Abstract
Telomerase and telomeres are attractive targets for anticancer therapy. This is supported by the fact that the majority of human cancers express the enzyme telomerase which is essential to maintain their telomere length and thus, to ensure indefinite cell proliferation – a hallmark of cancer. Tumours have relatively shorter telomeres compared to normal cell types, opening the possibility that human cancers may be considerably more susceptible to killing by agents that inhibit telomere replication than normal cells. Advances in the understanding of the regulation of telomerase activity and the telomere structure, as well as the identification of telomerase and telomere associated binding proteins have opened new avenues for therapeutic intervention. Here, we review telomere and telomerase biology and the various approaches which have been developed to inhibit the telomere/telomerase complex over the past decade. They include inhibitors of the enzyme catalytic subunit and RNA component, agents that target telomeres, telomerase vaccines and drugs targeting binding proteins. The emerging role of telomerase in cancer stem cells and the implications for cancer therapy are also discussed.
Keywords: telomerase, telomeres, telomere targeting agents, G-quadruplex, telomere uncapping, hTERT, hTERC
Introduction
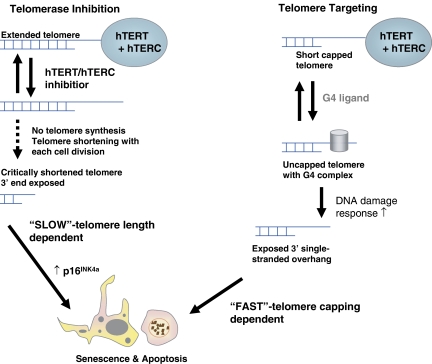
The most prominent difference between tumour and normal cells is their proliferative capacity. While normal cells have a limited life span and show replicative senescence, tumour cells are immortal (Goldstein, 1990; Hayflick, 1992). Limitless replicative potential is one of the six hallmarks of cancer as defined by Hanahan and Weinberg (2000). The biochemical mechanism responsible for tumour cell immortalization, and conversely for normal cell senescence, involves an enzyme termed telomerase (Greider, 1991; Blackburn, 1991, 1992; Shippen, 1993; Kim et al., 1994; Holt et al., 1996). This enzyme permits cancer cells to overcome one of the fundamental limitations to mammalian cell immortality, the progressive loss of telomeric DNA from the ends of the cell's chromosomes that occurs during each round of cell division (Figure 1). Since all chromosomes begin life with a limited amount of telomeric DNA, there are a finite number of cell divisions that a cell can undergo before it reaches an irreducible lower limit of telomeres in the absence of telomerase (Blackburn, 2000). Telomerase has therefore emerged as a very attractive target for therapeutic intervention of cancer and has been a major focus in cancer research since the mid-1990s.
Figure 1.
Consequences of telomerase enzyme inhibition compared to direct telomere targeting. ‘Pure' enzyme inhibitors require a lag time (several cell divisions) before telomeres shorten to a critical length. This process is slow and depends on the initial telomere length. Cell death is predominately caused by cellular senescence. Telomere targeting agents, such as G-quadruplex ligands, uncap telomeres and induce a p53-independent telomere-associated DNA-damage response. This process is fast and can induce senescence as well as apoptosis.
The most important criterion for developing better cancer therapies is tumour selectivity. During malignant transformation cancer cells acquire genetic mutations that override the normal mechanisms controlling cellular proliferation. It became clear in the early 1990s (Fearon and Vogelstein, 1990) that cancer is a multistep process and that normal cells can be converted into tumour cells by coexpression of co-operating oncogenes. Such experiments were mainly performed with mouse cell lines since it was very difficult to obtain transformed human cells. In 1999, Weinberg and co-workers were the first to create human tumour from normal cells with defined genetic elements. The key step in this process was the immortalization by ectopic expression of the catalytic subunit of the enzyme telomerase (hTERT) (Hahn et al., 1999a). Then, it became clear why normal mouse cells would transform by addition of oncogenes alone – they express as compared to normal human cells telomerase activity (Blasco et al., 1997; Hahn et al., 1999a).
In this review, we will give an overview of current telomere and telomerase biology and highlight evolving targets in the telomere/telomerase complex as well as therapeutic approaches.
Telomeres and telomerase in normal versus cancer cells
Human telomeres are non-coding DNA-sequences at the end of chromosomes, which are composed of (TTAGGG)n hexanucleotide repeats. During each cell division, telomeric DNA (30–100 bp) is lost because of the end-replication problem. Telomeres maintain chromosomal integrity and prevent replication of defective genes (Blackburn, 2000; Keith et al., 2001). When normal cells reach a critical telomere length, they exit the cell cycle, enter M2 (mortality stage 2) crisis and undergo senescence. This mechanism is thought to be the clock that determines human life span (Harley et al., 1990; Hayflick, 1992).
Different cell types have different telomere dynamics. The average available telomere length in normal somatic cells is 10 kilobases (kb), their telomeres erode resulting in senescence. Stem cells of renewal tissues have an average telomere length of 12 kb, which shorten at reduced rates, whereas germ cells and fetal tissues have 15–20 kb and maintain their telomeres. The average telomere length of cancer cells however, is only 5 kb (range ∼2–9 kb). During early tumourigenesis telomeres erode, but are then maintained at a stable length through, in the great majority of cases, the reactivation of telomerase (Hastie et al., 1990; Harley et al., 1990; Holt et al., 1996; Burger, 1999). Among cancers, it has been shown that telomere content (TC), a proxy for telomere length, has prognostic relevance. In prostate carcinomas, low TC predicts for metastasis and recurrence (Fordyce et al., 2005); in breast cancers low TC predicts for poor clinical outcome and a reduced 5-year breast cancer-free survival interval (Fordyce et al., 2006). Moreover, a recent meta-analysis of telomere length studies in solid tumours by Griffith and co-workers revealed that telomere length is reduced or elongated in a tumour type-specific manner (Bisoffi et al., 2006).
Telomerase is a ribonucleoprotein reverse transcriptase. It has a RNA component human telomerase RNA component (hTERC), which acts as the template for addition of new telomeric repeats, and a catalytic subunit human telomerase reverse transcriptase (hTERT) (Kim et al., 1994; Feng et al., 1995; Nakamura et al., 1997) (Figure 2). Telomerase permits cells to overcome one of the fundamental limitations to mammalian cell immortality, the progressive loss of telomeric DNA. Cell populations that continue dividing throughout life, such as germ and stem cells require the addition of new telomeres to their chromosomes to replace sequences lost during cell division (Shay and Bacchetti, 1997; Burger et al., 1997a). The latter and cancer cells, which have acquired a high proliferative potential, surpass replicative senescence by activation of the enzyme telomerase. As telomerase is reactivated, it can de novo synthesize TTAGGG hexanucleotide repeats onto shortened telomeres, thus maintaining them at a stable length. Cancer and stem cells have therefore an infinite capacity to proliferate and are immortal (Kim et al., 1994; Holt et al., 1996). Virtually, all human tumour cell lines and approximately 85% of human cancer tissues have been shown to possess telomerase activity. By contrast, normal tissues adjacent to tumour and human somatic tissues, other than stem cells, do not possess detectable levels of telomerase (Kim et al., 1994; Shay and Bacchetti, 1997).
Figure 2.
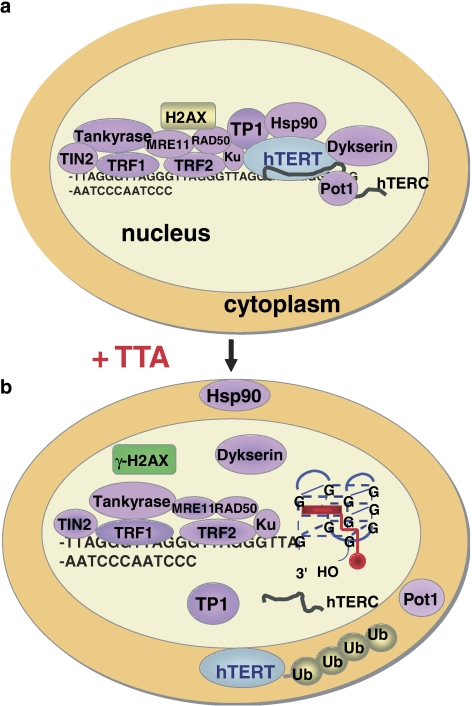
(a) The telomere cap is composed of telomerase and telomere-associated proteins as well as telomeric DNA repair proteins (see also Table 1). The 3′-end of telomeric DNA must be single-stranded for hybridization with hTERC. (b) G-quadruplex ligands (TTA, red) induce the 3′-end overhang to fold into a quadruplex structure, which inhibits hTERT and hTERC binding and stops telomere elongation. Unbound telomerase and telomere proteins (Pot1, Hsp90, etc.) translocate into the cytoplasm and undergo degradation via the ubiquitin (Ub) proteasome system (e.g. hTERT). ‘Unprotected' telomeres signal DNA damage, a very early indicator of this is the phosphorylation of H2AX and, thus, formation of γ-H2AX foci in nuclei of affected cells (Phatak et al., 2007; Salvati et al., 2007). Ligands need to be selective for this higher-order G-quadruplex structure to have minimal duplex DNA affinity so that drug concentrations that inhibit the enzyme activity to 50% (telEC50) << general cellular cytotoxicity. TTA, telomere targeting agent.
Although telomerase is the major mediator of telomere elongation, small fractions of yeast and mammalian cells that lack telomerase can operate a recombination-dependent mechanism, termed alternative lengthening of telomeres (ALT) (Bryan et al., 1995; Lundblad and Blackburn, 1993).
Telomere capping and uncapping
The telomere length set point and its regulation involve telomere binding proteins and other members of the telomere/telomerase complex. Telomeres and associated binding proteins provide protective capping for chromosome ends (Figure 2a, Table 1). According to the telomere capping/uncapping hypothesis proposed by Elizabeth Blackburn, capping is functionally defined as preserving the physical integrity of the telomere, allowing cell division to proceed. Regulated uncapping would occur normally in dividing cells with the crucial property that a functional telomere would rapidly switch back to a capped state. Left uncorrected for too long, the uncapped state will elicit cell-cycle arrest or other responses (Smith and Blackburn, 1999; Blackburn, 2000; Blackburn, 2001).
Table 1.
Telomere and telomerase associated proteins and their inhibitors
| Description | Inhibitors | References | |
|---|---|---|---|
| Telomerase components | |||
| hTERT | Human telomerase reverse transcriptase | AZT B1BR1532 Vaccine |
Strahl, 1996 Damm, 2001 Carpenter, 2006 |
| hTERC | Human telomerase RNA component | 2-5A antisense GRN163L GDEPT |
Kondo, 1998 Dikmen, 2005 Plumb, 2001 |
| Hsp90 | Heat shock 90 kDa protein | 17-AAG, 17-DMAG | Holt, 1999 |
| TP1 | Telomerase-associated protein 1 | NK | Keith, 2002 |
| Telomere proteins | |||
| TRF1 | Telomeric-repeat-binding factor 1 | NK | VanSteensel, 1997 |
| TRF2 | Telomeric-repeat-binding factor 2 | NK | Karlseder, 1999 |
| Tankyrase | Tankyrase, TRF1-interacting ankyrin-related poly(ADP-ribose) polymerase (PARP) | 3-aminobenzamide ABT-888 AG14361 |
Seimiya, 2005 Donawho, 2007 Thomas, 2007 |
| TIN2 | TRF1-interacting protein 2, negative regulator of telomere length | NK | Keith, 2002 |
| POT1 | Telomere-end-binding protein 1 | Telomestatin BRAC019 RHPS4 |
Gomez, 2006 Kelland, 2005 Salvati, 2007 |
| Dykserin | Catalyzes pseudouridylation, is associated with hTERC, has structural function | NK | Mochizuki, 2004 |
| Telomere repair | |||
| MRE11 | Meiotic recombination 11 homologue | NK | Blasco, 2003 |
| RAD50 | Rad50 (S. cerevisiae) homologue | NK | Blasco, 2003 |
| KU70 | Thyroid autoantigen 70 kDa (Ku antigen) | NK | Blasco, 2003 |
| XRCC5/KU80 | X-ray repair (double-strand-break rejoining; Ku autoantigen, 80 kDa) | NK | Blasco, 2003 |
| H2AX | Histone 2 AX | RHPS4 (induction of γ-H2AX) |
Phatak, 2007 Salvati, 2007 |
NK, not known; references list only the first author and year of publication.
Telomeres can become dysfunctional by several mechanisms including loss of telomere-binding proteins and direct telomeric DNA damage (Smith and Blackburn, 1999; Bisoffi et al., 2006) (Figures 1 and 2). Experimental evidence suggests that morphologically defective chromosomes are more likely to result from telomere uncapping than telomere erosion, thus challenging the paradigm that telomeres have to critically shorten to cause telomere dysfunction and senescence (Figure 1).
The telomere/telomerase complex
In vitro, hTERC and hTERT are sufficient to bestow telomerase activity (Feng et al., 1995; Nakamura et al., 1997). In vivo, these core subunits are augmented by additional factors that comprise the functional telomere/telomerase complex, the telomerase associated proteins and the telomere-binding proteins, which play a role in equilibrium length establishment by affecting the localization and activity of the enzyme (Figure 2, Table 1). TP1 (telomerase-associated protein 1) and the molecular chaperone Hsp90 are physically associated with the telomerase catalytic subunit protein hTERT. hTERT requires Hsp90-mediated chaperone conformational folding for maturation and functional activation (Holt et al., 1999; Forsythe et al., 2001) (Figure 2). Telomere binding proteins include but are not limited to the telomeric repeat binding factors 1 and 2 (TRF1 and TRF2), TRF-1 interacting protein 2 (TIN2), POT1 (protection of telomeres 1), as well as tankyrase (TRF1-inactivating ankyrin-related adenosine diphosphate (ADP)-ribose polymerase), a member of the PARP family proteins (Figure 2, Table 1) (van Steensel and de Lange, 1997; Smith et al., 1998; Karlseder et al., 1999). Dyskerin is a hTERC associated protein that has a structural function (Mochizuki et al., 2004).
Key components of the homologous recombination and non-homologous end-joining pathways for DNA double-strand break repair (DSB) are also found at mammalian telomeres, in particular they include a PARP (poly (ADP-ribose) polymerase (tankyrase)), MRE11, Ku70, Ku80, Histone 2AX and Rad50 (Table 1, Figure 2) (Keith et al., 2002; Blasco, 2003). The earliest measurable event following telomere-associated DNA-damage signalling is the phosphorylation of H2AX (γ-H2AX) (Figure 2) (d'Adda di Fagagna et al., 2003).
Telomeres consist predominantly of double-stranded telomeric repeats with only the extreme terminus containing some single-stranded G-rich sequences (G-strand or 3′-single-stranded overhang). Telomerase adds the single-strand TTAGGG extension, whereas double-stranded regions are replicated by conventional polymerases (Figure 2a). Two structures of the 3′-single stranded (TTAGGG)n overhang have been proposed: one suggesting the presence of a T-loop, which would result from a foldback of the 3′overhang into a loop of duplex DNA, the other proposes the formation of dimers and tetramers involving G-quadruplexes (de Lange, 2002). G-quadruplex formation seems to be accelerated in the presence of telomere binding proteins, which appear to act as chaperones (Fang and Czech, 1993). The G-quadruplex was recently crystallized and confers an energetically preferable conformation (Parkinson et al., 2002). However, both structures of the 3′-end overhang might coexist (Murti and Prescott, 1999). Nonetheless, the existence of a folding of the G-strand overhang into a G-quadruplex has to be considered most plausible in human cells based on the available experimental evidence.
Targets in the telomere/telomerase complex for anticancer therapy
Telomerase reactivation and the establishment of telomere length equilibrium are key steps during early tumourigenesis. Based on this and the facts described above, it seems very plausible that the inhibition of the enzyme telomerase or proteins involved in the telomere/telomerase complex by targeted small molecules or other telomerase-directed approaches would lead to inhibition of malignant transformation and/or tumour growth. Telomerase inhibition is likely to yield novel broad spectrum, but possibly specific anticancer drugs. The telomere/telomerase complex provides multiple possibilities for the development of inhibitors; various approaches are currently under advanced preclinical investigations or in early clinical trials (Figures 2 and 3). The targets are shown in Figure 2 and listed in Table 1(Kelland, 2001, 2005; Burger and Kelland, 2006; Burger, 2007). Compounds in Figure 3 represent the most specific and advanced telomerase inhibitors (see also Table 1).
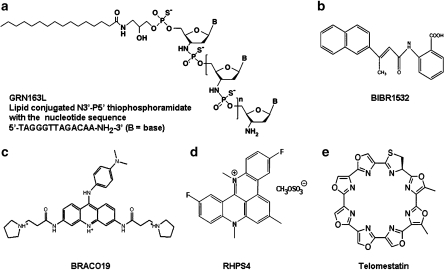
Figure 3.
Molecular structures of telomerase inhibitory agents. (a) GRN163L, (b) BIBR1532, (c) BRACO19, (d) RHPS4 and (e) telomestatin.
Telomerase as a cancer inhibitory target
Several genetically engineered model systems support the pivotal role of telomerase in cancer. Particularly useful to validate telomerase as a cancer inhibitory target were studies with hTERC knockout mice and ectopic expression of dominant-negative hTERT in human tumour cells (Blasco et al., 1997; Hahn et al., 1999b; Gonzalez-Suarez et al., 2000). Initial findings with the hTERC knockout mice were disappointing as they showed that fibroblasts derived from telomerase knockout mice were still capable of forming tumours upon transformation with oncogenes such as rasv12 or T-antigen and that the first-generation hTERC−/−mice appeared normal (Blasco et al., 1997). However, soon it became obvious that late generation mice (6th generation) were infertile due to defects in spermatogenesis or had abnormalities in other telomerase-dependent cells such as the marrow and spleen. These mice were then also significantly less prone to develop skin tumours upon chemical induced carcinogenesis (Gonzalez-Suarez et al., 2000), hence, suggesting a lag time of the telomere shortening process dependent on initial telomere length, which is very high in murine tissues (∼50 kb) (Figure 1).
Telomere length-dependent inhibition of tumour growth was further shown in human cells. The expression of a catalytically inactive form of the human TERT subunit in cancer-derived cell lines disrupted telomerase activity and was coincident with decrease in telomere length (Hahn et al., 1999b). This resulted in proliferative defects with successive population doublings. Time to growth arrest correlated with initial telomere length. To the contrary, a cell line that maintained its telomeres independent of telomerase by the ALT mechanism was resistant to growth inhibitory effects of double negative (DN)-hTERT transfection (Hahn et al., 1999b).
Agents targeting hTERC
The use of antisense oligonucleotides (ODNs) for targeting hTERC was first reported in 1995, and they represent the first telomerase inhibitors. ODNs are short single-stranded DNA sequences that are complementary to a target RNA such as hTERC. An antisense construct expressing 185 nucleotides of hTERC was able to shorten telomeres in HeLa cells over 23–26 population doublings via the reduction of telomerase activity and induced apoptosis (Feng et al., 1995). ODNs tagged with 2′-5′-oligoadenylate (2–5A) to bind and cleave hTERC caused apoptosis in glioma, prostate, cervical, bladder and ovarian cancer cells within 4–5 days (Kondo et al., 1998; Kushner et al., 2000; Koga et al., 2001; Yatabe et al., 2002). To enhance the binding properties to hTERC sequences and stability, 2′-O-methyl-RNA oligomers were developed (Herbert et al., 1999). The lead compound of the latter efforts was GRN163. Cells treated with this oligomer took more than 100 days to eventually die, but inhibited telomerase activity at very low concentrations (Figure 1; see principle of telomerase inhibition) (Herbert et al., 1999). GRN163L (Figure 3a), is a 13-mer oligonucleotide N3′ → P5′ thio-phosphoramidate in a lipid carrier (L), which has demonstrated promising preclinical in vitro and in vivo anti-tumour activity and has just entered clinical trials. GRN163L (Geron Corp., CA, USA) is the first anti-telomerase agent to enter the clinic (Dikmen et al., 2005; Gellert et al., 2006; Jackson et al., 2007). Safety and dose finding studies are underway in patients with refractory and relapsed solid malignancies. The drug is administered weekly and secondary objectives of the trial are the determination of its pharmacokinetics and preliminary antineoplastic activity. Correlative end points are not planned in this study (ClinicalTrials.gov, NCT00310895).
Another approach to using hTERC as a cancer-specific target that has been pursued is gene-directed enzyme pro-drug therapy (GDEPT). Keith and co-workers have exploited the transcriptional regulatory sequences of the hTERC gene to regulate expression of the bacterial nitroreductase enzyme in combination with the pro-drug CB1954 in a suicide gene therapy strategy. In cancer cells, hTERC promoter activity is 300-fold higher compared to normal cells, thus by placing the nitroreductase gene under the control of the telomerase gene promoter, sensitization to the pro-drug (CB1954 is a potent alkylating agent when released) with cell death was observed. The latter was restricted to cancer cells exhibiting high levels of promoter activity and this effect was retained in xenograft models in vivo (Plumb et al., 2001). The hTERC GDEPT concept is promising and currently under advanced preclinical investigation (Table 1).
Agents targeting hTERT
The telomerase catalytic subunit hTERT holds the greatest promise for a very specific targeted intervention. However, despite significant efforts made by pharmaceutical industry to identify inhibitors of hTERT, only a few approaches have yielded results. Often, small molecule hTERT inhibitors displayed a non-specific inhibition of DNA polymerases and as a consequence were dropped (Kelland, 2001). The most specific synthetic hTERT inhibitor known to date is BIBR1532 (2-[(E)-3-naphthalen-2-yl-but-2-enoylamino]-benzoic acid) (Figure 3b). BIBR1532 inhibits telomerase activity in vitro at inhibitory concentration 50% in the low nanomolar range (Damm et al., 2001). Treatment of DU145 (prostate), MDA-MB-231 (breast), HT1080 (fibrosarcoma) and NCI-H460 (lung) cells with BIBR1532 shortened telomeres progressively while lacking acute cytotoxicity. Typical of what one would expect for a ‘pure' hTERT inhibitory agent (see Figure 1), cancer cells underwent growth arrest only after a substantial lag time and when telomeres had shortened to a critical length, thus causing lethal mitotic and chromosomal defects (Damm et al., 2001).
The nucleoside analogue AZT (3-azido-2′-3′-dideoxythymidine) has also been shown to inhibit the telomerase reverse transcriptase activity, hTERT. However, high drug concentrations are required (>100 μM) for enzyme inhibition and its antiproliferate activity is weak (Strahl and Blackburn, 1996; Brown et al., 2003).
Similar to hTERC, gene therapy-based strategies have been developed utilizing hTERT. hTERT promoter GDEPT was equally effective as hTERC GDEPT (Plumb et al., 2001). In addition, an adenovirus expressing a ribozyme directed against the T-motif of hTERT significantly reduced telomerase activity 3 days after transduction and this was accompanied by a massive cell loss in ovarian cancer cell lines, but not in telomerase-negative human fibroblasts (Saretzki et al., 2001).
However, carefully conducted experiments that have employed genetic (dominant negative) hTERT inhibition, together with the data resulting from studying the synthetic hTERT inhibitor BIBR1532 and hTERC template antagonists such as GRN163L clearly indicate that enzyme inhibition requires a substantial lag time – dependent on the initial length of telomeres – before cell kill by replicative senescence occurs (Hahn et al., 1999b; Damm et al., 2001; Dikmen et al., 2005) (Figure 1). If this scenario is operable in vivo in patients, telomerase inhibition alone is probably insufficient for effective tumour growth inhibition and would require combination with other targeted or cytotoxic agents.
Telomere targeting agents
Most recently, experimental evidence has accumulated indicating that telomere uncapping will lead to more rapid cell kill (Figure 1). DNA damage signals at the G-strand overhang and the corresponding lagging strand could lead to uncapping of the chromosome and rapidly occurring apoptosis or genomic instability. In addition, telomere-based senescence and premature senescence due to static telomere conditions can also contribute to a much reduced lag time as compared to telomere shortening by telomerase enzyme inhibition alone (Blackburn, 2000; de Lange, 2002; Shay, 2003). Thus, agents that ‘uncap' and/or directly target telomeres are likely to be more effective as a monotherapy and can act faster as compared to ‘pure' enzyme inhibitors (Figure 1).
Telomere targeting agents (TTA) effectively accomplish both: the shortening and uncapping of telomeres as well as the inhibition of telomerase activity (Burger et al., 2005; Cookson et al., 2005a). TTAs have been rationally designed based on crystal and/or NMR solution structures (Wang and Patel, 1993; Parkinson et al., 2002; Cookson et al., 2005b) to interact with the G-rich single-stranded telomeric overhang by inducing it to form and stabilize G-quadruplexes (Kelland, 2005) (Figure 2b).
Agents that can specifically target telomeric repeat sequences are termed G-quadruplex ligands. They include trisubstituted (BRACO19, Figure 3c) and pentacyclic (RHPS4, Figure 3d) acridines, cationic porphyrins (TMPyP4), ethidum derivatives and telomestatin (Figure 3e, Table 1) (Kelland, 2005; Gowan et al., 2001; Read et al., 2001; Riou et al., 2002; Pennarun et al., 2005). Unlike the other G-quadruplex ligands, telomestatin is a natural product and is the most potent of these agents in terms of telomerase inhibition (low nanomolar range) (Kim et al., 2002).
We and others have shown that as a result of G-quadruplex formation, telomerase (hTERT) and telomere-associated proteins (for example, POT1 and TRF2) are displaced from the telomeres, translocate into the cytoplasm where they render non-functional (degradation via the ubiquitin proteasome system) and trigger telomere-associated DNA-damage response (Burger et al., 2005; Gomez et al., 2006; Phatak et al., 2007; Salvati et al., 2007) (Figure 2b). DNA damage response follows DSB signalling that is induced by phosphorylation of H2AX (γ-H2AX) (Figure 2b). This might in part explain their single agent activity and rapid onset of activity against in vivo human tumour models with relatively short telomeres (Burger et al., 2005; Phatak et al., 2007; Salvati et al., 2007). RHPS4 and BRACO19 are TTAs that have the potential for clinical development. They are currently undergoing preclinical toxicology and are scheduled for entry into phase I clinical trials (Kelland, 2005).
Interestingly, the standard anticancer drug cis-diamminedichloroplatinum (CDDP, cisplatin) could be considered as a non-specific TTA. Cisplatin is known to form N7-Pt-N7 guanine–guanine intrastrand crosslinks. The G-rich sequence of telomeres (TTAGGG)n, which extends beyond the C-rich strand for around ∼130–210 base pairs, has been shown to react with cisplatin. It has been proposed that cisplatin might specifically poison telomeres, because no transcription products from telomeres exist and thus damage to telomeric DNA will not be repaired by the transcription-coupled nucleotide excision repair system, whereas double-stranded coding DNA will be repaired (Burger et al., 1997b; Ishibashi and Lippard, 1998). Hence, the telomere targeting property of cisplatin should be utilized and further studied in clinical treatment modalities.
Agents that target other components of telomere/telomerase complex
Additional ‘less specific' targets in the telomere/telomerase complex are telomerase associated and telomere binding proteins respectively. Several drugs from the latter categories are in early clinical trials. They include the heat-shock protein 90 inhibitor 17-allylaminogeldanamycin, which was shown to downregulate telomerase activity as a result of loss of Hsp90 chaperone function (Holt et al., 1999; Villa et al., 2003), and PARP inhibitory compounds (Seimiya et al., 2005; Wright and Shay, 2005). The telomere binding protein tankyrase is a PARP family member (Figure 2, Table 1), which can be inhibited by broad-spectrum PARP inhibitory compounds such as 3-aminobenzamide (Seimiya et al., 2005). The PARP inhibitors were found to be potent chemosensitizers, and essentially lack single agent activity. PARP inhibitors under clinical investigation include ABT-888 (developed by Abbott Laboratories, Abbott Park, IL, USA and the US-National Cancer Institute, Bethesda, MD, USA) and AG14361 (developed by Pfizer, Cambridge, MA, USA and the University of Newcastle, Newcastle, UK) (Donawho et al., 2007; Thomas et al., 2007; No authors listed).
hTERT vaccines
Vaccines directed against hTERT as a ‘general' tumour antigen, have been generated and are being studied in the clinic. Several phase I trials of hTERT immunotherapy have been completed in patients with breast, prostate, lung and other cancers and clinical and immunological results are encouraging (Carpenter and Vonderheide, 2006). Patients with metastatic tumours were immunized against a short peptide sequence of the human telomerase reverse transcriptase catalytic subunit (hTERT, aa 540–548 and 611–626 respectively), which is recognized by cytotoxic T-lymphocytes (Parkhurst et al., 2004; Vonderheide et al., 2004). The overall concept of this approach is to lyse telomerase-positive tumours with hTERT-specific T-lymphocytes. While an US-National Cancer Institute trial concluded that the targeted hTERT peptide is not presented on the surface of tumour cells and will not be useful for immunotherapy of patients with cancer (Parkhurst et al., 2004), others reported objective tumour responses, suggesting that vaccinating patients against telomerase is immunologically feasible (Vonderheide et al., 2004; Brunsvig et al., 2006).
Telomerase and cancer stem cells
Telomerase expression and telomere maintenance are key to the limitless proliferative potential of embryonic and adult stem cells. Overexpression of the catalytic subunit of telomerase, hTERT, has been found to promote stem cell mobilization, whereas short telomeres have been reported to cause stem cell failure (Hao et al., 2005; Sarin et al., 2005). In cancer, stem cell biology is best understood in haematological malignancies. While telomere length maintenance in primitive human haematopoietic cells is dissociated from telomerase activity, telomerase-dependent telomere shortening appears to be involved in the chromosomal instability and transformation of haematopoietic stem cells into leukaemic stem cells (Ju and Rudolph, 2006). Despite the inherent presence of telomerase in normal stem cells, cancer stem cells arising from the latter require markedly higher telomerase levels that are more efficient at telomere maintenance (Armanios and Greider, 2005). Thus, cancer stem cells may be more susceptible to loss of functional telomerase by telomere uncapping, thereby providing promising therapeutic targets.
Colony formation in a semi-solid matrix such as soft agar is an established surrogate for stem cell growth (Hamburger and Salmon, 1977). The hTERC inhibitor GRN163L and the TTA RHPS4 were shown to be potent inhibitors of clonogenic tumour cell growth (colony formation). For both agents the stem cell inhibitory properties did translate into in vivo antitumour activity (Dikmen et al., 2005; Phatak et al., 2007). Interestingly, RHPS4 was 1–2 log-folds more active against clonogenic tumour cells as compared to bulk tumour mass and to normal adult stem cells, such as colony forming units (CFU) from cord blood. The latter suggests that the TTA can target cancer stem cells (Phatak et al., 2007). Similar observations were made in an hTERT vaccine study that was designed to assess the effects of hTERT vaccination on haematopoietic stem cells. There was no significant decline in the frequency of granulocyte, macrophage or erythroid CFU in bone marrow from patients receiving the vaccine. In nonobese diabetic/severe combined immunodeficient mouse repopulation assays, human haematopoietic reconstitution was easily detected, without quantitative or qualitative differences between pre- and post-vaccine samples (Danet-Desnoyers et al., 2005).
These observations strongly indicate that human tumour stem cells can be differentially targeted by telomerase inhibitors and are in agreement with recent findings that hTERT is a ‘stemness' gene and cancer stem cell target.
Conclusions and future perspectives
Since the first seminal study on the ‘specific association of telomerase activity with immortal cells and cancer' has been published by Kim et al. (1994), the knowledge of the function and regulation of the ribonucleoprotein enzyme has dramatically increased and it is widely studied in the field of cancer research.
Although the essential role of telomerase in immortalization has been clearly established (Hahn et al., 1999a, 1999b), its value as a cancer inhibitory target is still debated and has not fully been proven by clinical studies that report an actual significant therapeutic benefit in cancer patients. After the initial discovery that telomerase activity is found in more than 85% of all tumours, telomerase was proposed as a universal target (Morin, 1995). However, soon it became evident that several normal cell types, in particular somatic and haematopoietic stem cells, do express telomerase (Holt et al., 1996, Burger et al., 1997a). Nevertheless, owing to differences in telomere length and telomere dynamics of stem cell type normal cells and cancer cells, a therapeutic window for telomerase inhibitors and telomere targeting agents seems to exist. As the agents described in this review become more broadly available it will be very interesting to combine for example, TTAs and hTERT or hTERC inhibitors; or TTAs, Hsp90 inhibitors and/or the hTERT vaccine. It is very possible that they might synergize. Additionally, by testing a cancer stem cell targeting approach, telomerase therapeutics could be combined with debulking agents (our standard cytotoxics). In fact, the TTA RHPS4 acts synergistically with the Hsp90 inhibitor 17-AAG and Taxol in cell culture (Cookson et al., 2005a).
With hTERT vaccines and ODNs under clinical investigation, and several synthetic small molecules entering clinical trials, telomerase therapeutics may soon become an integral part of cancer chemotherapy regimens.
Acknowledgments
This work was supported by a University of Maryland Cancer Research Grant through the Maryland Cigarette Restitution Fund Programme and the European Organization for Research and Treatment of Cancer Drug Discovery Committee.
Abbreviations
- ALT
alternative lengthening of telomeres; ODN, antisense oligonucleotides
- AZT
3-azido-2′-3′-dideoxythymidine
- BIBR1532
2-[(E)-3-naphthalen-2-yl-but-2-enoylamino]-benzoic acid
- CDDP
cis-diamminedichloro-platinum
- DN
double negative
- DSB
double-strand break repair
- GDEPT
gene-directed enzyme pro-drug therapy
- G
guanine
- HR
homologous recombination
- hTERC
human telomerase RNA component
- hTERT
human telomerase reverse transcriptase
- kb
kilobase
- NHEJ
non-homologous end joining
- PARP
poly (ADP-ribose) polymerase
- POT1
protection of telomeres 1
- TP1
telomerase-associated protein 1
- TRF1 and TRF2
telomeric repeat binding factors 1 and 2
- TIN2
TRF-1 interacting protein 2
- TC
telomere content
- TTA
telomere targeting agents
Conflict of interest
These authors state no conflict of interest.
References
- Armanios M, Greider CW. Telomerase and cancer stem cells. Cold Spring Harb Symp Quant Biol. 2005;70:205–208. doi: 10.1101/sqb.2005.70.030. [DOI] [PubMed] [Google Scholar]
- Bisoffi M, Heaphy CM, Griffith JK. Telomeres: prognostic markers for solid tumours. Int J Cancer. 2006;119:2255–2260. doi: 10.1002/ijc.22120. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Structure and function of telomeres. Nature. 1991;305:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Switching, signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, et al. Telomere shortening and tumour formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Blasco MA. Telomeres in cancer and aging: lessons from the mouse. Cancer Lett. 2003;194:183–188. doi: 10.1016/s0304-3835(02)00705-x. [DOI] [PubMed] [Google Scholar]
- Brown T, Sigurdson E, Rogatko A, Broccoli D. Telomerase inhibition using azidothymidine in the HT-29 colon cancer cell line. Ann Surg Oncol. 2003;10:910–915. doi: 10.1245/aso.2003.03.032. [DOI] [PubMed] [Google Scholar]
- Brunsvig PF, Aamdal S, Gjertsen MK, Kvalheim G, Markowski-Grimsrud CJ, Sve I, et al. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553–1564. doi: 10.1007/s00262-006-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240–4248. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger AM, Bibby MC, Double JA. Telomerase activity in normal and malignant mammalian tissues: feasibility of telomerase as target for cancer chemotherapy. Br J Cancer. 1997a;75:516–522. doi: 10.1038/bjc.1997.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger AM, Dai F, Schultes CM, Reszka AP, Moore MJ, Double JA, et al. The G-quadruplex interactive molecule BRACO19 inhibits tumour growth, consistent with telomere binding and interference with telomerase function. Cancer Res. 2005;65:1489–1496. doi: 10.1158/0008-5472.CAN-04-2910. [DOI] [PubMed] [Google Scholar]
- Burger AM, Double JA, Newell DR. Inhibition of telomerase activity by cisplatin in human testicular cancer cells. Eur J Cancer. 1997b;33:638–644. doi: 10.1016/s0959-8049(96)00521-7. [DOI] [PubMed] [Google Scholar]
- Burger AM, Kelland LR.Telomere targeting agents Prostate Cancer: Translational and Emerging Therapies 2006Taylor & Francis Group, LLC: NY; 195–208.In: Dawson N, Kelly (eds).Chapter 12 [Google Scholar]
- Burger AM. Telomerase in cancer diagnosis and therapy. BioDrugs. 1999;12:413–422. doi: 10.2165/00063030-199912060-00001. [DOI] [PubMed] [Google Scholar]
- Burger AM. Highlights in experimental therapeutics. Cancer Lett. 2007;245:11–21. doi: 10.1016/j.canlet.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Carpenter EL, Vonderheide RH. Telomerase-based immunotherapy of cancer. Expert Opin Biol Ther. 2006;6:1031–1039. doi: 10.1517/14712598.6.10.1031. [DOI] [PubMed] [Google Scholar]
- Cookson JC, Dai F, Smith V, Heald RA, Laughton CA, Stevens MFG, et al. Pharmacodynamics of the G-quadruplex-stabilizing telomerase inhibitor 3,11-difluoro-6,8,13-trimethyl-8H-quino[4,3,2-kl]acridinium methosulfate (RHPS4) in vitro: activity in human tumour cells correlates with telomere length and can be enhanced, or antagonized, with cytotoxic agents. Mol Pharmacol. 2005a;68:1551–1558. doi: 10.1124/mol.105.013300. [DOI] [PubMed] [Google Scholar]
- Cookson JC, Heald RA, Stevens MF. Antitumour polycyclic acridines, 17, synthesis, and pharmaceutical profiles of pentacyclic acridinium salts designed to destabilize telomeric integrity. J Med Chem. 2005b;48:7198–7207. doi: 10.1021/jm058031y. [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- Damm K, Hemmann U, Garin-Chesa P, Hauel N, Kauffmann I, Priepke H, et al. A highly selective telomerase inhibitor limiting human cancer cell proliferation. EMBO J. 2001;20:6958–6968. doi: 10.1093/emboj/20.24.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet-Desnoyers GA, Luongo JL, Bonnet DA, Domchek SM, Vonderheide RH. Telomerase vaccination has no detectable effect on SCID-repopulating and colony forming activities in the bone marrow of cancer patients. Exp Hematol. 2005;33:1275–1280. doi: 10.1016/j.exphem.2005.07.011. [DOI] [PubMed] [Google Scholar]
- De Lange T. Protection of mammalian telomeres. Oncogene. 2002;21:532–540. doi: 10.1038/sj.onc.1205080. [DOI] [PubMed] [Google Scholar]
- Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65:7866–7873. doi: 10.1158/0008-5472.CAN-05-1215. [DOI] [PubMed] [Google Scholar]
- Donawho CK, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- Fang G, Czech TR. Characterization of a G-quartet formation reaction promoted by the beta-subunit of the Oxytricha telomere-binding protein. Biochemistry. 1993;32:11646–11657. doi: 10.1021/bi00094a022. [DOI] [PubMed] [Google Scholar]
- Fearon EA, Vogelstein BA. A genetic model for colorectal carcinogenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Feng J, Funk WD, Wang SS, Weinrich AA, Avilon CP, Chiu CP, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Fordyce CA, Heaphy CM, Bisoffi M, Wyaco JL, Joste NE, Mangalik A, et al. Telomere content correlates with stage and prognosis in breast cancer. Breast Cancer Res Treat. 2006;99:193–202. doi: 10.1007/s10549-006-9204-1. [DOI] [PubMed] [Google Scholar]
- Fordyce CA, Heaphy CM, Joste NE, Smith AY, Hunt WC, Griffith JK. Association between cancer-free survival and telomere DNA content in prostate tumours. J Urol. 2005;173:610–614. doi: 10.1097/01.ju.0000143195.49685.ce. [DOI] [PubMed] [Google Scholar]
- Forsythe HL, Jarvis JL, Turner JW, Elmore LW, Holt SE. Stable association of hsp90 and p23, but not hsp70, with active human telomerase. J Biol Chem. 2001;276:15571–15574. doi: 10.1074/jbc.C100055200. [DOI] [PubMed] [Google Scholar]
- Gellert GC, Dikmen ZG, Wright WE, Gryaznov S, Shay JW. Effects of a novel telomerase inhibitor, GRN163L, in human breast cancer. Breast Cancer Res Treat. 2006;96:73–81. doi: 10.1007/s10549-005-9043-5. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Gomez D, Wenner T, Brassart B, Douarre C, O'Donohue MF, El Khoury V, et al. Telomestatin-induced telomere uncapping is modulated by POT1 through G-overhang extension in HT1080 human tumour cells. J Biol Chem. 2006;281:38721–38729. doi: 10.1074/jbc.M605828200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez E, Samper E, Flores JM, Blasco MA. Telomerase deficient mice with short telomeres are resistant to skin tumourigenesis. Nat Genet. 2000;26:114–117. doi: 10.1038/79089. [DOI] [PubMed] [Google Scholar]
- Gowan S, Heald R, Stevens MFG, Kelland LR. Potent inhibition of telomerase by small-molecule pentacyclic acridines capable of interacting with G-quadruplexes. Mol Pharmacol. 2001;60:981–988. doi: 10.1124/mol.60.5.981. [DOI] [PubMed] [Google Scholar]
- Greider CW. Chromosome first aid. Cell. 1991;67:645–647. doi: 10.1016/0092-8674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999a;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Stewart SA, Brooks MW, York SG, Eaton E, Kurachi A, et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med. 1999b;5:1164–1170. doi: 10.1038/13495. [DOI] [PubMed] [Google Scholar]
- Hamburger AW, Salmon SE. Primary bioassay of human tumour stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:50–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Harley CB, Fuchter BA, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie ND, Dempster M, Dunlop MC, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Aging, longevity, and immortality in vitro. Exper Gerontol. 1992;27:363–368. doi: 10.1016/0531-5565(92)90066-9. [DOI] [PubMed] [Google Scholar]
- Herbert B, Pitts AE, Baker SI, Hamilton WE, Wright WE, Shay JW, et al. Inhibition of human telomerase in immortal human cells leads to progressive telomere shortening and cell death. Proc Natl Acad Sci USA. 1999;96:14276–14281. doi: 10.1073/pnas.96.25.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SE, Aisner DL, Baur J, Tesmer VM, Dy M, Ouellette M, et al. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 1999;13:817–826. doi: 10.1101/gad.13.7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt SE, Shay JW, Wright WE. Refining the telomere-telomerase hypothesis of aging and cancer. Nat Biotechnol. 1996;14:1734–1741. doi: 10.1038/nbt0796-836. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Lippard SJ. Telomere loss in cells treated with cisplatin. Proc Natl Acad Sci USA. 1998;95:4219–4223. doi: 10.1073/pnas.95.8.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SR, Zhu CH, Paulson V, Watkins L, Dikmen ZG, Gryaznov SM, et al. Antiadhesive effects of GRN163L – an oligonucleotide N3′->P5′ thio-phosphoramidate targeting telomerase. Cancer Res. 2007;67:1121–1129. doi: 10.1158/0008-5472.CAN-06-2306. [DOI] [PubMed] [Google Scholar]
- Ju Z, Rudolph KL. Telomeres and telomerase in cancer stem cells. Eur J Cancer. 2006;42:1197–1203. doi: 10.1016/j.ejca.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. P53 and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- Keith NW, Evans JTR, Glasspool RM. Telomerase and Cancer: time to move from a promising target to a clinical trial. J Pathol. 2001;195:404–414. doi: 10.1002/path.1001. [DOI] [PubMed] [Google Scholar]
- Keith WN, Bilsland A, Evans TR, Glasspool RM. Telomerase-directed molecular therapeutics. Expert Rev Mol Med. 2002;22:1–25. doi: 10.1017/S1462399402004507. [DOI] [PubMed] [Google Scholar]
- Kelland LR. Telomerase: biology and phase I trials. Lancet Oncol. 2001;2:95–102. doi: 10.1016/S1470-2045(00)00226-6. [DOI] [PubMed] [Google Scholar]
- Kelland LR. Overcoming the immortality of tumour cells by telomere and telomerase based cancer therapeutics-current status and future prospectus. Eur J Cancer. 2005;41:971–979. doi: 10.1016/j.ejca.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Kim MY, Vankayalapati H, Shin-Ya K, Wierzba K, Hurley LH. Telomestatin, a potent telomerase inhibitor that interacts quite specifically with the human telomeric intramolecular G-quadruplex. J Am Chem Soc. 2002;124:2098–2099. doi: 10.1021/ja017308q. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Koga S, Kondo Y, Komata T, Kondo S. Treatment of bladder cancer cells in vitro and in vivo with 2–5 A anti telomerase RNA. Gene Therapy. 2001;8:654–658. doi: 10.1038/sj.gt.3301449. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kondo Y, Li G, Silverman RH, Cowel JK. Targeted therapy of human malignant glioma in a mouse model by 2–5A antisense directed against telomerase RNA. Oncogene. 1998;16:3323–3330. doi: 10.1038/sj.onc.1201885. [DOI] [PubMed] [Google Scholar]
- Kushner DM, Paranjape JM, Bandyopadhyay B, Cramer H, Leaman DW, Kennedy AW, et al. 2–5A antisense directed against telomerase RNA produces apoptosis in ovarian cancer cells. Gynecol Oncol. 2000;76:183–192. doi: 10.1006/gyno.1999.5668. [DOI] [PubMed] [Google Scholar]
- Lundblad V, Blackburn EH. An alternative pathway for yeast telomere maintenance rescues est-1 senescence. Cell. 1993;73:347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc Natl Acad Sci USA. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin GB. Is telomerase a universal cancer target? J Natl Cancer Inst. 1995;87:859–861. doi: 10.1093/jnci/87.12.859. [DOI] [PubMed] [Google Scholar]
- Murti KG, Prescott DM. Telomeres of polytene chromosomes in a ciliated protozoan terminate in duplex DNA loops. Proc Natl Acad Sci USA. 1999;96:14436–14439. doi: 10.1073/pnas.96.25.14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- No authors listed NIH Clinical Research StudiesProtocol Number, 06-C-0172
- Parkhurst MR, Riley JP, Igarashi T, Li Y, Robbins PF, Rosenberg SA. Immunization of patients with the hTERT:540–548 peptide induces peptide-reactive T lymphocytes that do not recognize tumours endogenously expressing telomerase. Clin Cancer Res. 2004;10:4688–4698. doi: 10.1158/1078-0432.CCR-04-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson GN, Lee MP, Neidle S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature. 2002;417:876–880. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]
- Pennarun G, Granotier C, Gauthier LR, Gomez D, Hoffschir F, Mandine E, et al. Apoptosis related to telomere instability and cell cycle alterations in human glioma cells treated by new highly selective G-quadruplex ligands. Oncogene. 2005;24:2917–2928. doi: 10.1038/sj.onc.1208468. [DOI] [PubMed] [Google Scholar]
- Phatak P, Cookson JC, Dai F, Smith V, Gartenhaus RB, Stevens MF, et al. Telomere uncapping by the G-quadruplex ligand RHPS4 inhibits clonogenic tumour cell growth in vitro and in vivo consistent with a cancer stem cell targeting mechanism. Br J Cancer. 2007;96:1223–1233. doi: 10.1038/sj.bjc.6603691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb JA, Bilsland A, Kakani R, Zhao J, Glasspool RM, Knox RJ, et al. Telomerase-specific suicide gene therapy vectors expressing bacterial nitroreductase sensitize human cancer cells to the pro-drug CB1954. Oncogene. 2001;20:7797–7803. doi: 10.1038/sj.onc.1204954. [DOI] [PubMed] [Google Scholar]
- Read M, Harrison RJ, Romagnoli B, Tanious FA, Gowan SH, Reszka AP, et al. Structure-based design of selective and potent G quadruplex-mediated telomerase inhibitors. Proc Natl Acad Sci USA. 2001;98:4844–4849. doi: 10.1073/pnas.081560598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou J-F, Guittat L, Mailliet P, Laoui A, Renou E, Petitgenet O, et al. Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proc Natl Acad Sci USA. 2002;99:2672–2677. doi: 10.1073/pnas.052698099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvati E, Leonetti C, Rizzo A, Scarsella M, Mottolese M, Galati R, et al. Telomere damage promotes antitumoural activity of the G-quadruplex ligand RHPS4 J Clinc Invest 2007(in press)
- Saretzki G, Ludwig A, Zglinicki T, Runnebaum IB. Ribozyme-mediated telomerase inhibition induced immidiate cell loss but not telomere shortening i ovarian cancer cells. Cancer Gene Ther. 2001;8:827–834. doi: 10.1038/sj.cgt.7700383. [DOI] [PubMed] [Google Scholar]
- Sarin KY, Cheung P, Gilison D, Lee E, Tennen RI, Wang E, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seimiya H, Muramatsu Y, Tomokazu O, Tsuruo T. Tankyrase 1 as a target for telomere-directed molecular cancer therapeutics. Cancer Cell. 2005;7:25–37. doi: 10.1016/j.ccr.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Shay JW. Telomerase therapeutics: telomeres recognized as a DNA damage signal. Clin Cancer Res. 2003;9:3521–3525. [PubMed] [Google Scholar]
- Shippen DE. Telomeres and telomerases. Curr Opinion Genet Devel. 1993;3:759–763. doi: 10.1016/s0959-437x(05)80095-4. [DOI] [PubMed] [Google Scholar]
- Smith CD, Blackburn EH. Uncapping and deregulation of telomeres lead to detrimental cellular consequences in yeast. J Cell Biol. 1999;145:203–214. doi: 10.1083/jcb.145.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose)polymerase at human telomeres. Science. 1998;282:1484–1487. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- Strahl C, Blackburn EH. Effects of reverse transcriptase inhibitors on telomere length and telomerase activity in two immortalized human cell lines. Mol Cell Biol. 1996;16:53–65. doi: 10.1128/mcb.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas HD, Calabrese CR, Batey MA, Canan S, Hostomsky Z, Kyle S, et al. Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther. 2007;3:945–956. doi: 10.1158/1535-7163.MCT-06-0552. [DOI] [PubMed] [Google Scholar]
- Van Steensel B, de Lange T. Control of telomere length by the human telomeric binding protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- Villa R, Folini M, Porta CD, Valentini A, Pennati M, Daidone MG, et al. Inhibition of telomerase activity by geldanamycin and 17-allylamino, 17-demethoxygeldanamycin in human melanoma cells. Carcinogenesis. 2003;24:851–859. doi: 10.1093/carcin/bgg028. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–839. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Patel DJ. Solution structure of the human telomeric repeat d[AG3(T2AG3)3] G-tetraplex. Structure. 1993;1:263–282. doi: 10.1016/0969-2126(93)90015-9. [DOI] [PubMed] [Google Scholar]
- Wright WE, Shay JW. Mechanism-based combination telomerase inhibition therapy. Cancer Cell. 2005;7:1–2. doi: 10.1016/j.ccr.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Yatabe N, Kyo S, Kondo S, Kanaya T, Wang Z, Maida Y, et al. 2–5A antisense therapy directed against human telomerase RNA inhibits telomerase activity and induces apoptosis without telomere impairment in cervical cancer cells. Cancer Gene Ther. 2002;9:624–630. doi: 10.1038/sj.cgt.7700479. [DOI] [PubMed] [Google Scholar]