Abstract
The discovery of antibiotics some 60 years ago was anticipated to herald the end of infectious diseases. However, microbial evolution and genetic jugglery have dispelled this notion; the constant increase in the appearance of resistant strains has not been matched by the introduction of new therapeutic agents. On the contrary, the dire need for novel antibiotics has coincided with a reduction in antibiotic discovery programs in the pharmaceutical industry. As a result, the treatment of microbial diseases has reached a point where many infections are essentially untreatable by the antimicrobial agents currently available. At the present time, numerous initiatives are being undertaken by physicians and by governments in an attempt to redress this situation. In addition, alternative approaches to antibiotics for the treatment of infectious diseases are being explored intensively.
Key Words: Alternative therapies, Antibiotic resistance, Mechanisms
The discovery of penicillin in 1929 and streptomycin in 1943 heralded the age of antibiotics and, coincidentally, the founding of the American pharmaceutical industry. Within a decade after World War II, a number of important antibiotics were discovered and developed for therapeutic use. They became the foundation for the treatment of infectious disease (Table 1). This, along with the introduction of better hygiene, led to a dramatic reduction in worldwide morbidity and mortality due to bacterial infections.
TABLE 1.
Principal antibiotics in use today
| Class | Year discovered |
|---|---|
| Sulfonamides | 1937 |
| Penicillins | 1940 |
| Polymyxin | 1947* |
| Chloramphenicol | 1949 |
| Tetracyclines | 1953 |
| Cephalosporins (four generations) | 1953 |
| Aminoglycosides | 1957 |
| Vancomycin | 1958* |
| Clindamycin | 1966 |
| Rifamycin | 1971 |
| Trimethoprim/sulfamethoxazole | 1973 |
| Carbapenems | 1976 |
| Monobactams | 1982 |
| Linezolid | 1987* |
| Daptomycin | 1987* |
| Synercid | 1992* |
Recently reintroduced
The period from 1950 to 1960 was truly the golden age of antibiotic discovery, as one-half of the drugs commonly used today were discovered in this period. Unfortunately, the increasing use of antibiotics for human and nontherapeutic animal use (growth promotion) led all too soon to the development of resistant bacterial pathogens. Recognizing the correlation between antibiotic use and resistance development, much of subsequent antibiotic research has been devoted to the discovery and design of new compounds effective against the successive generations of resistant pathogens. It is interesting to note that microbial geneticists in the 1950s thought that the development of antibiotic-resistant strains concomitant with antibiotic use would be an unlikely and rare event at most!
In the year 2000, antibiotic production in the United States totalled 50 million pounds. Accurate figures are hard to obtain, but assuming this level of production for the past 20 years, it can be estimated that one billion pounds were made during this time. When we consider that the United States is not the principal antibiotic manufacturer (China, India and other countries are heavily involved), the quantity of antibiotics produced and used worldwide may be at least three times greater. The total amount of antibiotics produced since the beginning of the antibiotic era in 1950 is obviously very considerable, and one wonders if it may be significantly more than what is produced naturally in the biosphere, given that antibiotics are made in barely detectable amounts in soil. With respect to distribution, approximately 50% has been devoted to human use, with the remainder applied in animal husbandry, agriculture and aquaculture, etc.
It is hard to envision the effects of this flood of bioactive molecules on the environment and on microbial ecology. Responding to the selective pressure for survival of the microbial population in the face of this onslaught, bacteria have prevailed and even flourished through a variety of mechanisms of previously unsuspected genetic jugglery, with the concomitant selection of very high levels of antibiotic-resistant organisms in the biosphere.
Transferable resistance was first identified in Japan in the 1950s, although it took a while to decipher the mechanisms involved. The combination of mutation and horizontal gene transfer (HGT) has provided a remarkable collection of biochemical defense mechanisms against antibiotic action in bacterial cells (Table 2). The likelihood that additional mechanisms of resistance exist cannot be discounted. Recognition of the fundamental role of HGT in the dissemination of antibiotic resistance in bacteria (by transformation, transduction or conjugation of the encoding genes) has proven to be a finding of great significance. There is convincing evidence that it has been a major factor in prokaryotic evolution, and this has completely changed current concepts of phylogenetic relationships within the prokaryotes: the branches of the ‘trees’ have become interconnected (1)! There are also indications that HGT was critical in the evolution of eukaryotes.
TABLE 2.
Biochemical mechanisms of antibiotic resistance
| Increased efflux | Decreased efflux |
| Enzymatic inactivation | Sequestration |
| Target modification | Target bypass |
| Target repair | Target amplification |
| Biofilm formation | Intracellular localization |
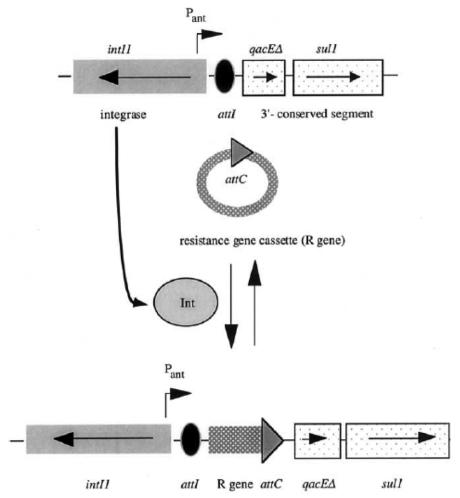
It is common to find clusters of resistance genes constructed on transferable vectors by recombination. One well-known structure is the integron, a vehicle by which open reading frames (gene cassettes) can be converted into resistance genes by acquisition and insertion adjacent to strong promoters, ensuring efficient transcription (Figure 1). The result is that multiple antibiotic resistance is common, perhaps encompassing all useful therapies for certain infections. Multidrug-resistant integrons are common in the Enterobacteriaceae family (2).
Figure 1.
The process of resistance gene capture by an integron. The genetic structure of the basic type I integron is shown at the top of the figure. Pant is the promoter that transcribes any gene sequence inserted at the attachment site (attI). The resistance gene cassette (middle) is inserted into the integron structure as a result by recombination between the attC and attI sequences catalyzed by the integrase (intI1 gene). Once integrated, the resistance gene is expressed. Multiple antibiotic resistance occurs by the insertion of additional resistance gene cassettes at the attI site. sul1 encodes resistance to sulfonamide drugs, and gacEΔ is a defective export system. The latter two are found on all type I integrons. This figure was kindly provided by Dr Patrice Courvalin (Institut Pasteur, Paris, personal communication)
Similar types of gene-trapping systems have been detected in Gram-positive pathogens (3). Resistance is often associated with genes for pathogenicity and other survival functions in composite structures known as genomic islands. Thus, the acquisition of antibiotic resistance genes by bacteria can lead to enhanced virulence and vice versa. A supreme example is found in the recent appearance of multidrug-resistant strains of Acinetobacter baumannii, which have become a major nosocomial pathogen; some strains possess a genomic island of 85 genes that encodes resistance to six different antibiotic classes (4). The only effective treatment for multidrug-resistant Acinetobacter species is colistin, a drug rarely used because of its toxicity. In addition to complicating the problems of therapy, the frequent appearance of these gene-trapping systems in genome sequencing studies has overturned accepted concepts of genome evolution.
Bacteria cannot be considered as independent, isolated colonies on Petri plates. Different environments are components of a huge bacterial network including environmental populations (soil, water), clinical populations (hospital, intensive care unit, community) and commensals of other living species (Figure 2). The movement of bacterial strains and extensive gene flux takes place between these different populations, such that they can share survival characteristics, typically antibiotic resistance and virulence. An advantageous function from one environment can rapidly make its way throughout the bacterial kingdom. The evidence for reservoirs of potential antibiotic resistance genes in soil is very convincing (5). Biofilms are a good example of an evolutionary mechanism by which mixed populations of prokaryotes acquire a collective advantage in avoiding antibiotics (6). The widespread distribution of antibiotics in hospitals and agricultural environments leads to a situation of constant selective pressure for survival.
Figure 2.
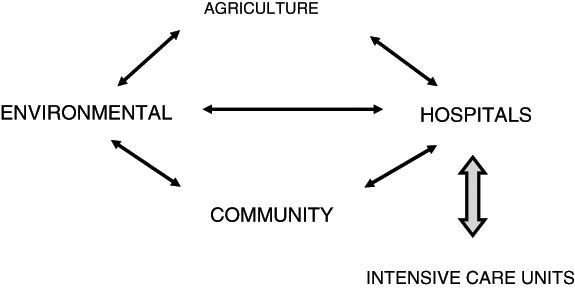
The interactive network of pathogens and antibiotic resistance genes
Antibiotic resistance has occurred in our lifetimes. Within two to three years after the introduction of a new antibiotic treatment, resistance usually develops (although there have been a few notable exceptions – penicillin resistance in streptococci, for example). This is nowhere more apparent than in the steady evolution of beta-lactamases (enzymes that detoxify the beta-lactam antibiotics) by point mutation under the selective pressure of successive introductions of new beta-lactamase-resistant penicillins, cephalosporins, carbapenems and monobactams (7). A single nucleotide substitution leading to an amino acid change in such an enzyme necessitates the development of a new antibiotic derivative at a cost of tens of millions of dollars! We often speak of the ‘cost’ of antibiotic resistance to the bacterium, but this is real cost in real time.
To the pharmaceutical industry, the ceaseless, losing battle with microbes has changed from disillusionment over the economics of antibiotic discovery and development to virtual resignation (8). The cost of drug discovery and the stringent Food and Drug Administration requirements in the clinical trial process have increased significantly, while the success rate of discovery has gone down. It has proven ever more difficult to find novel, active compounds with the desired characteristics for use as antibiotics. Even if a potent new compound were to be discovered, it most likely will not be applied in general therapy but will be put on the reserve list for serious, difficult-to-treat infections. There is good reason for this strategy as a means to avoid overuse, thereby limiting resistance development and thus extending the life of the new antibiotic, but limited use also prevents profit! It is difficult for pharmaceutical companies to recoup the expenses of long-term clinical trials; they can more successfully achieve financial gain by producing ‘quality-of-life’ drugs. At the present time, the many reasons for the pharmaceutical industry’s disinterest in antibiotic discovery (Table 3) are cause for great concern among members of the infectious diseases community. A number of proposals have been made to encourage a variety of government-led actions to reinstate antibiotic discovery programs and to seek viable alternatives to antibiotics (9,10).
TABLE 3.
Why the pharmaceutical industry is abandoning antibiotics
| • | No new antibiotics have been found by traditional approaches. |
| • | High-throughput screening/genomic approaches have been a |
| scientific failure and a financial disaster. | |
| • | Combinatorial chemistry libraries are limited sources of chemical diversity. |
| • | It takes too much money and too much time to find antibiotics. |
| • | Are new antibiotics really needed when the old ones still work (mostly)? |
| • | Food and Drug Administration approval is arduous and risky. |
| • | Novel antibiotics are restricted to last-resort use (low sales). |
| • | Quality-of-life ‘drugs’ are more marketable and more profitable; only four |
| antibiotics among 290 agents are currently in clinical development. | |
| • | There is no academic discovery pipeline because of insufficient funding. |
| • | The threat of litigation. |
Nonetheless, antibiotics are an irreplaceable component in the control and treatment of infectious diseases; we cannot do without them. Nature has provided the majority of effective treatments available at this time. But there are millions of bioactive small molecules made by bacteria and fungi awaiting discovery; they have gone through millennia of natural, evolutionary selection to target specific cellular components. This microbial world has barely been mined, mainly because a large proportion of the organisms cannot be grown in the laboratory. However, using modern molecular genetic procedures (such as metagenomic techniques) as a new approach to drug discovery (11), novel microbial strains with novel antibiotic-producing potential can be found, and more and more ‘unculturable’ microbes can be grown in the laboratory. Accessing the rich reservoirs of bioactive microbial products, together with advances in the technology of structure identification, should bring about rapid changes in this field of discovery (12). New antibiotics and other therapeutics will be found.
One important problem remains: will we be able to use these new medicinals in an effective manner so as to preserve their value for longer periods of time? Or will overuse, overprescription and misuse continually plague their application? Numerous suggestions for appropriate antibiotic use have been made, and with diligence, it should be possible to extend the useful lifetimes of antibacterial and other drugs; alternatives, such as vaccines, must be sought (Table 4). Several European countries have applied restrictions to the use of antibiotics as animal growth promotants, a policy that has been shown to work: resistance levels in both animal and human populations have declined (13). It is evident that different strategies must be applied and combined to arrive at effective solutions.
TABLE 4.
Avoiding and overcoming antibiotic resistance
| • | Optimal use of all antimicrobials through selection, cycling, combination |
| and restriction | |
| • | Novel antimicrobials and their prudent use |
| • | Alternative approaches (immunity, phage, probiotics) |
| • | Better understanding of pathogen, commensal and host biology |
| • | Increased surveillance and epidemiology of resistance |
| • | Improved public and health care specialist education |
| • | Improved hygiene (back to Semmelweis) |
| • | Banning of nontherapeutic uses of antimicrobials |
| • | Reduction in bactericide use |
A final question: it is generally accepted that antibiotics are overprescribed, but are they also being misused in a biological sense? We have been using antibiotics for over half a century without having a clue as to their biology and roles in microbial ecology. If more research was devoted to studying their natural functions, we may be better equipped to discover new antibiotics and use them to their best advantage in health care.
Acknowledgments
The author thanks Dorothy Davies for her help in preparing this paper. Financial support for work performed in the author’s laboratory was provided by the Canadian Institute for Health Research and the Canadian Bacterial Diseases Network.
References
- 1.Rowe-Magnus DA, Mazel D. Integrons: Natural tools for bacterial genome evolution. Curr Opin Microbiol 2001;4:565-9. [DOI] [PubMed] [Google Scholar]
- 2.Fluit AC, Schmitz FJ. Resistance integrons and super-integrons. Clin Microbiol Infect 2004;10:272-88. [DOI] [PubMed] [Google Scholar]
- 3.Skurray RA, Firth N. Molecular evolution of multiply- antibiotic-resistant staphylococci. Ciba Found Symp 1997;207:167-83. [DOI] [PubMed] [Google Scholar]
- 4.Fournier P-E, Vallenet D, Barbe V, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet 2006;2:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the antibiotic resistome. Science 2006;311:374-7. [DOI] [PubMed] [Google Scholar]
- 6.Parsek MR, Fuqua C. Biofilms 2003: Emerging themes and challenges in studies of surface-associated microbial life. J Bacteriol 2004;186:4427-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacoby G, Bush K. Beta-lactam resistance in the 21st century. In: White DG, Alekshun MN, McDermott PF, eds. Frontiers in Antimicrobial Resistance. Washington, DC: ASM Press, 2005:53-65. [Google Scholar]
- 8.Projan SJ. Why is big pharma getting out of antibacterial drug discovery? Curr Opin Microbiol 2003;6:427-30. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C. Antibiotics at the crossroads. Nature 2004;431:899-902. [DOI] [PubMed] [Google Scholar]
- 10.Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: An update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 2006;42:657-68. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie DE, Brady SF, Bettermann AD, et al. Isolation of antibiotics turbomycin A and B from a metagenomic library of soil microbial DNA. Appl Environ Microbiol 2002;68:4301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clardy J, Walsh C. Lessons from natural molecules. Nature 2004;432:829-37. [DOI] [PubMed] [Google Scholar]
- 13.Wegener HC. Antibiotics in animal feed and their role in resistance development. Curr Opin Microbiol 2003;6:439-45. [DOI] [PubMed] [Google Scholar]