunist, causing pneumonia and systemic infection almost exclusively in immunocompromised patients or following near-drowning. Limited awareness of this organism has led to delayed diagnosis, therapeutic failures and poor outcomes. Confusion with F tularensis may lead to false biohazard alarms, as occurred in the present case.
Case Presentation
A 10-year-old boy with CGD and on long-term itraconazole and trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis presented with a two-week history of fatigue and intermittent fever, followed by the appearance of tender, left-sided submandibular swelling. He subsequently developed a nonproductive cough and was brought to his physician for assessment. His medical history noted that he had never travelled outside of Atlantic Canada and had no animal contacts. Just before the illness, a friend had tossed a saltwater crab at the patient, striking him on the left cheek and leaving an abrasion.
Initial physical examination noted a febrile, tired-appearing boy with a dry cough and no respiratory distress. Several oral ulcerations were noted, and palpation of the submandibular swelling revealed a mildly tender 2 cm left-sided submandibular lymph node. Crackles were detected at the right lung base. Examination was otherwise normal. A chest x-ray was interpreted as normal. On complete blood count testing, the peripheral leukocyte count was 15x109/L with 77% neutrophils. Blood and urine cultures were collected.
Hospital admission was arranged, and empirical therapy with intravenous cefuroxime and oral azithromycin was initiated. Fever persisted over the next 72 h, prompting the replacement of cefuroxime with intravenous cefotaxime. Blood and urine cultures were reported as negative. Despite broad-spectrum antimicrobial therapy, the patient failed to defervesce, had ongoing tenderness of the node and, of more concern, became tachypneic. Examination of a follow-up chest x-ray revealed a small effusion at the right lung base.
A computed tomography scan of the neck and thorax was performed. An inspection of the images noted extensive noncaseating enlargement of several submandibular and deep axillary nodes and a wedge-shaped consolidation in the right lung (Figure 1) accompanied by several pulmonary nodules (Figure 2).
Figure 1.
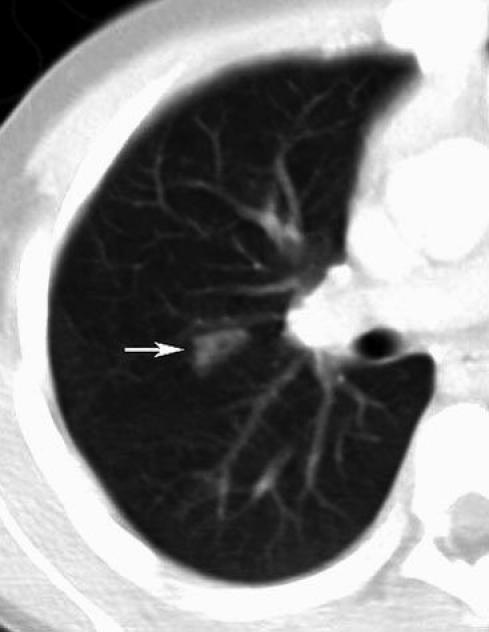
Computed tomography examination of the thorax with intravenous contrast enhancement shows a small triangular airspace opacity in the posterior segment of the right upper lobe (arrow)
Figure 2.
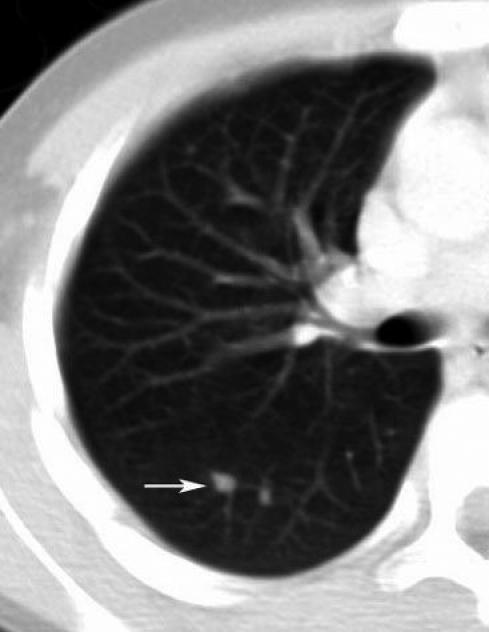
Computed tomography image of the right lung demonstrating pulmonary nodules (arrow)
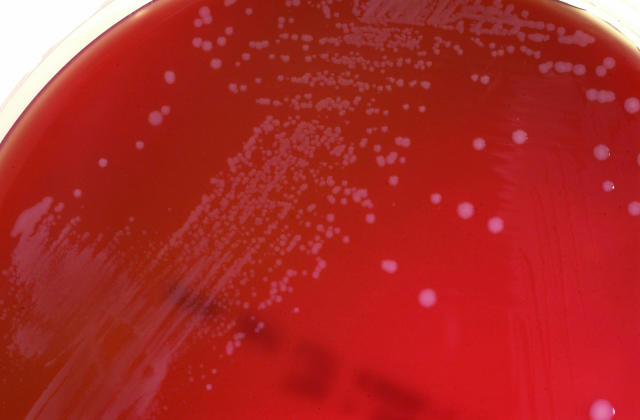
A submandibular node was surgically excised, sent for histological examination and cultured for bacteria, mycobacteria and viruses. The histopathology report described a mildly reactive histiocytosis of the node without granuloma formation. Acid-fast and Gram stains of tissue were negative. Within 48 h, tiny, weakly catalase-positive colonies were observed on sheep blood agar incubated aerobically and chocolate agar incubated in 5% CO2 (Figure 3).
Figure 3.
Typical Francisella philomiragia colonies on sheep blood agar have a smooth, white and convex appearance
Microscopy of Gram-stained organisms noted bizarre, amorphous, Gram-negative material. Closer examination revealed very tiny, poorly stained, Gram-negative coccobacilli. These were initially (and incorrectly) interpreted as oxidase-negative, prompting consideration of the organism as F tularensis, which is a biohazard level three agent. The organism was transferred to a level three laboratory for definitive identification, and the patient's antibiotic regimen was changed to ciprofloxacin monotherapy.
Four laboratory staff had handled the organism on an open bench. Due to potential F tularensis exposure, these staff members were assessed at the infectious diseases clinic of the Queen Elizabeth II Health Sciences Centre (Nova Scotia) and placed on prophylactic doxycycline pending identification of the organism.
Subsequently, the oxidase test was repeated and determined to be weakly positive (ruling out F tularensis). The organism grew as a nonmotile strict aerobe that produced hydrogen sulfide on triple sugar iron agar slants (Figure 4). Acid production from glucose and sucrose was demonstrated and gelatin was slowly hydrolyzed. The isolate was tentatively identified as F philomiragia and confirmed as such by 16S ribosomal RNA sequencing.
Figure 4.
This thin line of hydrogen sulfide production (arrow) in a triple sugar iron slant is one of the key features in the identification of Francisella philomiragia
While susceptibility testing remains nonstandardized for Francisella species, both Kirby-Bauer and broth microdilution minimum inhibitory concentration (MIC) methods performed at the IWK Health Centre (Nova Scotia) suggested TMP-SMX resistance (no zone of inhibition surrounding the TMP-SMX disc and an MIC greater than 2/38 µg/mL). MIC testing further suggested ampicillin resistance (MIC greater than 16 µg/mL) and susceptibility to both cefotaxime (MIC less than 8 µg/mL) and ciprofloxacin (MIC less than 0.5 µg/mL).
The patient improved dramatically after starting ciprofloxacin and was discharged five days later to complete a four to six week course. At follow-up, symptoms had resolved completely, and a repeat computed tomography scan documented resolution of the initial findings.
Materials and Methods
All English-language literature was reviewed to identify reported cases of F philomiragia infection. The data extracted from each case are summarized in Table 1.
TABLE 1.
Demographic and clinical features of patients infected with Francisella philomiragia
| Patient | Age/sex (reference) | Date of isolation | Geographic source | Clinical source | Underlying illness | Clinical presentation | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 18/male (1) | 12/27/74 | California, USA | Lung biopsy | CGD | Pneumonia | Chloramphenicol, sulfisoxazole, penicillin G |
| 2 | 39/male (1) | 10/21/76 | Colorado, USA | Pleural fluid | Pleural effusion | Fever | None |
| 3 | NA/male (1) | 10/04/77 | New York, USA | Blood | Near-drowning | NA | NA |
| 4 | 39/male (1) | 9/13/78 | California, USA | Blood | Near-drowning | Pneumonia | Cephalothin, gentamicin |
| 5 | 6/male (1) | July 1979 | Switzerland | Blood, bone marrow, ascitic fluid | CGD | Fever, sepsis | Cefotaxime, amikacin, trimethoprim- sulfamethoxazole |
| 6 | 68/female (1) | 1/16/80 | Pennsylvania, USA | Blood | Agnogenic myeloid metaplasia | Fever | Cephalexin, tobramycin |
| 7 | 86/female (1) | 7/15/80 | Connecticut, USA | Blood | Near-drowning | Pneumonia | Oxacillin, gentamicin |
| 8 | 75/male (1) | 8/19/80 | Connecticut, USA | Blood | Near-drowning | Pneumonia | Cephalothin, gentamicin |
| 9 | 5/male (1) | 1/30/81 | New York, USA | Blood | CGD | Fever | NA |
| 10 | 12/female (1) | 9/13/81 | California, USA | Lung biopsy | CGD | Pneumonia | Erythromycin, rifampin |
| 11 | 34/female (1) | 8/15/84 | New Mexico, USA | Ascitic fluid | None | Peritonitis | Clindamycin, gentamicin |
| 12 | 28/male (1) | 5/14/85 | Virginia, USA | Blood | Near-drowning | Sepsis | NA |
| 13 | 47/female (1) | 9/16/86 | New York, USA | Blood, pericardial fluid | Hodgkin’s disease | Fever, sepsis | Erythromycin, tobramycin, trimethoprim- sulfamethoxazole |
| 14 | 16/male (1) | 8/23/86 | Massachusetts, USA | Cerebrospinal fluid | CGD | Meningitis | Vancomycin, gentamicin, others |
| 15 | 19/male (3) | ‘Summer’ | Maryland, USA | Blood | CGD | Fever | Cefotaxime, gentamicin, ciprofloxacin |
| 16 | 10/male* | 8/20/03 | Nova Scotia | Lymph node | CGD | Adenitis, pneumonia | Ciprofloxacin |
| 17 | 24/male (5) | ‘Summer’ | Turkey | Blood | CGD | Pneumonia, sepsis | Cefuroxime, ciprofloxacin, meropenem, others |
This patient is from the present report. CGD Chronic granulomatous disease; NA Not available; USA United States of America
Results
Seventeen cases of F philomiragia infection were identified (1-5). As noted by Wenger et al (1), patients fell into three main categories: CGD patients (mean age 14 years); patients that experienced near-drownings in saltwater; and adults with hematological malignancy. Only two cases had no discernable risk factor. CGD, the main predisposing factor, was present in eight of 17 (47%) cases. Saltwater immersions and hematological malignancies were present in five of 17 (29%) and two of 17 (11%) cases, respectively. Fifteen of 17 (88%) reported cases occurred within 50 miles of a saltwater coastline, often involving direct contact with saltwater. Most cases occurred during summer or fall, both of which are peak times for recreational saltwater exposure. Only three of 17 cases occurred during the other half of the year (from November to April). Recurring clinical presentations included pneumonia (seven of 17 cases), nonlocalized fever (four of 17 cases) and sepsis (three of 17 cases). All three cases of pneumonia in CGD patients required an invasive procedure (biopsy) to recover the organism. Including the present case, three patients with CGD developed F philomiragia infection during TMP-SMX prophylaxis. While there are little published data on the susceptibility of this organism to TMP-SMX, the authors' isolate appeared to be completely resistant, exhibiting no zone of inhibition on Kirby-Bauer disc susceptibility testing.
Discussion
In 1969, Yersinia philomiragia was first reported as a novel muskrat pathogen (6). Today, the organism is categorized as a Francisella species and is recognized as an opportunistic pathogen in humans. It shares unique fatty acids, marked DNA homology and phenotypic features with F tularensis; both appear in the laboratory as tiny, nonmotile, strictly aerobic Gram-negative coccobacilli (1,7). Both take up Gram's counterstain poorly, have weakly positive catalase tests and grow on chocolate or cysteine-supplemented agar, yielding smooth, raised, moist colonies. F philomiragia is cysteine-independent and yields white colonies (Figure 3). In contrast, F tularensis is fastidious, with colonies typically appearing gray. Identification of F philomiragia may be achieved by demonstrating a positive Kovac's oxidase test (100% of strains positive), the production of hydrogen sulfide in triple sugar iron agar (100% positive) and delayed gelatin hydrolysis (80% positive). DNA amplification methods and sequencing can confirm species identification.
Despite genetic similarities, F philomiragia infection is epidemiologically distinct from diseases due to F tularensis. Neither animal nor arthropod vectors are implicated for F philomiragia and although initial isolates of the organism came from freshwater, human cases have often been associated with saltwater exposure. Tularemia, in contrast, is acquired primarily through contact with infected animals or ticks or through ingestion of contaminated freshwater or meat. F philomiragia is an opportunist, while most F tularensis infections occur in previously healthy people.
Similar disease manifestations for the Francisella species imply shared routes of pathogenesis. While F tularensis is clearly more virulent, both organisms are facultative intracellular organisms capable of infecting reticuloendothelial tissues. Penetration of F tularensis through intact or abraded skin is followed by localized inflammation. Failure to contain the infection results in spread to regional lymph nodes. Lymphohematogenous dissemination may then occur, causing the varied presentations of tularemia. The crab-induced abrasion and subsequent local adenitis observed in the present patient suggests that, like tularemia, cutaneous inoculation is also a probable route of F philomiragia acquisition. This first report of lymph node involvement during F philomiragia infection is directly comparable to 'glandular' tularemia. Although both species of Francisella are now recognized to cause pulmonary infections, micronodular disease as seen in the present patient has not been previously reported with either organism. Cavitating pulmonary lesions have been reported as a rare complication of tularemia - perhaps the end result of a process that started as micronodules (8). F philomiragia cases presenting with pneumonia or sepsis parallel 'pneumonic' and 'typhoidal' forms of tularemia. Meningitis and peritonitis, present in one case each of F philomiragia infection, have also been reported with tularemia. Because lung (or in the present case, node) biopsy has been required to isolate the organism from CGD patients with pneumonia, F philomiragia infection may be more common than reported cases suggest.
The contribution of lipopolysaccharide (LPS) to the virulence of Francisella species has been examined in recent animal models (9). When characterized by LPS chemotypes, wild strains of F tularensis cause rapid death following intraperitoneal injection in mice. A single F tularensis bacillus has been shown to cause death in mice, guinea pigs and hamsters. In contrast, F philomiragia, which produces lipooligosaccharide, behaves like LPS-deficient F tularensis strains and has caused significantly less morbidity and mortality in animal models.
Although susceptibility testing of Francisella species remains nonstandardized, the Clinical Laboratory and Standards Institute (Pennsylvania, USA; formerly the National Committee for Clinical Laboratory Standards, or NCCLS) have formed a Working Group on Susceptibility Testing of Fastidious Organisms that may soon provide standardized breakpoints for interpretation. Until then, laboratory data should be used with caution. Therapeutic failures involving F philomiragia infection have been documented, for example, despite apparent in vitro susceptibility to cephalosporins (5). While all 14 of the isolates initially described by Wenger et al (1) were susceptible to third-generation cephalosporins, all produced beta-lactamase and were ampicillin-resistant. A more recent study (3) provided the first clinical report of in vitro cefotaxime resistance. To date, there have been no clinical isolates reported with resistance to ciprofloxacin, and it may be prudent to consider this agent as part of a therapeutic regimen in moderately to severely ill patients. The best therapeutic plan, however, remains contingent on early diagnosis through clinical suspicion and rapid laboratory diagnosis of this rare pathogen.
References
- 1.Wenger JD, Hollis DG, Weaver RE, et al. Infection caused by Francisella philomiragia (formerly Yersinia philomiragia). A newly recognized human pathogen. Ann Intern Med 1989;110:888-92. [DOI] [PubMed] [Google Scholar]
- 2.Seger RA, Hollis DG, Weaver RE, Hitzig WH. Chronic granulomatous disease: Fatal septicemia caused by an unnamed gram-negative bacterium. J Clin Microbiol 1982;16:821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sicherer SH, Asturias EJ, Winkelstein JA, Dick JD, Willoughby RE. Francisella philomiragia sepsis in chronic granulomatous disease. Pediatr Infect Dis J 1997;16:420-2. [DOI] [PubMed] [Google Scholar]
- 4.Polack FP, Harrington SM, Winkelstein JA, Merz WG, Willoughby RE. Recurrent Francisella philomiragia sepsis in chronic granulomatous disease. Pediatr Infect Dis J 1998;17:442-3. [DOI] [PubMed] [Google Scholar]
- 5.Friis-Moller A, Lemming LE, Valerius NH, Bruun B. Problems in identification of Francisella philomiragia associated with fatal bacteremia in a patient with chronic granulomatous disease. J Clin Microbiol 2004;42:1840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen WI, Owen CR, Jellison WL. Yersinia philomiragia sp. n., a new member of the Pasteurella group of bacteria, naturally pathogenic for the muskrat (Ondatra zibethica). J Bacteriol 1969;100:1237-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollis DG, Weaver RE, Steigerwalt AG, Wenger JD, Moss CW, Brenner DJ. Francisella philomiragia comb. nov. (formerly Yersinia philomiragia) and Francisella tularensis biogroup novicida (formerly Francisella novicida) associated with human disease. J Clin Microbiol 1989;27:1601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozak AJ, Hall WH, Gerding DN. Cavitary pneumonia associated with tularemia. Chest 1978;73:426-7. [DOI] [PubMed] [Google Scholar]
- 9.Onoprienko NN, Pavlovich NV. [The role of lipopolysaccharide in toxicity of Francisella genus bacteria]. Mol Gen Mikrobiol Virusol 2003;(3):25-8. [PubMed] [Google Scholar]