Abstract
Background and purpose:
Calcitonin gene-related peptide (CGRP), a capsaicin-sensitive neuromodulator of splanchnic vascular tone in several animal species, remains poorly investigated in mouse models. We therefore assessed whether endogenous CGRP is a non-adrenergic/non-cholinergic (NANC) neuromodulator in the mesenteric vascular bed of the mouse.
Experimental approach:
Arterial and venous changes in perfusion pressure in response to perivascular nerve stimulation (PNS) were monitored in the mouse mesenteric bed under basal conditions or precontracted with KCl (artery) or U46619 (vein) in circuits pretreated with guanethidine, atropine, indomethacin and prazosin. Arterial responses to NANC were also characterized with a CGRP1 antagonist, hαCGRP8−37. Finally, the PNS-induced release of arterial CGRP was measured by enzyme immunoassay.
Key results:
HαCGRP8−37 enhanced PNS-induced arterial increases in perfusion pressure under basal tone. PNS-induced stimulation of NANC triggered an hαCGRP8−37 or capsaicin- sensitive reduction in perfusion pressure of the pre-contracted arterial bed only. Chemical removal of the endothelium inhibited PNS- and hαCGRP- induced reduction in perfusion pressure in the arterial mesenteric bed. Responses to NANC nerves were reduced by guanylate and adenylate cyclase inhibitors (1H-[1,2,4]oxadiazole[4,3-a] quinoxalin-1-one (ODQ)) and [9-(tetrahydro-2-furanyl)-9H-purin-6-amine] (SQ 22 536), respectively. A neuronal NOS inhibitor (7-nitroindazole; 7-NI) also enhanced the response to NANC in vessels from wild-type, eNOS KO but not iNOS KO mice. Finally, PNS enhanced the release of immunoreactive CGRP from the perfused arterial mesenteric bed.
Conclusions and implications:
Our study demonstrates a role for CGRP in the NANC-dependent reduction in perfusion pressure of the arterial but not venous mesenteric bed of the mouse.
Keywords: CGRP, hαCGRP8−37, neuronal nitric oxide synthase, arterial mesenteric vasculature, mouse model
Introduction
Primary afferent neurons, initiating in the dorsal root ganglia, provide a perivascular network of sensory fibres around the arterial system throughout the body (Holzer et al., 1995). Trans-mural complexes, composed of those sensory nerve fibres releasing calcitonin gene-related peptide (CGRP), substance P (SP), vasoactive intestinal peptide (VIP), nitric oxide (NO) and adenosine 5′-triphosphate (ATP) (Lundberg, 1996), act in most cases as modulators of the autonomic sympathetic and parasympathetic activity in vessels of the intestinal tract (Maggi and Meli, 1988; Mione et al., 1990).
Calcitonin gene-related peptide is a 37-amino-acid vasoactive neurotransmitter that has been shown to act as a physiological antagonist of autonomic nerve-dependent increases in vascular tone (Mione et al., 1990). For example, CGRP is known as the main neuropeptide involved in the capsaicin-sensitive nerve-induced vasodilatation of the rat mesenteric vascular bed (Kawasaki et al., 1988, 1990). Binding of capsaicin to specific neuronal receptors, prompts release of neurotransmitters such as CGRP from the peripheral ending of sensory nerves followed by a desensitization of the mechanism (Rubino and Burnstock, 1996).
Calcitonin gene-related peptide-dependent responses in the vascular system are usually mediated by a G-protein-coupled receptor; calcitonin receptor-like receptor (CL), associated with a receptor activity-modifying protein (CL/RAMP1) (McLatchie et al., 1998). We have also previously shown that exogenously administered human αCGRP (hαCGRP), as well as the NANC-stimulated vasodilatation in the arterial but not venous mesenteric bed of the rat depends on the activation of CGRP1 receptors (Claing et al., 1992).
Our group has developed and characterized a mouse counterpart of the rat double-perfused mesenteric bed, initially reported by Warner (1990), and has shown in this model that bradykinin induces an endothelium and dose-dependent vasodilatation of the arterial and venous circuits of wild-type but not B2 knockout mice (Berthiaume et al., 1997). More recently, Grant et al. (2004) reported that exogenously administered hαCGRP triggers, in the arterial mesenteric bed of the mouse, a marked vasodilatation which was abolished by BIBN4096BS (1-piperidinecarboxamide, N-[2-[ [5-amino-1-[ [4-(4-pyridinyl)-1-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl) methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)-[R-(R*,S*)]-), a αCGRP antagonist with high affinity for the CGRP1 receptor (Doods et al., 2000). It is however worthy of mention that the contribution of the endothelium in these vasoactive responses to CGRP in the mouse mesenteric circuit was not explored by this group.
In addition, Hatanaka et al. (2006) have recently reported that neuronal nitric oxide synthase (nNOS) physiologically represses the vasoactive response to adrenergic neurotransmission in the rat arterial mesenteric bed. Such modulator roles of the nNOS isoform on NANC responses to PNS however remain to be investigated.
In the present study, we used the murine model developed by Berthiaume et al. (1997) for the pharmacological and biochemical characterization of the putative NANC neurotransmitter involved in the modulation of mesenteric arterial and venous perfusion pressure in the mouse. We also addressed the putative modulator roles of the NOS isozymes (nNOS, iNOS and eNOS) on the NANC evoked response in the murine vascular circuit.
Our results confirm that CGRP is a pivotal NANC neuromodulator, which acts via cGMP/cAMP pathways as a physiological antagonist of perivascular nerve stimulation (PNS)-induced contraction of the mouse arterial but not venous mesenteric bed. Our data, in addition, identifies complex interactions between the different NOS isoforms and highlights the role of endothelium-derived vasoactive factors other than NO in the physiological modulation of NANC activity in this particular system.
Methods
Experimental design
Animals. Animal care and experimentations were approved by the Ethics Committee on Animal Research of the Université de Sherbrooke.
Wild-type (WT) mice on C57BL/6J background were purchased from Charles River (Montreal, QC, Canada). Breeding couples of homozygous iNOS KO (Laubach et al., 1995) or eNOS KO (Shesely et al., 1996) mice, with the same C57BL/6J genetic background, were obtained from Jackson Laboratory (Bar Harbour, ME, USA). All animals were kept at constant room temperature (±23 °C) and humidity (∼78%) under a controlled light–dark cycle (0600–1800 hours) with free access to food and water.
Mouse simultaneously perfused superior mesenteric arterial and venous vascular bed. As previously described (Berthiaume et al., 1997), male and female mice weighing 25–35 g were anaesthetized with injection of ketamine/xylaxine (87/13 mg kg−1; i.m.) and then killed by aortic exsanguination.
The abdomen was opened and the ileocolic and colic branches of the superior mesenteric artery were tied. The portal and superior mesenteric veins were freed of connective and adipose tissues and cannulated with hypodermic blunt needles (23G.5, Strategic Applications Inc., Libertyville, IL, USA). The superior mesenteric artery was cannulated and then perfused at 200 μl min−1 for 5 min with Krebs' solution (at room temperature) containing heparin (100 U ml−1). The composition of the Krebs' solution was as follows (mmol l−1): NaC1, 118; KCl, 4.7; KH2PO4, 1.2; MgSO4·7H2O, 1.2; CaCl2·6H2O, 2.5; NaHCO3, 25; and glucose, 5.5. Following this initial perfusion period, the mesentery was separated from the intestine by cutting close to the intestinal border and the preparation was supported by a heated bath (37.5 °C). The venous and arterial vasculature were then perfused independently with peristaltic pumps (model Ismatec Cole-Parmer; Chicago, IL, USA) at a constant flow rate of 400 μl min−1 with warm (37.5 °C), oxygenated (95% O2: 5% CO2) Krebs' solution either containing or not a cocktail of inhibitors composed of a non-selective cyclooxygenase inhibitor, indomethacin (5 μmol l−1), an adrenergic neurone blocker, guanethidine (5 μmol l−1), a muscarinic antagonist, atropine (1 μmol l−1), and a α1-adrenoceptor selective antagonist, prazosin (1 μmol l−1). The cocktail used, with the exception of the added prazosin, has been previously reported by our laboratory in the rat, guinea-pig and mouse mesenteric beds (Claing et al., 1992; D'Orléans-Juste et al., 1996; Berthiaume et al., 1997). The changes in perfusion pressures were measured with pressure transducers Statham (Model P-23A) and recorded on a Grass physiograph (Model 7-D).
After 45–50 min of stabilization, a steady baseline perfusion pressure was reached on both sides of the preparation. A dual platinum electrode was then placed near the superior mesenteric artery and vein proximal to the cannulae for electrical stimulation (stimulator Grass, Model S5) of the perivascular nerves (PNS).
Experimental protocols
Responses to PNS of the non-precontracted venous and arterial mesenteric vasculature. After stabilization, perivascular electrical stimulation was applied to the arterial (2 and 30 Hz; 60 V; 5 ms duration for 1 min) or venous (30 and 80 Hz; 80 V; 5 ms duration for 1 min) mesenteric vasculature in the absence and presence of the CGRP1 receptor antagonist hαCGRP8−37 (2.5 μmol l−1) (Chiba et al., 1989) infused for 15 min prior to and during electrical challenges. Vasoconstriction to PNS was expressed as increase in perfusion pressure from baseline (mm Hg).
NANC-dependent responses of the pre-contracted arterial and venous mesenteric vasculature. The perfusion pressure of the cocktail-treated arterial and venous mesenteric vasculature was increased by infusing a solution of KCl (200 mmol l−1) or U46619, a thromboxane mimetic (20 μmol l−1), respectively. When a plateau was reached, the arterial and venous mesenteric arteries were separately stimulated (0.5–80 Hz;. 60 V; 5 ms duration for 1 min). To avoid tachyphylaxis, each mesenteric bed was electrically stimulated once. Arterial or venous responses were expressed as percent change of induced perfusion pressure increases following infusions of KCl or U46619, respectively.
Sodium nitroprusside (50 nmol) was systematically administered at the end of all experiments to ascertain the reactivity of the mesenteric circuits to guanylate cyclase stimulation (that is cyclic GMP-dependent reduction in perfusion pressure).
Modulatory roles of NOS isoforms in arterial mesenteric bed responses to PNS- or hαCGRP-dependent arterial vasodilator responses. The KCl pre-contracted and cocktail-treated arterial mesenteric vasculatures of control, eNOS or iNOS KO mice were perfused with vehicle or 7-nitroindazole (7-NI) (100 μmol l−1, for 15 min prior to and during the entire experiments) (Alderton et al., 2001; Ralevic, 2002) to evaluate the implication of neuronal NOS in the reduction in perfusion pressure afforded by PNS. Dose-dependent responses to hαCGRP administered as a bolus (in 1 μl) were also assessed in the arterial mesenteric vasculature of control or endothelial NOS knockout mice as well as in 7-NI pretreated vascular circuits derived from wild-type animals.
Contribution of the endothelium and nitric oxide in arterial mesenteric bed responses to PNS- or hαCGRP-dependent arterial vasodilator responses. The KCl pre-contracted and cocktail-treated arterial mesenteric vasculatures of wild-type mice were perfused with vehicle, the detergent sodium deoxycholate (SD; 0.45 mg ml−1, for 45 s prior to stimulation) (Eguchi et al., 2004) or with the non-selective NOS inhibitor L-NAME (400 μmol l−1, for 45 min) prior and during electrical or pharmacological stimulation, to evaluate the involvement of endothelium and NO, respectively, in the vasodilator response to PNS and hαCGRP.
Pharmacological characterization of the arterial vasodilator response to PNS by hαCGRP8–37. To characterize pharmacologically the vasodilator response to PNS, the CGRP1 receptor antagonist, hαCGRP8–37, was infused in KCl precontracted arterial mesenteric vasculature (2.5 μmol l−1) 15 min before and during PNS (30 Hz; 60 V; 5 ms duration for 1 min) or bolus (1 μl) injections of hαCGRP (1 nmol).
Contribution of C-sensory/neurogenic fibres in the NANC-dependent arterial vasodilator responses to PNS. The contribution of C-sensory nerve fibres in NANC responses was assessed by pre-treating the arterial mesenteric bed with capsaicin (5 μmol l−1), which was infused for 25 min. This concentration of capsaicin has been previously shown to deplete neurotransmitters from peripheral ending of sensory nerves (Rubino and Burnstock, 1996). The cocktail-treated mesenteric circuits were subsequently washed out for 30 min before being pre-contracted with infusion of KCl and finally stimulated by PNS (30 Hz; 60 V; 5 ms duration for 1 min) or by bolus injections of hαCGRP (1 nmol). In another series of experiments, PNS, hαCGRP and NaNP induced changes in perfusion pressure were monitored in arterial mesenteric beds derived from wild-type mice pretreated with either vehicle or with tetrodotoxin (1 μmol l−1, 15 min prior to stimulations) (Phillips et al., 1998) to ascertain the neurogenic nature of PNS and NANC-induced responses of the mouse mesenteric circuits.
Contribution of cAMP and cGMP transduction pathways in the vasodilator responses to PNS. The guanylate and adenylate cyclase inhibitors, ODQ (Garthwaite et al., 1995; Sobey and Faraci, 1997) and SQ 22 536 (Harris et al., 1979), respectively, were infused either individually or concomitantly at a concentration of 10 μmol l−1 each for 15 min prior to and during PNS or bolus injection of hαCGRP in KCl pre-contracted and cocktail-treated arterial mesenteric vasculature of control mice.
Enzyme immunoassay (EIA) for CGRP. For measurements of immunoreactive CGRP release after PNS, an enzyme immunoassay (EIA) for rat CGRP (EIA Kit no 589001, Cayman Chemicals, distributed by Cedor Lane, Canada) with a 100% cross-reactivity with the same peptide of murine origin (Arai et al., 2003 and Cayman Chemicals) was used. After stabilization, 10 min (basal) perfusates were collected before and following PNS. All perfusates were acidified with equal volumes of 4% acetic acid. Extraction was performed in solid-phase sorbent using a Sep-Pak C-18 cartridge (Waters, Milford, MA, USA). Immunoreactive CGRP content was determined as described by the supplier.
Statistical analysis
Results are shown as mean values±s.e.mean for n experiments. Student's unpaired t-test and one-way analysis of variance (ANOVA), followed by Bonferroni's multiple comparisons tests were used when appropriate in order to determine the significance of differences between means when P<0.05.
Materials
Indomethacin, atropine, bradykinin, prazosin, 7-NI, SQ 22 536, ODQ, sodium nitroprusside, sodium deoxycholate, U46619 and capsaicin were purchased from Sigma Chemical Co. (St Louis, MO, USA). Guanethidine sulphate was from TCI America (Portland, OR, USA). The hαCGRP and hαCGRP8–37 were generous gifts from Dr A Fournier (IAF-INRS-Santé, Dorval, Québec, Canada). Rat EIA kit for CGRP was purchased from Cayman Chemical (Ann Arbor, MI, USA). Atropine and indomethacin were dissolved in ethanol, prazosin in methanol, 7-NI and ODQ in dimethyl sulphoxide (DMSO). The Krebs' concentration of ethanol, methanol and DMSO did not exceed 0.05%. The other drugs were dissolved in phosphate-buffered saline pH 7.4 or in distilled water. All vehicles were tested and were found to be without any significant effects on the mesenteric responses to hαCGRP or PNS.
Results
Typical responses of the pre- and post-capillary mesenteric circuit of the mouse to PNS
The basal perfusion pressure on the arterial or venous mesenteric bed of wild-type mice averaged at 10.6±0.96 or 1.29±0.15 mm Hg, respectively, when perfused at a flow of 400 μl min−1 (n=55) (Table 1).
Table 1.
Basal perfusion pressures in arterial and venous mesenteric beds as well as in KCl-treated arterial beds
| Perfusion pressure | C57BL/6J | eNOSKO | iNOSKO | C57BL/6J+7-NI |
|---|---|---|---|---|
| Arterial (basal) | 10.6±0.96 | 10.1±0.62 | 7.75±0.83 | 10.2±2.42 |
| Arterial+KCl (200 mmol l−1) | 37.7±1.95 | 49.9±2.29*** | 31.9±2.66 | 36.4±1.43 |
| Venous (basal) | 1.29±0.15 | 1.20±0.11 | 1.98±0.40 | 0.77±0.12 |
| n | 55 | 46 | 12 | 14 |
n: number of experiments in arterial beds.
Each value is expressed in mean±s.e.mean mm Hg.
***P<0.001 when compared to C57BL/6J.
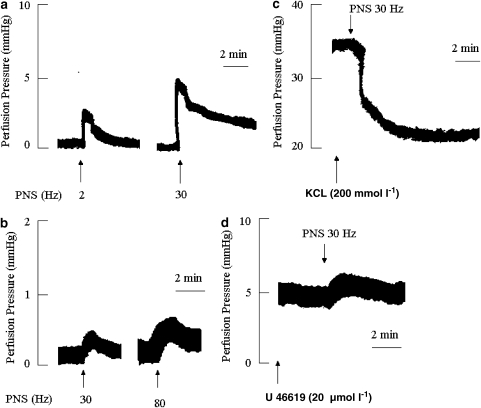
Figures 1a and b illustrate typical arterial and venous responses to PNS in the non-pre-contracted mesenteric bed. It is noticeable that the venous circuit is less responsive to electrical stimulation than the arterial bed, as the latter responds at lower thresholds of field stimulation (2 vs 30 Hz, in the arterial and venous bed, respectively).
Figure 1.
Typical traces illustrating the vasoconstrictor responses of non-pre-contracted arterial (a) and venous (b) mesenteric beds to perivascular nerve stimulation (PNS). (c) and (d) illustrate the effect of a PNS (30 Hz)-induced NANC response in KCl (200 mmol l−1) and U46619 (20 μmol l−1) pre-contracted arterial and venous mesenteric bed, respectively. The mesenteric beds were perfused, in panels c and d, with a Krebs' solution containing atropine (1 μmol l−1), guanethidine (5 μmol l−1), prazosin (1 μmol l−1) and indomethacin (5 μmol l−1).
It is also noteworthy that KCl was found to be the most stable enhancer of vascular tone in the arterial mesenteric bed, when compared to noradrenaline or U46619. Indeed, the later two stimulants infused at concentrations of 10 and 20 μmol l−1, respectively, induced an increase in perfusion pressure which was not sustained for more than 2 min (results not shown). In contrast, KCl (200 mmol l−1) induced a protracted perfusion pressure of 37.7±1.95 mm Hg which lasted for at least 50 min in the arterial mesenteric beds tested (n=127, see Table 1). Table 1 also summarizes the basal perfusion pressure of the arterial and venous mesenteric beds as well as the increases in perfusion pressure triggered by KCl in C57BL/6J mice treated with vehicle or 7-NI or in circuits derived from eNOS KO or iNOS KO mice.
Figure 1c shows that in mesenteric beds pretreated with guanethidine (5 μmol l−1), prazosin (1 μmol l−1), indomethacin (5 μmol l−1) and atropine (1 μmol l−1), and pre-contracted with KCl, the arterial circuits respond to PNS by a marked vasodilatation. In contrast, the venous circuit responds to U46619 (20 μmol l−1) by a 3±0.5 mm Hg increase in vascular tone (Figure 1d) when compared to 0.58±0.14 and 0.43±0.07 mm Hg (n=5 each) in non-contracted post-capillary beds treated with vehicle or with the cocktail of inhibitors, respectively.
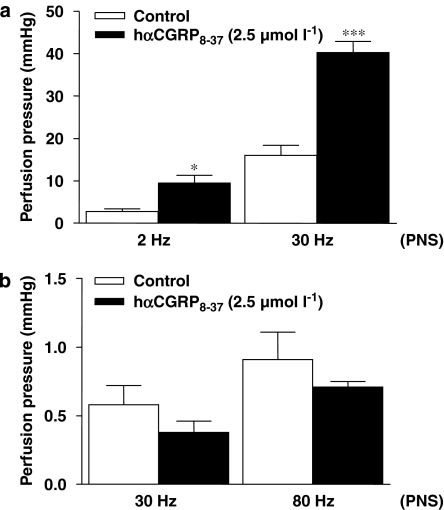
CGRP8−37 potentiates the contractile response to PNS in the arterial but not venous mesenteric bed of the mouse
Perivascular nerve stimulation in non-pre-contracted vessels triggered a frequency-dependent increase in pre- and post-capillary perfusion pressures, which were significantly potentiated by the CGRP1 antagonist, hαCGRP8–37 (2.5 μmol l−1), in the arterial (P<0.001, n=5) but not venous mesenteric bed of the mouse (Figures 2a and b).
Figure 2.
Effects of the CGRP1 receptor antagonist, hαCGRP8–37 (2.5 μmol l−1), on the perfusion pressure of arterial and venous mesenteric beds in response to perivascular nerve stimulation (PNS) in non-pre-contracted tissues (PNS: arterial side (a) 2 and 30 Hz, venous side (b) 30 and 80 Hz). *P<0.05 and ***P<0.001 when compared to controls (Student's t-test). Values are means±s.e.mean of at least five independent experiments.
Considering the lack of potentiating properties of hαCGRP8−37 on the venous constrictor response to PNS, as well as the absence of veno-dilator response afforded by the same electrical stimulus, subsequent experiments were limited to the arterial mesenteric bed.
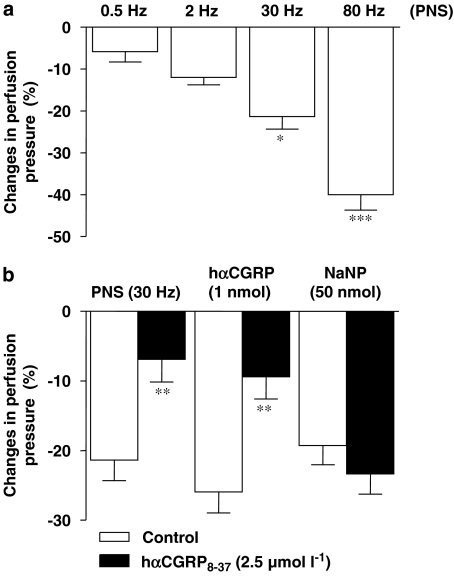
Pharmacological characterization of the pre-contracted pre-capillary mesenteric responses to PNS and hαCGRP
Upon stabilization of the KCl-induced contraction (15–20 min), PNS (0.5–80 Hz) triggered a pronounced reduction of arterial perfusion pressure in a frequency-dependent fashion (Figure 3a).
Figure 3.
Frequency-dependent NANC responses of the arterial mesenteric bed to PNS (0.5–80 Hz) (a). (b) Illustrates the effects of the CGRP1 receptor antagonist, hαCGRP8−37 (2.5 μmol l−1), on the NANC-dependent reduction in perfusion pressure induced by PNS (30 Hz), as well as on the same vasoactive responses triggered by hαCGRP (1 nmol) and sodium nitroprusside (NaNP;50 nmol). (*P<0.05, **P<0.01 and ***P<0.001 against 0.5 Hz or control; ANOVA followed by Bonferroni's comparison test and Student's t-test). Values are means±s.e.m. of at least 6–10 independent experiments.
In the following series of experiments, hαCGRP8−37 pre-infused at 2.5 μmol l−1 for 15 min markedly reduced the vasoactive response of the arterial mesenteric bed to PNS (30 Hz) and exogenously administered hαCGRP (1 nmol), but not to sodium nitroprusside (50 nmol) (Figure 3b).
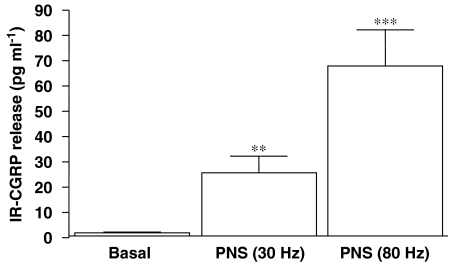
In addition, Figure 4 shows that PNS triggered a frequency-dependent release of immunoreactive CGRP, as detected by specific EIA, following 60-sec long trains of electrical stimulations at 30 and 80 Hz.
Figure 4.
Measurements of immunoreactive CGRP (IR-CGRP) from perfusates collected for 10 min before (basal) or after PNS (30–80 Hz) stimulation in arterial mesenteric beds of control mice. **P<0.01 and ***P<0.001 when compared to basal (ANOVA). Values are means±s.e.mean of at least 10–14 independent experiments.
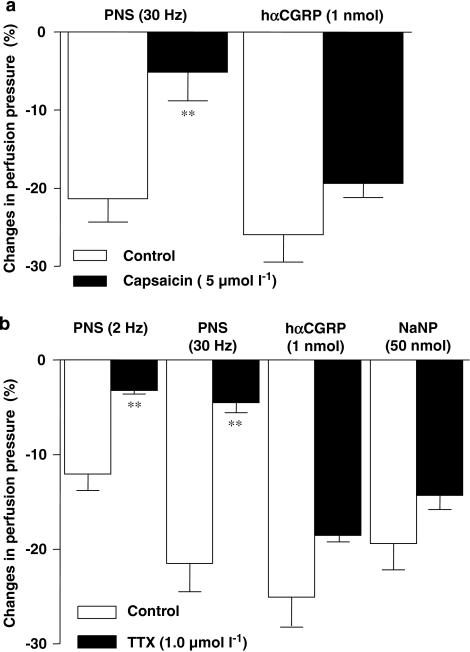
Figure 5a illustrates that a 25-min pre-treatment of the mesentery vasculature with capsaicin (5 μmol l−1) reduced the vasodilatation triggered by PNS (30 Hz) by 76±17%, yet had no effect on the arterial response to exogenously administered hαCGRP (1 nmol). Finally, a 15 min pre-treatment of the mesenteric circuit with tetrodotoxin (1 μmol l−1) markedly reduced the frequency-dependent decrease in arterial perfusion pressure afforded by PNS without affecting the same response to hαCGRP (1 nmol) or sodium nitroprusside (50 nmol) (Figure 5b). TTX and capsaicin experiments were limited to PNS stimuli of up to 30 Hz the latter triggering a quantitatively similar reduction of perfusion pressure as afforded by the highest dose of exogenously administered hαCGRP (1 nmol).
Figure 5.
Effects of capsaicin (a) (5 μmol l−1) or tetrodotoxin (b) (1 μmo l−1) treatments on NANC-dependent reduction in perfusion pressure induced by PNS (30 Hz) or hαCGRP (1 nmol) (panel a). Panel b illustrates the effect of tetrodotoxin (TTX: 1.0 μmol l−1) on PNS (2 or 30 Hz) as well as hαCGRP (1 nmol) or NaNP (50 nmol)-induced reduction in perfusion pressures. These experiments were performed in arterial mesenteric bed of control (C57BL/6J) mice. **P<0.01 when compared to controls (Student's t-test). Values are means±s.e.mean of at least 5–10 independent experiments.
Contribution of the endothelium and of nitric oxide in arterial mesenteric bed responses to PNS- or hαCGRP-dependent arterial vasodilator responses
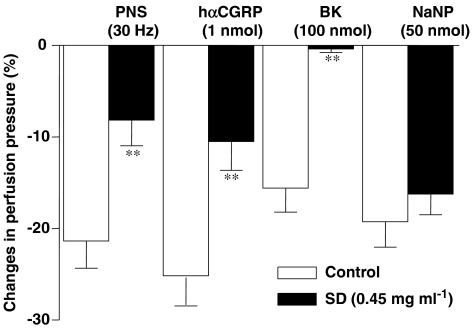
Figure 6 shows that responses to PNS (30 Hz) and to hαCGRP (1 nmol) were significantly reduced in mesenteric beds pre-treated with sodium deoxycholate (for chemically removing the endothelium) when compared to vehicle-treated circuits. The same treatment abolished the reduction in perfusion pressure triggered by bradykinin (100 nmol) without affecting that induced by sodium nitroprusside (50 nmol) (Figure 6). In an additional series of experiments, the non-selective NOS inhibitor L-NAME, significantly potentiated the increases in perfusion pressure induced by KCl (45.8±3.49 mm Hg; ***P<0.001, n=10) when compared to circuits not treated with L-NAME (Table 1). L-NAME, in contrast, inhibited the decrease of perfusion pressure induced by PNS (30 Hz) (+vehicle: 21.4±2.98; +L-NAME: 10.7±4.14 %, P<0.05, n=6) and hαCGRP at 100 pmol (+vehicle: 18.8±3.27; +L-NAME: 7.18±1.65% P<0.05, n=6 and 5, respectively) but not that at a higher dose of 1 nmol of the same peptide (+vehicle: 25.94±2.99; +L-NAME: 21.07±2.55% n=11). Finally, L-NAME potentiated the reduction in perfusion pressure afforded by sodium nitroprusside (+vehicle: 19.3±2.76; +L-NAME: 28.3±2.08%, P<0.05, n=10).
Figure 6.
Histogram showing the effects of vehicle (Control) or sodium deoxycholate (SD; 0.45 mg ml−1), on NANC-dependent reduction of perfusion pressure (induced by PNS, 30 Hz), or on the vasoactive response to exogenous hαCGRP (1 nmol), bradykinin (BK; 100 nmol) or sodium nitroprusside (NaNP; 50 nmol) in the arterial mesenteric bed of control mice. **P<0.01 when compared to vehicle-treated mice (Student's t-test). Values are means±s.e.mean of at least six independent experiments.
Modulatory contributions of iNOS, eNOS and nNOS in the mesenteric response to PNS or hαCGRP
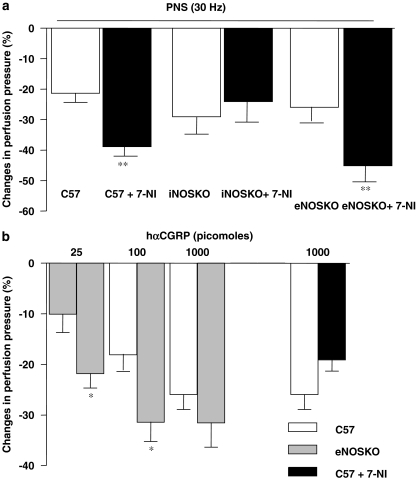
Figure 7a shows that the response to PNS is enhanced by 7-NI (100 μmol l−1) in KCl-pre-contracted circuits derived from wild-type and eNOS KO, but not iNOS KO mice. In contrast, responses to hαCGRP in 7-NI pre-treated vascular mesenteric circuits of wild-type animals did not differ from those of vehicle-treated vessels (Figure 7b). Responses of the same circuit to exogenously administered hαCGRP to the lower two (25 and 100 pmol) but not at the highest dose of 1000 pmol were also potentiated in vessels derived from eNOS KO mice (Figure 7b).
Figure 7.
(a) NANC-dependent reduction in perfusion pressure induced by PNS (30 Hz) in vehicle or 7-nitroindazole-treated (7-NI; 100 μmol l−1) arterial circuits derived from C57BL/6J (C57), iNOS KO mice and eNOS KO mice; (b) reduction in perfusion pressure induced by exogenous hαCGRP in mesenteric beds from C57, eNOS KO mice or in C 57 beds treated with 7-NI. *P<0.05 and **P<0.01 when compared to C57BL/6 J mice treated with vehicle (Student's t-test). Values are means±s.e.mean of at least 6–10 independent experiments.
Contribution of cAMP and cGMP in the vasodilator response to PNS and hαCGRP
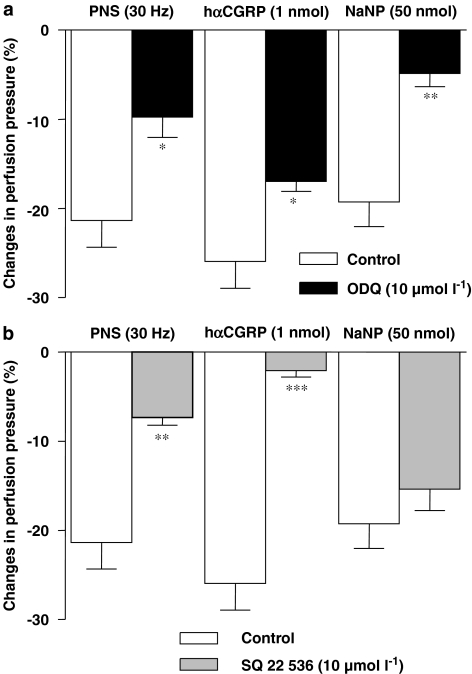
In a final series of experiments, the guanylate cyclase inhibitor ODQ (10 μmol l−1) (Figure 8a) or the adenylate cyclase inhibitor SQ 22 536 (10 μmol l−1) (Figure 8b) were tested against the vasodilator responses to exogenous hαCGRP or the NANC-dependent response of the arterial mesenteric bed of the mouse to PNS. Administered alone, ODQ or SQ 22 536 reduced the vasodilator responses to hαCGRP or PNS. In a series of control experiments, ODQ but not SQ 22 536 markedly inhibited the vasorelaxation induced by sodium nitroprusside (Figures 8a and b). Finally, when combined, ODQ and SQ 22 536 reduced the response to PNS in an additive fashion (control PNS (30 Hz): 21.36±2.98; PNS+ODQ and SQ 22 536: 4.87±1.95 mm Hg, (Student's t-test; *** P<0.001, n=6, when compared to ODQ or SQ 22 536 administered separately) (Figures 8a and b).
Figure 8.
Effects of vehicle, ODQ (10 μmol l−1) (a) or SQ 22 536 (10 μmol l−1) (b) on NANC-dependent reduction in perfusion pressure induced by PNS (30 Hz) as well as by hαCGRP (1 nmol) or sodium nitroprusside (NaNP; 50 nmol) in the arterial mesenteric bed of control mice. *P<0.05, **P<0.01 and ***P<0.001 when compared to vehicle-treated vessels derived from C57BL/6J mice (control) (Student's t-test). Values are means±s.e.mean of at least 6–10 independent experiments.
Discussion
To our knowledge, this is the first report demonstrating an important role for endogenous CGRP in the NANC-dependent vasodilatation of the arterial but not venous mesenteric bed of the mouse. We have also shown that removal of the endothelium or treatment with a non-selective NOS inhibitor markedly reduced the response to NANC stimulation whereas 7-NI (a putative nNOS inhibitor) enhanced the same response of the mesenteric arterial bed. In addition, genetic repression of the eNOS gene potentiated the response to exogenous CGRP yet had no significant effect on NANC-induced responses. Finally, the reduction of perfusion pressure afforded by NANC stimulation was found to be sensitive to adenylate and guanylate cyclase inhibitors.
Our study shows that NANC stimulation triggers capsaicin-sensitive vasodilatation in the arterial mesenteric bed of the mouse, as recently revisited in the same circuit in the rat (Ralevic et al., 2000). It is noteworthy that a depolarizing concentration of KCl was used in our model, to establish a stable and protracted increase in perfusion pressure. Even in a depolarized state, the mouse arterial mesenteric bed responded to PNS via a TTX- or capsaicin-sensitive reduction of perfusion pressure.
Interestingly, capsaicin activates the transient receptor potential vanilloid 1 (TRPV1) as recently reviewed by Begg et al. (2005) whereas Raisinghani et al. (2005) have suggested that binding of capsaicin to its receptors can occur in a voltage-dependent fashion in patch-clamped-single-cell experiments. Our results on NANC-induced reduction in perfusion pressure in a complex vascular circuit under depolarized conditions can therefore be explained by the voltage-dependent increases in TRPV1 activation as previously put forward by Raisinghani et al. (2005).
In contrast, venous mesenteric beds of the mouse responded to PNS in vehicle or cocktail-treated vasculatures by an increase in perfusion pressure suggesting a direct venous constrictive response to field stimulation in our model. Interestingly, Ahluwalia and Vallance (1997) have shown a capsaicin-dependent vasodilatation of small veins derived from the rat mesenteric bed. The conclusions drawn by Ahluwalia and Vallance (1997) allow us to argue in favour of regional differences in mesenteric vascular reactivity to various stimuli, since we have reported that the rat venous mesenteric bed responded to NANC stimulation by a capsaicin-insensitive increase in perfusion pressure (Claing et al., 1992). With regards to the observations summarized above, further pharmacological characterizations of the venous mesenteric bed of the mouse are currently ongoing in our laboratory.
It is also of interest that blockade of CGRP1 receptors potentiated the increase in arterial but not venous resistance triggered by the catecholaminergic/cholinergic responses of the non-pre-contracted mesenteric bed of the mouse to PNS. Such stimulation also triggers the release of immunoreactive CGRP from the arterial mesenteric bed, as previously reported also in a mouse gastric mucosa model (Arai et al., 2003) but not in the venous circuit. Thus, we suggest that the endogenous neuropeptide CGRP acts as a physiological antagonist of adrenergic/cholinergic-induced increases of perfusion pressure in the mesenteric pre-capillary circuits of the mouse. It is yet to be determined whether this NANC-dependent physiological antagonism of sympathetic/ parasympathetic tone is caused by a direct vascular effect of CGRP and/or through the interference of this neuropeptide with noradrenaline/acetylcholine release from perivascular nerve terminals. In support of the latter concept, Oh-hashi et al. (2001) have reported increased urinary levels of catecholamine metabolites in mice lacking the αCGRP gene. In contrast, Maynard and Burnstock (1994) have shown that purines but not CGRP act as the main pre-junctional modulator of tritiated noradrenaline release in the rabbit ear artery model.
We have also shown that reductions in perfusion pressure of the mouse arterial mesenteric circuit by endogenous CGRP (via NANC stimulation) are modulated by the neuronal isoform of the NO producing enzyme, NOS. It is noteworthy that Ralevic (2002) showed that the release of transmural NO represses NANC-dependent vasodilatation, albeit the specific contribution of the neuronal isoform was not identified. In addition, Hatanaka et al. (2006) have recently reported that nNOS is a potent repressor of adrenergic-dependent vasoconstriction following perivascular nerve stimulation of the rat arterial mesenteric bed. Therefore, nNOS may significantly depress the overall (that is autonomic and C-fibre-dependent) perivascular nervous control of mesenteric tone in the mouse model.
The source of neuronal NOS in the mesenteric bed of the mouse remains to be histologically confirmed. In circuits of larger animal species, NO derived from nNOS has been suggested to act either as a co-transmitter with CGRP or as a single neuromodulator released from nitrergic nerve terminals, as recently revisited by Hatanaka et al. (2006). Assuming that in our model, 7-NI selectively inhibits the neuronal isoform, our results would support an important role for nNOS as a pre-junctional modulator of the CGRP-releasing nerve terminals since the inhibitor potentiated the response to NANC stimulation. This concept is also reinforced by the fact that 7-NI did not alter the reduction of perfusion pressure induced by exogenously administered hαCGRP in circuits derived from wild-type animals.
The selectivity of 7-NI as a neuronal NOS inhibitor varies depending on the bioassay investigated (Moore and Handy, 1997; Reiner and Zagvazdin, 1998). In in vivo settings however, several groups have used 7-NI in the characterization of nNOS selective mechanisms (Di Matteo et al., 2006; Lidington et al., 2007; Tai et al., 2007; Song et al., 2007). In the present study, we suggest a selective neuronal uptake of 7-NI (as previously suggested by Southan and Szabó, 1996), unlike vascular rings or in isolated cells, since the mesenteric bed assay possesses complex and functional autonomic nerve networks.
It is also worthy of notice that 7-NI did not potentiate PNS response in mesenteric beds derived from iNOS KO as opposed to wild-type and eNOS KO mice. If 7-NI was non-selective, one would have expected a potentiating effect of this inhibitor in all vascular beds tested. Alternatively, we suggest that knocking out the iNOS gene interferes with nNOS-dependent neuromodulation. Little is known of the putative crosstalk between nNOS and iNOS, apart from the study of Martins-Pinge et al. (2007) reporting a positive cooperativity of both NOS isoforms within the rostral ventrolateral medulla in the sympathetic control of cardiovascular functions.
We have also shown that removal of the endothelium with sodium deoxycholate and overall inhibition of NO production with L-NAME markedly inhibited both NANC—and hαCGRP-induced reduction in arterial perfusion pressure in the mouse mesenteric bed (only at the lower dose tested for the later stimulant). These results firstly highlight that both endogenous CGRP (that is NANC induced) and the exogenously administered peptide requires a functional endothelium to optimally act as vasoactive factors in the murine mesenteric circuit.
Secondly, although NO appears to mediate the mouse mesenteric response to PNS as well as to a lower dose of hαCGRP, the response to the highest dose of the same peptide may be dependent on other endothelial-derived factors such as epoxyeicosatrienoic acids (Campbell, 2000), C-type Natriuretic peptide (Sandow and Tare, 2007), hydroperoxides (Shimokawa and Matoba, 2004) and the charybdotoxin and apamin-sensitive endothelial-derived hyperpolarizing factor (EDHF) (for recent review see Félétou and Vanhoutte, 2006). Among those four factors, the last appears the most unlikely candidate considering that the high KCl concentrations used in our study would hamper hyperpolarization of potassium channels by EDHF in the underlying mesenteric smooth muscle (Félétou and Vanhoutte, 2006).
Thirdly, removal of the endothelium abolished the reduction of perfusion pressure induced by bradykinin but only reduced that following hαCGRP and PNS suggesting an endothelium-independent component in the vasoactive properties of the latter peptide as suggested by Amerini et al. (1993) and Gangula et al. (2003).
In contrast, the reduction in perfusion pressure afforded by CGRP, at the two lowest doses tested, was potentiated in eNOS KO mice. We suggest that the opposite effects of endothelium removal and NO inhibition versus deletion of endothelial NOS on the responses to CGRP are due to nNOS compensatory mechanisms previously reported in the eNOS KO mouse. In support of this concept, Loesch and Burnstock (1998) have immunohistologically identified both nNOS and eNOS in the cytoplasm of endothelial cells. Sanz et al. (2001) have subsequently reported that nNOS is importantly involved in leukocyte–endothelial cells interactions in eNOS KO mice.
Considering that 7-NI also potentiated the NANC response to PNS in arterial mesenteric circuits of eNOS KO mice, our results support a functional role for the nNOS isozyme as a neuromodulator of PNS even in absence of the eNOS.
It is also possible that the enhanced response to the lower doses of CGRP, but not to NANC stimulation reported here, in mesenteric bed of eNOS KO mice may be due to the fact that the exogenous peptide must permeate through the endothelial barrier, whereas the capsaicin-sensitive response to PNS may be targeting firstly the underlying smooth muscle.
It is also important to point out that we have focused mainly on the involvement of the NOS pathways in the reactivity of the mouse mesenteric bed to NANC stimulation. One cannot exclude, however that NANC stimulation triggers the release of several other peptides and autacoids (Lundberg, 1996) which are unaccounted for by the pharmacological approaches exploited in our study.
For example, the systematic use of a cyclooxygenase inhibitor, did not allow the identification of a modulatory role for endogeneous eicosanoids in the response of the mouse mesenteric bed to NANC stimulation. Kodama et al. (1995), had reported an indomethacin-sensitive component in the constrictor properties of PGF2α and U46619 in mesenteric microvessels of the rat. The contribution of COX-derived metabolites as modulators of the mouse mesenteric vascular tone remains, therefore, to be investigated.
Finally, the present study also illustrates the significant contribution of cAMP in the reduction in arterial mesenteric perfusion pressure, following NANC stimulation. Brain and Grant (2004) had initially demonstrated cAMP-dependent vasodilatory responses to CGRP. Our data however also suggest that cGMP may be an additional intracellular signalling nucleotide involved in the mouse mesenteric bed response to NANC stimulation. Considering that both endothelial removal and L-NAME blunted markedly the response of the arterial mesenteric bed to NANC stimulation, it is suggested that the release of endogenous CGRP following PNS triggers the release of endothelial-derived NO which subsequently activates cGMP production in the underlying vascular smooth muscle (Murad, 2006). It is noteworthy that the cGMP-dependent vasodilator, sodium nitroprusside, was considered in our model, as a reliable control towards the assessment of guanylate cyclase-dependent vasoactive responses in the mouse mesenteric bed.
In conclusion, our study reports that endogenous CGRP is an important mediator, following activation of CGRP1 receptors and via cAMP/cGMP pathways, of NANC responses in the pre-capillary but not post-capillary circuits of the mouse mesenteric bed. Our results also suggest that the neuronal but not the endothelial NOS may limit the NANC response to PNS in these murine vascular beds. In contrast, NO and other endothelial-derived factors, are also involved as mediators of the responses to both NANC-induced stimulation and exogenous hαCGRP in the same circuit. Finally, we suggest that, in accordance with previous reports in vascular models derived from larger animal species (Kawasaki et al., 1990; Rubino and Burnstock, 1996; Brain and Grant, 2004) NANC perivascular nerves act as a physiological antagonist of the autonomic nerve-dependent increase of perfusion pressure in the arterial mesenteric bed of the mouse.
Acknowledgments
We acknowledge the financial support of the Canadian Institutes for Health Research. We also express our gratitude to Dr Nathalie Berthiaume (Department of Pharmacology, Université de Sherbrooke) for technical support, Dr Alain Cadieux (Department of Pharmacology, Université de Sherbrooke) for constructive comments on this manuscript and Dr Robert Dumaine (Department of Physiology, Université de Sherbrooke) for supplying TTX. PDJ is a National Scholar of the Fonds de la Recherche en Santé du Québec.
Abbreviations
- BIBN4096BS, 1-piperidinecarboxamide, N-[2-[ [5-amino-1-[ [4-(4-pyridinyl)-1-piperazinyl]carbonyl]pentyl]amino]-1-[(3,5-dibromo-4-hydroxyphenyl) methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)- [R-(R*
S*)]-
- CGRP
calcitonin gene-related peptide
- EDHF
endothelium-derived hyperpolarizing factor
- EIA
enzyme immunoassay
- hαCGRP8–37
human fragment 8–37 of alpha calcitonin gene-related peptide
- L-NAME
NG-nitro-L-arginine methyl ester
- 7-NI
7-nitroindazole
- nNOS
neuronal nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- ODQ, 1H-[1,2,4]oxadiazole[4
3-a] quinoxalin-1-one
- PNS
perivascular nerve stimulation
- SD
sodium deoxycholate
- SQ 22 536
[9-(tetrahydro-2-furanyl)-9H-purin-6-amine]
- TRPV1
transient receptor potential vanilloid receptor 1
- TTX
tetrodotoxin
Conflict of interest
The authors state no conflict of interest.
References
- Ahluwalia A, Vallance P. Evidence for functional responses to sensory nerve stimulation of rat small mesenteric veins. J Pharmacol Exp Ther. 1997;281:9–14. [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerini S, Mantelli L, Ledda F. Nitric oxide is not involved in the effects induced by non-adrenergic non-cholinergic stimulation and calcitonin gene-related peptide in the rat mesenteric vascular bed. Neuropeptides. 1993;25:51–55. doi: 10.1016/0143-4179(93)90068-l. [DOI] [PubMed] [Google Scholar]
- Arai K, Ohno T, Saeki T, Mizuguchi S, Kamata K, Hayashi I, et al. Endogenous prostaglandin I2 regulates the neural gene related peptide emergency system through release of calcitonin. Gut. 2003;52:1242–1249. doi: 10.1136/gut.52.9.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Berthiaume N, Hess F, Chen A, Regoli D, D'Orleans-Juste P. Pharmacology of kinins in the arterial and venous mesenteric bed of normal and B2 knockout transgenic mice. Eur J Pharmacol. 1997;333:55–61. doi: 10.1016/s0014-2999(97)01096-0. [DOI] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Campbell WB. New role for epoxyeicosatrienoic acids as anti-inflammatory mediators. Trends Pharmacol Sci. 2000;21:125–127. doi: 10.1016/s0165-6147(00)01472-3. [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamaguchi A, Yamatani T, Nakamura A, Morishita T, Inui T, et al. Calcitonin gene-related peptide receptor antagonist human CGRP-(8–37) Am J Physiol. 1989;256:E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- Claing A, Telemaque S, Cadieux A, Fournier A, Regoli D, D'Orleans-Juste P. Nonadrenergic and noncholinergic arterial dilatation and venoconstriction are mediated by calcitonin gene-related peptide1 and neurokinin-1 receptors, respectively, in the mesenteric vasculature of the rat after perivascular nerve stimulation. J Pharmacol Exp Ther. 1992;263:1226–1232. [PubMed] [Google Scholar]
- Di Matteo V, Benigno A, Pierucci M, Giuliano DA, Crescimanno G, Esposito E, et al. 7-Nitroindazole protects striatal dopaminergic neurons against MPP+-induced degeneration: an in vivo microdialysis study. Ann N Y Acad Sci. 2006;1089:462–471. doi: 10.1196/annals.1386.015. [DOI] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, et al. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orléans-Juste P, Berthiaume N, Plante GE, Bkaily G, Claing A. Comparison of the pre- and post-capillary vascular reactivity in the rat and guinea pig perfused mesenteric bed. Can J Physiol Pharmacol. 1996;74:811–817. [PubMed] [Google Scholar]
- Eguchi S, Tezuka S, Hobara N, Akiyama S, Kurosaki Y, Kawasaki H. Vanilloid receptors mediate adrenergic nerve- and CGRP-containing nerve-dependent vasodilation induced by nicotine in rat mesenteric resistance arteries. Br J Pharmacol. 2004;142:1137–1146. doi: 10.1038/sj.bjp.0705773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Gangula PR, Thota C, Wimalawansa SJ, Bukoski RD, Yallampalli C. Mechanisms involved in calcitonin gene-related peptide-induced relaxation in pregnant rat uterine artery. Biol Reprod. 2003;69:1635–1641. doi: 10.1095/biolreprod.103.016725. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Grant AD, Tam CW, Lazar Z, Shih MK, Brain SD. The calcitonin gene-related peptide (CGRP) receptor antagonist BIBN4096BS blocks CGRP and adrenomedullin vasoactive responses in the microvasculature. Br J Pharmacol. 2004;142:1091–1098. doi: 10.1038/sj.bjp.0705824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DN, Asaad MM, Phillips MB, Goldenberg HJ, Antonaccio MJ. Inhibition of adenylate cyclase in human blood platelets by 9-substituted adenine derivatives. J Cyclic Nucleotide Res. 1979;5:125–134. [PubMed] [Google Scholar]
- Hatanaka Y, Hobara N, Honghua J, Akiyama S, Nawa H, Kobayashi Y, et al. Neuronal nitric-oxide synthase inhibition facilitates adrenergic neurotransmission in rat mesenteric resistance arteries. J Pharmacol Exp Ther. 2006;316:490–497. doi: 10.1124/jpet.105.094656. [DOI] [PubMed] [Google Scholar]
- Holzer P, Wachter C, Heinemann A, Jocic M, Lippe IT, Herbert MK. Sensory nerves, nitric oxide and NANC vasodilatation. Arch Int Pharmacodyn Ther. 1995;329:67–79. [PubMed] [Google Scholar]
- Kawasaki H, Nuki C, Saito A, Takasaki K. Adrenergic modulation of calcitonin gene-related peptide (CGRP)-containing nerve-mediated vasodilation in the rat mesenteric resistance vessel. Brain Res. 1990;506:287–290. doi: 10.1016/0006-8993(90)91263-g. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Kodama T, Marmon LM, Vargas R, Farhat M, Hoy GR, Ramwell PW. The interaction between endothelium-derived relaxing factor (EDRF) and eicosanoids in the regulation of the mesenteric microcirculation. J Surg Res. 1995;58:227–232. doi: 10.1006/jsre.1995.1035. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci USA. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidington D, Li F, Tyml K. Deletion of neuronal NOS prevents impaired vasodilation in septic mouse skeletal muscle. Cardiovasc Res. 2007;74:151–158. doi: 10.1016/j.cardiores.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Loesch A, Burnstock G. Perivascular nerve fibers and endothelial cells of the rat basilar artery: immuno-gold labelling of antigenic sites for type I and type III nitric oxide synthase. J Neurocytol. 1998;27:197–204. doi: 10.1023/a:1026493425977. [DOI] [PubMed] [Google Scholar]
- Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Martins-Pinge MC, Garcia MR, Zoccal DB, Crestani CC, Pinge-Filho P. Differential influence of iNOS and nNOS inhibitors on rostral ventrolateral medullary mediated cardiovascular control in conscious rats. Auton Neurosci. 2007;131:65–69. doi: 10.1016/j.autneu.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Maynard KI, Burnstock G. Evoked noradrenaline release in the rabbit ear artery: enhancement by purines, attenuation by neuropeptide Y and lack of effect of calcitonin gene-related peptide. Br J Pharmacol. 1994;112:123–126. doi: 10.1111/j.1476-5381.1994.tb13040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Mione MC, Ralevic V, Burnstock G. Peptides and vasomotor mechanisms. Pharmacol Ther. 1990;46:429–468. doi: 10.1016/0163-7258(90)90027-y. [DOI] [PubMed] [Google Scholar]
- Moore PK, Handy RLC. Selective inhibitors of neuronal nitric oxide synthase—is no NOS really good NOS for the nervous system? Trends Pharmacol Sci. 1997;18:204–211. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- Murad F. Shattuck lecture. Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- Oh-hashi Y, Shindo T, Kurihara Y, Imai T, Wang Y, Morita H, et al. Elevated sympathetic nervous activity in mice deficient in alphaCGRP. Circ Res. 2001;89:983–990. doi: 10.1161/hh2301.100812. [DOI] [PubMed] [Google Scholar]
- Phillips JK, McLean AJ, Hill CE. Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentery. Br J Pharmacol. 1998;124:1403–1412. doi: 10.1038/sj.bjp.0701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisinghani M, Pabbidi RM, Premkumar LS. Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. J Physiol. 2005;567:771–786. doi: 10.1113/jphysiol.2005.087874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V. Endothelial nitric oxide modulates perivascular sensory neurotransmission in the rat isolated mesenteric arterial bed. Br J Pharmacol. 2002;137:19–28. doi: 10.1038/sj.bjp.0704837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Hogestatt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Zagvazdin Y. On the selectivity of 7-nitroindazole as an inhibitor of neuronal nitric oxide synthase. Trends Pharmacol Sci. 1998;19:348–350. doi: 10.1016/s0165-6147(98)01194-8. [DOI] [PubMed] [Google Scholar]
- Rubino A, Burnstock G. Capsaicin-sensitive sensory-motor neurotransmission in the peripheral control of cardiovascular function. Cardiovasc Res. 1996;31:467–479. [PubMed] [Google Scholar]
- Sandow SL, Tare M. C-type natriuretic peptide: a new endothelium-derived hyperpolarizing factor? Trends Pharmacol Sci. 2007;28:61–67. doi: 10.1016/j.tips.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Sanz MJ, Hickey MJ, Johnston B, McCafferty DM, Raharjo E, Huang PL, et al. Neuronal nitric oxide synthase (NOS) regulates leukocyte–endothelial cell interactions in endothelial NOS deficient mice. Br J Pharmacol. 2001;134:305–312. doi: 10.1038/sj.bjp.0704234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H, Matoba T. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pharmacol Res. 2004;49:543–549. doi: 10.1016/j.phrs.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Faraci FM.Effects of a novel inhibitor of guanylyl cyclase on dilator responses of mouse cerebral arterioles Stroke 199728837–842.discussion 842–843 [DOI] [PubMed] [Google Scholar]
- Song MY, Zwemer CF, Whitesall SE, D'Alecy LG. Acute and conditioned hypoxic tolerance augmented by endothelial nitric oxide synthase inhibition in mice. J Appl Physiol. 2007;102:610–615. doi: 10.1152/japplphysiol.00894.2006. [DOI] [PubMed] [Google Scholar]
- Southan GJ, Szabó C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- Tai MH, Weng WT, Lo WC, Chan JY, Lin CJ, Lam HC, et al. Role of nitric oxide in alpha-melanocyte-stimulating hormone-induced hypotension in the nucleus tractus solitarii of the spontaneously hypertensive rats. J Pharmacol Exp Ther. 2007;321:455–461. doi: 10.1124/jpet.106.118299. [DOI] [PubMed] [Google Scholar]
- Warner TD. Simultaneous perfusion of rat isolated superior mesenteric arterial and venous beds: comparison of their vasoconstrictor and vasodilator responses to agonists. Br J Pharmacol. 1990;99:427–433. doi: 10.1111/j.1476-5381.1990.tb14720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]