Abstract
Background
Linezolid is approved for the treatment of designated infections caused by methicillin-resistant and -susceptible Staphylococcus aureus and vancomycin-resistant Enterococcus faecium.
Objective
To characterize linezolid utilization since its launch in Canada in 2001.
Methods
Demographics, antimicrobial regimens, and clinical and resource utilization data for linezolid-treated patients were collected retrospectively by hospital pharmacists at nine tertiary care hospitals in four provinces. Statistics describing linezolid utilization were calculated and the appropriateness of use was assessed according to a treatment algorithm based on recommendations of the Infectious Diseases Pharmacy Specialty Network in 2001.
Results
Ninety-nine linezolid courses were prescribed for 103 infections in 95 patients (mean age 57.8 years, 52.6% male) with an average length of hospital stay of 40.6 days. Fifty-three per cent of patients had an allergy to at least one antibiotic other than linezolid. The major use of linezolid was for treatment of skin and soft tissue infections (32.0%), followed by bacteremia (15.5%). The most prevalent pathogen was methicillin-resistant S aureus, identified in 44.7% of infections. Linezolid was primarily prescribed as the oral form following other intravenous anti-infectives (55.6% of courses) for an average duration of 14.4 days. The rate of appropriate utilization was 53% (range 25% to 75% by site). In 93.5% of courses deemed inappropriate, recommended first-line therapies were not attempted before linezolid.
Conclusions
Linezolid was prescribed appropriately in approximately one-half of cases reviewed. The rate of appropriate utilization is similar to those rates reported in other Canadian antibiotic reviews.
Key Words: Drug utilization, Linezolid, Staphylococcus aureus
Linezolid is an oxazolidinone antibiotic that inhibits bacterial protein synthesis with activity against Gram-positive bacteria, including multidrug-resistant, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VREF) and Enterococcus faecalis (1-3). Linezolid was approved for use in Canada on April 4, 2001, for treatment of adults with VREF, group A and B streptococcus, methicillin-susceptible and -resistant S aureus, and penicillin-susceptible Streptococcus pneumoniae. Linezolid is available in both intravenous (IV) and 100% bioavailable oral forms. Currently, microbial resistance to linezolid is not significant in Canada (4).
Rates of inappropriate antibiotic use are high in Canada (5-10). This is particularly concerning because it has been demonstrated that inappropriate use increases costs related to treatments (5-7). The present study’s primary objective was to characterize linezolid utilization in selected provinces in Canada and determine its rate of appropriate use as defined in the Infectious Diseases Pharmacy Specialty Network (ID-PSN) recommendations. A secondary objective was to examine associated resource utilization.
Methods
Study Investigators and Sites
Nine hospitals from across Canada were involved in the study, including four sites in Quebec, three sites in Ontario, one site in Alberta and one site in British Columbia. These provinces were chosen for feasibility reasons – it was likely that linezolid use would be observed in these provinces due to the fact that they have higher MRSA incidence rates than the other Canadian provinces (11).
No sample size calculations were conducted. Because there had been relatively limited use of the product over the 12-month period before the study, it was estimated that 10 treatment courses per site would be the inclusion criteria and provide a representative sample size. This is consistent with published antibiotic drug utilization review (DUR) studies conducted in Canada (5-7,9,10,12).
A pharmacist at each site was designated as the study investigator (see Appendix). The study protocol was approved by the governing ethics review board of each site. Study procedures were standardized among sites by the use of a single instruction guide at each site, a uniform training session for each pharmacist before initiation of data collection and by the provision of telephone support throughout the study to address questions.
Patient Population
A pharmacy database at each site was used to identify hospitalized patients who received IV and/or oral linezolid treatment for an active infection. The course of linezolid may have been prescribed during hospitalization or at discharge from hospital. Each chart identified through the database search was screened for study eligibility. The inclusion and exclusion criteria were minimal to mimic real-life linezolid use. Patients were eligible for the study if they were at least 18 years of age, hospitalized at a study site and had received at least one linezolid treatment course (IV and/or oral) between October 1, 2001, and December 31, 2002. To discriminate between the number of linezolid treatment courses received by a patient, a single course was defined as continuous treatment with an interruption of no greater than 72 h. Patients were excluded from the study if they were enrolled in a clinical trial during hospitalization or treatment. If a site could not identify 10 eligible patients, a different site with higher linezolid use was asked to recruit more patients, with up to 20 patients per site.
Procedure
Data relevant to the determination of the level of appropriate linezolid use were abstracted from each chart. These data included the following: site and type of infection; culture and sensitivity results; antimicrobials prescribed before, simultaneous to or succeeding linezolid treatment for the same infection (start and stop dates, dose and route of administration); details of linezolid use (start and stop dates, dose and route of administration); and antibiotic contraindications (allergy history and significant rifampin drug interactions). Antibiotic contraindications also included a history of vancomycin intolerance. This intolerance was defined as any of the following: nephrotoxicity due to vancomycin (defined as two consecutive tests showing an absolute rise in serum creatinine levels [sCr] by 44 µmol/L or a relative rise in sCr by 50% from baseline, where baseline was the first sCr measured from the day of initiating vancomycin therapy); signs and symptoms of ototoxicity attributed to vancomycin; and vancomycin hypersensitivity reactions. The definition of vancomycin intolerance was based on the clinical judgment of the study investigators. Data on basic demographics and resource utilization (defined as duration of hospitalization) were also collected.
Data Analysis
Data from all sites were pooled for all analyses except for the appropriate utilization analysis, in which case total and site-specific analyses were conducted. For provincial appropriateness ratings, data from Alberta and British Columbia were combined to represent the western provinces.
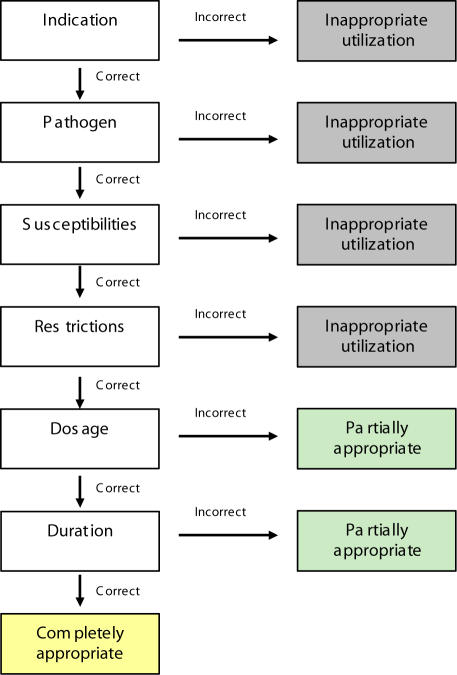
The proportion of treatment courses were classified as appropriate, partially appropriate or inappropriate. Treatment course classification was based on a decision algorithm shown in Figure 1. The decision algorithm was based on predefined treatment recommendations suggested by the ID-PSN (Table 1), which were reviewed and refined by the clinical expert and study advisors (see Appendix). Appropriateness of linezolid use was evaluated based on infection site, pathogen, sensitivities, contraindications, dose, dosage interval and duration of treatment. A treatment course was deemed completely appropriate if all criteria were met. A course was deemed partially appropriate if the dose and/or duration of treatment criteria were not met. A treatment course was considered inappropriate if it violated the criteria for appropriate indication, pathogen, sensitivities or contraindications.
Figure 1.
Appropriate use decision algorithm template
TABLE 1.
Summary of Infectious Disease Pharmacy Specialty Network recommendations for appropriate use of linezolid*
| Indication | Infecting pathogen | Restrictions | Dose (IV or PO) | Duration of therapy† |
|---|---|---|---|---|
| Skin and soft tissue infections (complicated and uncomplicated) | Methicillin-susceptible Staphylococcus aureus | Allergy or resistance to other first-line agents for which culture and sensitivity is reported by the institution’s microbiology laboratory (beta-lactams, cephalosporins, clindamycin, cotrimoxazole, fluoroquinolones, macrolides, tetracyclines), including allergy or intolerance to vancomycin | ≤30 kg adult 300 mg every 12 h, >30 kg adult 600 mg every 12 h | ≤28 days |
| Methicillin-resistant S aureus | Allergy or resistance to other first-line agents for which culture and sensitivity is reported by the institution’s microbiology laboratory (fusidic acid combination therapy, rifampin combination therapy [unless there is rifampin drug interaction], cotrimoxazole, clindamycin), including allergy or intolerance to vancomycin | |||
| Pneumonia | Pneumonia (PSSP, PRSP and multiresistant species) | Allergy or resistance to other first-line agents for which culture and sensitivity is reported by the institution’s microbiology laboratory (beta-lactams, cephalosporins, cotrimoxazole, fluoroquinolones, macrolides, tetracyclines), including allergy or intolerance to vancomycin | ≤30 kg adult 300 mg every 12 h, >30 kg adult 600 mg every 12 h | ≤28 days |
| Methicillin-susceptible S aureus | Allergy or resistance to other first-line agents for which culture and sensitivity is reported by the institution’s microbiology laboratory (beta-lactams, cephalosporins, clindamycin, cotrimoxazole, fluoroquinolones, macrolides, tetracyclines), including allergy or intolerance to vancomycin | |||
| Methicillin-resistant S aureus | Allergy or resistance to other first-line agents for which culture and sensitivity is reported by the institution’s microbiology laboratory (fusidic acid combination therapy, rifampin combination therapy [unless there is rifampin drug interaction], cotrimoxazole, clindamycin), including allergy or intolerance to vancomycin | |||
| Infection (including osteomyelitis and prosthetic joint infection) | Vancomycin-resistant enterococci | Infectious diseases specialist consultation required | ≤30 kg adult 300 mg every 12 h, >30 kg adult 600 mg every 12 h | ≤28 days |
| Osteomyelitis and prosthetic joint infection | Methicillin-resistant S aureus or coagulase-negative methicillin-resistant staphylococci | Allergy or resistance to other first-line agents for which culture and sensitivity is reported by the institution’s microbiology laboratory (fusidic acid combination therapy, rifampin combination therapy [unless rifampin drug interaction], cotrimoxazole, clindamycin), including allergy or intolerance to vancomycin | ≤30 kg adult 300 mg every 12 h, >30 kg adult 600 mg every 12 h | ≤28 days |
Please see disclaimer at the end of the article;
Could be longer, if clinically necessary. IV Intravenous form; PO Oral form; PRSP Penicillin-resistant Streptococcus pneumoniae; PSSP Penicillin-susceptible S pneumoniae
If a decision on the level of appropriateness could not be made because a treatment course did not fit the decision algorithm, a blinded adjudication process was used. In summary, two study advisors (see Appendix) were independently presented with all clinical data collected for each treatment course that did not fall within one of the appropriateness categories; the advisors then classified them according to the classification system. For treatment courses where disagreement remained, the data were forwarded to the clinical expert (see Appendix) for a final decision.
The total duration of hospitalization was calculated as the number of days between hospital admission and discharge. Infection-related hospitalization was estimated and defined as the number of days from the first day of anti-infective treatment received in hospital until the date of successful infection treatment in hospital, the date of discharge or until the patient died in hospital, whichever occurred first.
Results
The study enrolled 95 patients who received 99 linezolid courses for treatment of 103 infections. Most patients were from sites in Ontario and Quebec (38.9% and 36.8%, respectively). Patient demographics are summarized in Table 2. Bone and joint surgeries comprised 41.2% of procedures performed in patients with surgeries in the previous six months. Debridement and/or irrigation of various sites comprised 35.9% of all procedures performed in patients with surgery during hospitalization. For the nine patients who expired during hospitalization, the causes of death were bacteremia (three patients), respiratory conditions (respiratory failure, broncho-aspiration, invasive aspergillosis and idiopathic pulmonary fibrosis, one patient each) and cancer relapse (one patient). One death was due to an unknown cause.
TABLE 2.
Patient demographics (n=95)
| Mean age, years (range) | 57.8 (24–100) |
| Male, n (%) | 50 (52.6) |
| Patients with surgery in previous six months, n (%) | 24 (25.3)* |
| Patients with surgery during hospitalization, n (%) | 39 (41.1)† |
| Deaths during hospitalization, n (%) | 9 (9.5) |
| Mean linezolid treatment courses per patient | 1.04 |
Data missing for one patient;
Data missing for three patients
The pathogens identified by type of infection are shown in Table 3. The major use of linezolid was for treatment of skin and soft tissue infections (SSTI) (32.0%), followed by bacteremia (15.5%). The most prevalent pathogen was MRSA, identified in 44.7% of infections.
TABLE 3.
Percentage of infections with major pathogen identified
| Pathogen | Infection type | |||||
|---|---|---|---|---|---|---|
| SSTI | Pneumonia | Osteomyelitis | Prosthetic joint | Other | Unknown | |
| Total infections, n | 33 | 11 | 6 | 11 | 40 | 2 |
| Staphylococcus aureus, % | 54.5 | 72.7 | 83.3 | 27.3 | 55.0 | 0 |
| Methicillin-susceptible | 22.2 | 0 | 20.0 | 33.3 | 18.2 | 0 |
| Methicillin-resistant | 77.8 | 100 | 80.0 | 66.6 | 81.8 | 0 |
| Streptococcus pneumoniae, % | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillin-susceptible | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillin-resistant | 0 | 0 | 0 | 0 | 0 | 0 |
| Multidrug-resistant | 0 | 0 | 0 | 0 | 0 | 0 |
| Enterococcus species, % | 18.2 | 9.1 | 0 | 18.2 | 27.5 | 50.0 |
| Vancomycin-susceptible | 50.0 | 100 | 0 | 100 | 63.6 | 0 |
| Vancomycin-resistant | 33.3 | 0 | 0 | 0 | 36.4 | 100 |
| Unknown | 16.7 | 0 | 0 | 0 | 0 | 0 |
| Coagulase-negative staphylococci, % | 21.2 | 0 | 0 | 36.4 | 15.0 | 50.0 |
| Methicillin-susceptible | 28.6 | 0 | 0 | 25.0 | 16.7 | 0 |
| Methicillin-resistant | 57.1 | 0 | 0 | 75.0 | 83.3 | 100 |
| Unknown | 14.3 | 0 | 0 | 0 | 0 | 0 |
| Infections with no pathogen identified, n | 5 | 3 | 1 | 2 | 3 | 0 |
SSTI Skin and soft tissue infections
In 55.8% of patients, anti-infective treatment began before admission and continued following discharge from hospital. An allergy history to one or more antibiotic was reported in 52.6% of patients. Allergies to penicillin, vancomycin and the cephalosporins were the most common. A history of vancomycin intolerance was reported in 24% of patients and 18.9% of patients were unable to receive rifampin due to potential drug interactions.
The mean duration of total anti-infective treatment was 34.8 days (range one to 180 days). The mean durations of in-hospital and out-of-hospital anti-infective treatment were 24.2 days and 19.0 days, respectively. The duration of linezolid treatment and hospitalization are presented in Table 4. The majority of patients received some form of anti-infective drug before initiating linezolid treatment. In these patients, vancomycin and ciprofloxacin were the most common agents. Also, a small proportion received at least one other anti-infective following linezolid treatment. The most commonly initiated anti-infectives were vancomycin, rifampin and ciprofloxacin. Linezolid was used concomitantly (more than a two-day overlap) with other anti-infective drugs in 27.4% of patients.
TABLE 4.
Anti-infective therapies and length of hospitalization (n=95)
| Resource | Patients, n (%) | Mean days ± SD |
|---|---|---|
| Anti-infective therapy | 80 (84.2) | 23.1±28.2 |
| Initiated before linezolid | ||
| Linezolid | 95 (100) | 14.4±11.8* |
| Oral form only | 59 (62.1) | 13.6±11.0 |
| Intravenous form only | 17 (17.9) | 11.4±10.0 |
| Intravenous and oral forms | 19 (20.0) | 22.4±15.8 |
| Initiated following linezolid | 24 (25.3) | 32.9±29.1 |
| Hospitalization | ||
| Total hospital length of stay | 95 (100) | 40.6±46.8 |
| Infection-related length of stay | 95 (100) | 24.1±21.1 |
| Total rehospitalization length of stay | 6 (6.3) | 9.5±6.4 |
Mean treatment duration based on 96 linezolid courses because dates not provided for three courses
Linezolid treatment courses lasted a mean of 14.4 days, with a minimum treatment period of one day (eg, due to treatment interruption for surgery) and a maximum of 57 days. Linezolid was primarily prescribed in the oral form. In 52.6% of patients, treatment of the infection with linezolid was successful. Linezolid was discontinued in 44.2% of patients, and the most common reason for discontinuation was the switch to another anti-infective (9.5%).
Patients spent a mean of 40.6 days in hospital and anti-infective treatment was administered 58.5% of this time. Of the 31.6% of patients who still required anti-infective treatment following discharge from hospital, the majority were discharged on linezolid in the oral form. Readmittance to the same hospital due to an event deemed to be associated with linezolid-treated infection occurred for 6.3% of patients and resulted in a mean of 9.0 additional days in hospital.
For the different linezolid formulations, the rate of appropriate use is presented in Table 5. The total linezolid treatment course was deemed appropriate in 53% of the 99 courses evaluated, partially appropriate in 1% of courses and inappropriate in 46% of courses. In 93.5% of courses deemed inappropriate, recommended first-line therapies were not attempted before linezolid. When the form of linezolid used was considered in addition to the sequence in which it was given, the highest rate of appropriate utilization occurred when linezolid was given following other anti-infectives in the IV to oral combination and during the switch to the oral form directly from other anti-infectives.
TABLE 5.
Linezolid utilization classification by form of linezolid prescribed
| Form used in linezolid treatment course | Appropriate courses, n (%) | Partially appropriate courses, n (%) | Inappropriate courses, n (%) | |
|---|---|---|---|---|
| Total linezolid courses | 52 (53) | 1 (1) | 46 (46) | 99 (100) |
| Linezolid used as initial therapy | 4 (25) | 0 | 12 (75) | 16 (16) |
| Oral form only | 1 (14) | 0 | 6 (86) | 7 (44) |
| Intravenous and oral forms | 3 (60) | 0 | 2 (40) | 5 (31) |
| Intravenous form only | 0 | 0 | 4 (100) | 4 (25) |
| Linezolid used following other anti-infectives | 48 (58) | 1 (1) | 34 (41) | 83 (84) |
| Oral form only | 35 (64) | 0 | 20 (36) | 55 (66) |
| Intravenous and oral forms | 10 (67) | 0 | 5 (33) | 15 (18) |
| Intravenous form only | 3 (23) | 1 (8) | 9 (69) | 13 (16) |
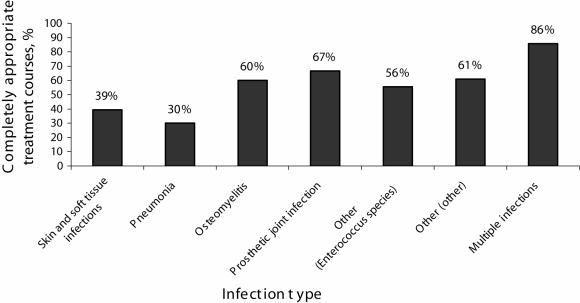
The rate of appropriate use for each infection type is shown in Figure 2. The highest rate of appropriate use included that of linezolid treatment for osteomyelitis, prosthetic joint infections, other infection types (due to Enterococcus species and other pathogens) and for treatment of patients with multiple infections. The rate of appropriate linezolid use also varied provincially and among sites. Quebec had the highest rate of appropriate linezolid use, followed by the western provinces and finally Ontario (59%, 57% and 44%, respectively). The rate of appropriate linezolid use was as low as 25% at one site and as high as 75% at another site.
Figure 2.
Linezolid utilization classification by infection type
Adjudication to determine the level of appropriateness was necessary in approximately 85% of the treatment courses. The first round of adjudication resolved 57% of these cases and the remainder were resolved by the next and final round of adjudication. Cases were adjudicated for the following reasons: infection and treatment characteristics; nonconfirmation of a pathogen; treatment of Enterococcus species infections when ampicillin susceptibilities were unknown or where no ampicillin contraindications existed; and for treatment of SSTI due to pathogens other than S aureus. Adjudication determined that in 51% of cases, linezolid use was appropriate, whereas nonadjudicated decisions deemed that in 60% of cases, linezolid use was appropriate.
Discussion
The present study characterized linezolid utilization in nine tertiary care centres across four provinces in Canada: Quebec, Ontario, Alberta and British Columbia. Overall, the rate of appropriate linezolid use was 53%. The mean duration of hospitalization was 40.6 days. Linezolid was primarily used to treat SSTI and bacteremias, with the most common pathogen being MRSA. The oral form of linezolid was most commonly prescribed following IV treatment with another anti-infective.
The level of appropriateness of linezolid utilization was assessed against recommendations developed by the ID-PSN pharmacists, with details finalized by the study advisors and the clinical expert. The recommendations reflected an evidence-based review of the existing clinical literature at the time the present DUR was conducted (2001), with the intent of publishing the guidelines. Infections considered by the guidelines included complicated and uncomplicated SSTI, pneumonia, infections due to vancomycin-resistant enterococci, osteomyelitis and prosthetic joint infections. Linezolid is not approved in Canada for use in pneumonia (caused by penicillin-resistant S pneumoniae and multiresistant species), prosthetic joint infections or for osteomyelitis. However, it was thought by the study advisors that, in light of the limited treatment alternatives available for these potentially life-threatening infections, the use of linezolid may be appropriate in certain settings when used after failed attempts by all other available agents.
The ID-PSN guidelines used to define appropriate use in the present study specify the prescribing of other anti-infective drugs before linezolid, except in certain situations. The majority of inappropriate linezolid use (93.5%) was due to use of linezolid as first-line therapy. Efforts could be made to clarify and emphasize proper first-line antibiotic therapy to improve linezolid use. The inclusion of an adjudication process and the large number of adjudicated cases (85%) in the present study reflects recognition by the clinical expert and study advisors that clinical practice does not always concretely fit into guidelines. As experience with linezolid increases, refinements to the guidelines will help to more completely define appropriate linezolid use.
The rate of inappropriate linezolid use in the present study is similar to those found in Canadian DUR studies of other antibiotics. The rate of inappropriate vancomycin use has been reported as 42% (7), 58.2% (6) and 65% (5). For other antibiotics, the rate of inappropriate use has been reported 42% for cefoxitin (6), 62% for metronidazole (10) and 80% for ciprofloxacin (8). Implementing drug utilization programs may reduce inappropriate antibiotic use. Several of these programs have been successfully implemented in Canada (13-15). The use of drug utilization programs may have accounted for some of the variations in inappropriate linezolid use at the different sites across Canada.
High rates of appropriate linezolid use were determined when linezolid was prescribed following treatment with another anti-infective drug. This high rate of appropriate use may be partially attributed to the stepdown to oral linezolid from IV treatment at the time of discharge to eliminate the use of IV treatment in outpatients. While this study was not designed to test whether this stepdown facilitated discharge from hospital, several Canadian studies have demonstrated that stepdown treatments reduce the duration of hospitalization (5,12,16,17). Indeed, Li et al (18) have shown that the duration of hospitalization is reduced during stepdown linezolid treatment compared with IV vancomycin treatment.
There are several limitations to the present study. Although the ID-PSN recommendations for appropriate use of linezolid were based on the literature available, they have not been published in a peer-reviewed journal nor has the appropriate use decision algorithm been validated. In addition, the relatively small sample size used in the present study, although likely capturing much of the total linezolid use in Canada at the time the study was conducted, may impact the ability to generalize the results. Furthermore, the use of a ‘convenience sample’ for selection of study sites further limits the ability of these results to be generalized with certainty across other regions of Canada. Despite these limitations, the present study was the first to assess the utilization of linezolid in selected Canadian provinces.
The present study provides an understanding of linezolid utilization in the four largest provinces in Canada. This information may be useful in identifying areas in which education could improve the use of linezolid. Rational use of this important medicine should contribute to extend its utility and optimize the occurrence of favourable patient outcomes.
Acknowledgments
The authors thank the investigators and significant contributors, particularly Dr Donald Low, Dr Karl Weiss and Dr Brian Conway. This was a Pharmacia Canada (now Pfizer Canada Inc)-sponsored study. The authors acknowledge the valuable contributions of Ms Angelina Wong and Ms Elizabeth Miller. Ms Wong and Ms Miller were employed by Pharmacia Canada at the time of the study. Dr Alissa Scalera is a paid employee of Pfizer Canada Inc. This article was prepared with the assistance of Script Medical Writing, Toronto, Ontario.
Disclaimer (Table 1)
Although the ID-PSN recommends the use of linezolid for pneumonia (caused by penicillin-resistant S pneumoniae and multiresistant species), prosthetic joint infections and osteomyelitis, these recommendations have not been approved by Health Canada; please consult the product monograph for linezolid for complete information. The ID-PSN recommendations were based on the literature available at the time the study was conducted. Clinicians should base their treatment practices on the product monograph.
Appendix
Individuals involved in the present study
Principal Advisors:
Dr Sandra Walker, Sunnybrook Health Sciences Centre, Toronto, Ontario; Dr Linda Dresser, Mount Sinai Hospital, Toronto, Ontario
Clinical Expert:
Dr Donald Low, Mount Sinai Hospital, Toronto, Ontario
Investigators:
Mr Luc Bergeron, Centre Hospitalier de l’Université Laval, Sainte-Foy, Quebec; Dr Anne Marie Bombassaro, London Health Sciences Centre, London, Ontario; Dr Glen Brown, St Paul’s Hospital, Vancouver, British Columbia; Ms Sylvie Carle, McGill University Health Centre, Montreal, Quebec; Ms Susan Fryters, Royal Alexandra Hospital, Edmonton, Alberta; Dr Dominique Richard, Hôpital Laval, Sainte-Foy, Quebec; Mr Barrie McTaggart, McMaster University Medical Centre, Hamilton, Ontario; Mr Michel Savoie, Hôpital Maisonneuve-Rosemont, Montreal, Quebec; Ms Rosemary Zvonar, Ottawa Hospital, Ottawa, Ontario
Adjudication Panel:
Dr Brian Conway, University of British Columbia, Vancouver, British Columbia; Dr Linda Dresser, Mount Sinai Hospital, Toronto, Ontairo; Dr Donald Low, Mount Sinai Hospital, Toronto, Ontario; Dr Sandra Walker, Sunnybrook Health Sciences Centre, Toronto, Ontario; Dr Karl Weiss, Hôpital Maisonneuve-Rosemont, Montreal, Quebec
References
- 1.Bozdogan B, Appelbaum PC. Oxazolidinones: Activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents 2004;23:113-9. [DOI] [PubMed] [Google Scholar]
- 2.Perry CM, Jarvis B. Linezolid: A review of its use in the management of serious gram-positive infections. Drugs 2001;61:525-51. [DOI] [PubMed] [Google Scholar]
- 3.Ford CW, Hamel JC, Stapert D, et al. Oxazolidinones: New antibacterial agents. Trends Microbiol 1997;5:196-200. [DOI] [PubMed] [Google Scholar]
- 4.Ballow CH, Jones RN, Biedenbach DJ; North American ZAPS Research Group. A multicenter evaluation of linezolid antimicrobial activity in North America. Diagn Microbiol Infect Dis 2002;43:75-83. [DOI] [PubMed] [Google Scholar]
- 5.Dranitsaris G, Pilla NJ, McGreer A. A vancomycin drug use evaluation and economic analysis in a cancer treatment centre. Can J Hosp Pharm 1994;47:59-64. [PubMed] [Google Scholar]
- 6.Lazor-Bajcar JM. Cefoxitin use review. Can J Hosp Pharm 1990;43:221-5,xxxii. [PubMed] [Google Scholar]
- 7.Madsen M, Taylor GD. Intravenous vancomycin usage in a tertiary care hospital. Can J Hosp Pharm 1989;42:153-6. [PubMed] [Google Scholar]
- 8.Mather JL, Bayliff CD, Reider MJ, Hussain Z, Colby WD. The impact of formulary reservations on drug utilization: A controlled trial. Can J Hosp Pharm 1994;47:111-6. [PubMed] [Google Scholar]
- 9.Piquette RK. A targeted review of vancomycin use. Can J Hosp Pharm 1991;44:83-7. [PubMed] [Google Scholar]
- 10.Wang JC, Conly JM, Shafran SD. Appropriateness of metronidazole use in a teaching hospital. Am J Hosp Pharm 1989;46:1385-9. [PubMed] [Google Scholar]
- 11.Simor AE, Ofner-Agostini M, Bryce E, et al. Canadian Nosocomial Infection Surveillance Program, Health Canada. The evolution of methicillin-resistant Staphylococcus aureus in Canadian hospitals: 5 years of national surveillance. CMAJ 2001;165:21-6. [PMC free article] [PubMed] [Google Scholar]
- 12.Frighetto L, Martinusen SM, Mamdani F, Jewesson PJ. Ciprofloxacin use under a reserved drug and stepdown promotion program. Can J Hosp Pharm 1995;48:35-42. [PubMed] [Google Scholar]
- 13.Blain L, O’Brodovich M. The effect of an education program on cefazolin prescribing. Can J Hosp Pharm 1989;42:69-71. [PubMed] [Google Scholar]
- 14.Gin AS, Lipinski LA, Honcharik N. Impact of a target drug monitoring program on the usage of clindamycin. Can J Hosp Pharm 1994;47:53-8. [PubMed] [Google Scholar]
- 15.Stewart J, Pilla J, Dunn L. Pilot study for appropriate anti-infective community therapy. Effect of a guideline-based strategy to optimize use of antibiotics. Can Fam Physician 2000;46:851-9. [PMC free article] [PubMed] [Google Scholar]
- 16.Jewesson P. Cost-effectiveness and value of an IV switch. Pharmacoeconomics 1994;5(Suppl 2):20-6. [DOI] [PubMed] [Google Scholar]
- 17.Zamin MT, Pitre MM, Conly JM. Development of an intravenous- to-oral route conversion program for antimicrobial therapy at a Canadian tertiary care health facility. Ann Pharmacother 1997;31:564-70. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Willke RJ, Pinto LA, et al. Comparison of length of hospital stay for patients with known or suspected methicillin-resistant Staphylococcus species infections treated with linezolid or vancomycin: A randomized, multicenter trial. Pharmacotherapy 2001;21:263-74. [DOI] [PubMed] [Google Scholar]