Abstract
Background and purpose:
Rho-kinase (ROCK) has been implicated in the pathophysiology of altered vasoregulation leading to hypertension. Here we describe the pharmacological characterization of a potent, highly selective and orally active ROCK inhibitor, the derivative of a class of azaindoles, azaindole 1(6-chloro-N 4-{3,5-difluoro-4-[(3-methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy]-phenyl}pyrimidine-2,4-diamine).
Experimental approach:
Pharmacological characterization of azaindole 1was performed with human recombinant ROCK in vitro. Vasodilator activity was determined using isolated vessels in vitro and different animal models in vivo.
Key results:
This compound inhibited the ROCK-1 and ROCK-2 isoenzymes with IC50 s of 0.6 and 1.1 nM in an ATP-competitive manner. Although ATP-competitive, azaindole 1was inactive against 89 kinases (IC50>10 μM) and showed only weak activity against an additional 21 different kinases (IC50=1 - 10 μM). Only the kinases TRK und FLT3 were inhibited by azaindole 1in the sub-micromolar range, albeit with IC50 values of 252 and 303 nM, respectively. In vivo, azaindole 1lowered blood pressure dose-dependently after i.v. administration in anaesthetized normotensive rats. In conscious normotensive and spontaneously hypertensive rats azaindole 1induced a dose-dependent decrease in blood pressure after oral administration without inducing a significant reflex increase in heart rate. In anaesthetized dogs, azaindole 1induced vasodilatation with a moderately elevated heart rate.
Conclusions and implications:
Azaindole 1is representative of a new class of selective and potent ROCK inhibitors and is a valuable tool for the elucidation of the role of ROCK in the cardiovascular system.
Keywords: Rho-kinase inhibitor, in vitro selectivity, vasorelaxation, blood pressure, heart rate, hypertension
Introduction
The small GTP-binding proteins of the Rho family regulate various cellular functions. One effector of Rho is Rho-kinase (ROCK), a serine/threonine kinase, which is comprised of two isoenzymes, ROCK-1 (or ROKβ) and ROCK-2 (or ROKα). It is well established that ROCK plays a pivotal role in the regulation of the tonic phase of smooth muscle cell contraction (Gong et al., 1996; Christ and Wingard, 2005). The contractile effect results from ROCK-mediated phosphorylation of the myosin-binding subunit (MBS), the regulatory subunit of myosin light chain (MLC) phosphatase. Phosphorylated MBS inhibits the phosphatase activity thus allowing an increase in the level of phosphorylated MLC and contraction at a constant intracellular calcium concentration (Kimura et al., 1996). This phenomenon is defined as calcium sensitization (Christ and Wingard, 2005).
ROCK activity or protein is dysregulated under certain cardiovascular pathophysiological conditions such as vasospasm (Masumoto et al., 2002), atherosclerosis (Shimokawa et al., 2001) and hypertension (Masumoto et al., 2001). The ROCK locus was identified in genome-wide linkage studies as a hypertension-associated gene (Mendelsohn, 2005), and homozygous carriers of a polymorphism in the ROCK gene (ROCK2-Thr431Asn) had an increased risk of hypertension (Seasholtz et al., 2006). Pharmacological inhibition of ROCK has beneficial effects in a variety of animal models of cardiovascular diseases (Christ and Wingard, 2005).
Previously, mainly two ROCK inhibitors, Y-27632 ((+)-R-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide dihydrochloride) (Uehata et al., 1997) and HA-1077 (fasudil), which belong to a series of homopiperazine-derived ROCK inhibitors (Ono-Saito et al., 1999; Nagumo et al., 2000), have been used to explore the function of ROCK in the cardiovascular system. In Japan, fasudil is already in use as a therapeutic agent to treat cerebral vascular spasm (Tachibana et al., 1999). In addition, fasudil has shown benefits for the treatment of patients with angina pectoris (Shimokawa et al., 2002). However, both Y-27632 and fasudil are weak inhibitors of ROCK with Ki-values of 140 and 330 nM, respectively. The selectivity profile of Y-27632 appears to be reasonable, but only 27 different kinases have been tested so far (Davies et al., 2000). Fasudil shows some selectivity for ROCK inhibition. However, it is also a relatively potent inhibitor of kinases such as protein kinase A (PKA), PKG and PKC that regulate important cellular functions (Ono-Saito et al., 1999; Naguma et al., 2000). Recently, it has been shown that hydroxyfasudil, the main metabolite of fasudil after oral administration, is more specific than the parent compound, but it is still a weak inhibitor of ROCK with a Ki-value of 170 nM (Shimokawa et al., 1999). Another analogue of fasudil, H-1152P, although a potent ROCK-2 inhibitor (Ki=1.6 nM), still retains inhibitory activity against several kinases in the submicromolar range (Tamura et al., 2005).
Here, we have characterized, for the first time, the pharmacological properties of the potent and orally available ROCK inhibitor azaindole 1 (6-chloro-N4-{3,5-difluoro-4-[(3-methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy]phenyl}pyrimidine-2,4-diamine). This compound is a highly specific ATP-competitive inhibitor with activity in the low nanomolar range. The compound potently relaxes rabbit precontracted isolated saphenous arteries in a concentration-dependent manner, increases coronary blood flow in the rat heart Langendorff model and dose dependently lowers blood pressure in normotensive anaesthetized rats and conscious hypertensive rats without an increasing heart rate. In addition, in anaesthetized dogs, azaindole 1 induced a marked decrease in blood pressure.
Methods
Molecular cloning and expression of protein kinases
To obtain the human protein kinases ROCK-1, ROCK-2 and zipper-interacting protein kinase (ZIP-kinase) and the murine ROCK-2 for the enzymatic assays, the following primers were used for modification of the 5′ and 3′ ends of the respective cDNA clones by PCR. Primer A: 5′-GGGATCCACCATGAGCCGGCCCCCGCCGACGGG-3′ (BamHI site underlined), primer B: 5′-GGGAATTCTTATGATCCATAATTTTGCTATATG-3′ (EcoRI site underlined), primer C: 5′-CACCATGTCGACTGGGGACAGTTTTGAGACTCG-3′, primer D: 5′-GGAATTCGCCCTTTTAACTAGTTTTTCCAG-3′, primer E: 5′-GGGATCCATGTCCACGTTCAGGCAGGAGGACG-3′ (BamHI site underlined), primer F: 5′-GGGCTCGAGCTAGCGCAGCCCGCACTCCACGCC-3′ (XhoI site underlined). The PCR fragments containing primers A and B and primers E and F were cloned into TOPO TA. The fragments containing primers C and D were cloned into pENTR TOPO.
The murine ROCK-2 cDNA was cloned into the EcoRI site of pFASTBAC HTa (Invitrogen, Karlsruhe, Germany). The human ROCK-2 cDNA was modified by PCR employing primers A and B and cloned into pFASTBAC HTb after digestion with BamHI and EcoRI. The human ROCK-1 cDNA was modified by PCR employing primers C and D and cloned into pDEST10 using GATEWAY technology (Invitrogen). Recombinant baculoviruses were prepared using the Bac-to-Bac system according to instructions from the manufacturer (Invitrogen). The human ZIP-kinase cDNA was modified by PCR employing primers E and F and cloned into the Escherichia coli expression vector pKa83a after digestion with BamHI and XhoI. pKa83a is an in-house developed vector containing a kanamycin-resistance gene and an isopropyl-β-D-thiogalactopyranoside inducible promoter. From this vector, the human ZIP-kinase is produced with an N-terminal His6 and a maltose-binding protein (MBP) tag. The final vector is transformed into the E. coli strain W3110.
Purification of recombinant proteins
His-tagged ROCK-1 and ROCK-2 proteins were purified from the cytosolic fraction of baculovirus-infected Sf9 insect cells. The cell pellet was resuspended in lysis buffer (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 20 mM imidazole, 2 mM Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), 0.5% Triton X-100, 0.1 mM Pefabloc, protease inhibitor cocktail Complete, 10% glycerol, pH 7.3) at 30 °C. The cells were disrupted and homogenized with an ultraturax followed by sonification. After centrifugation (10 000 g, 4 °C) the proteins were purified from the supernatant by Ni-immobilized metal affinity chromatography (IMAC) on a nickel-activated chelate sepharose column. The column was washed with IMAC buffer A (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 20 mM imidazole, 2 mM TCEP, 0.5% Triton X-100, 0.1 mM Pefabloc, 10% glycerol, pH 7.3) and the bound protein was eluted at a flow rate of 1.5 ml min−1 with IMAC buffer B (IMAC buffer A plus 500 mM imidazole).
The E. coli cell pellet containing ZIP-kinase was resuspended in lysis buffer (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 40 mM imidazole, 2 mM TCEP, 0.1 mM Pefabloc, protease inhibitor cocktail Complete, 10% glycerol, pH 7.5) at 30 °C. Homogenization and purification of the protein was carried out as already described for ROCKs. The nickel-activated chelate sepharose was washed with IMAC buffer A, and the bound protein was eluted at a flow rate of 10 ml min−1 with IMAC buffer B. The proteins were stored at −70 to −80 °C.
Kinase assays
All protein kinase activities were linear with respect to time and protein in every incubation. Human ROCK-1 and ROCK-2, murine ROCK-2, human ZIP-kinase and human MLC kinase (MLCK) (aa1425–1771) were preincubated for 10 min with test compounds in 0.05 ml of 50 mM Tris/HCl, pH 7.5, 1 mM EDTA, 5 mM MgCl2 and 0.06% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulphonate) (ROCK-1; ROCK-2; ZIP-kinase) or 50 mM HEPES, pH 7.4, 10 mM Mg acetate, 1 mM dithiothreitol, 0.3 mM CaCl2, 1 μM calmodulin (MLCK). As substrate following peptides were used: RRLSSLRA (ROCK; S6 peptide), KEAKEKRQEQIAKRRRLSSLRASTSKSGGSQK (ZIP-kinase; long S6 peptide) and KKRAARATSNVFA (MLCK; MLC peptide). After 10 min preincubation with dimethyl sulphoxide (DMSO) (0.1% final concentration) or with increasing amounts of azaindole 1 (final concentration 0.1 nM–3 μM), the assays were initiated with [33P]ATP (3000 mCi mmol−1) (10 μM final concentration, unless stated otherwise). After 20 min incubation, the reaction was terminated by incubation at 95 °C for 10 min. After centrifugation at 10 000 g for 1 min, an aliquot of each incubation was spotted on paper matts. The papers were dried and subsequently washed twice in distilled water. After being dried, the papers were coated with scintillator and counted for radioactivity. Inhibition curves were analysed by nonlinear regression using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA).
The activity of azaindole 1 against 112 kinases was investigated in collaboration with Upstate (Dundee, UK) using an ATP concentration of 100 μM.
ROCK-2 autophosphorylation and myosin-binding subunit phosphorylation
Human ROCK-2 (50 ng) was preincubated (final concentration 1–30 nM in DMSO) with the test compound either alone or in the presence of human MBS (aa654–880; 100 ng) as substrate in 0.05 ml of 50 mM Tris/HCl pH 7.5, 5 mM MgCl2, 1 mM EDTA, 0.06% CHAPS for 10 min at 37 °C. The final concentration of DMSO was 0.1%. The reaction was started by the addition of [33P]ATP (10 μM final concentration; 3000 Ci mmol−1). After incubation for 30 min at 37 °C, the reaction was terminated by adding 2 × Laemmli sample buffer. The solution was boiled for 5 min at 95 °C and then applied to an SDS–polyacrylamide gel electrophoresis using a 4–15% gradient SDS-gel and the PHAST System. The gel was dried and subjected to autoradiography using Kodak XAR-5 film.
Molecular modelling
Maestro (v. 7.0, Schrödinger, Portland, OR, USA) was used for protein overlays, inhibitor docking and the generation of modelling pictures. MacroModel (v. 9.0, Schrödinger) was used to perform energy minimizations (protein coordinates fixed, inhibitor flexible; solvent water; convergence on gradient, convergence threshold=0.2).
Rabbit isolated vessels
Chinchilla rabbits of either sex (Harlan Winkelmann, Borchen, Germany) (about 2–3 kg) were killed by an overdose of thiopental. The saphenous arteries were dissected and rings of the arteries (3 mm width) were suspended under an initial tension of approximately 4 g in 5 ml organ baths containing Krebs-Henseleit solution (containing 0.001% BSA) at 37°C. Contractions were measured isometrically with Statham UC2 strain gauges connected to a DAS1802HC data acquisition board (Keithley instruments, Germering, Germany). Rings were precontracted by phenylephrine (0.15 μM; submaximal contraction) four times. Each contraction was followed by a series of 11 washing cycles and 1 resting period of 25 min. The increasing concentrations of the test compound (0.1 nM–10 μM) in DMSO, final concentration 0.1%) were added to the organ baths. The concentration of the test compounds was increased by a factor of 10. Rings were subsequently contracted with phenylephrine (0.15 μM). The intact endothelium was functionally tested by the presence of the relaxant response to acetylcholine (0.5 μM).
Rat heart Langendorff preparation
The hearts of Wistar rats (200–250 g) were perfused according to Langendorff at 37 °C with a nonrecirculating system. The perfusion medium was filtered Krebs–Henseleit solution containing 11 mM glucose and 1.2 mM of CaCl2, equilibrated with O2+CO2 (95+5%), to give a pH of 7.4 and a pO2 of 650–700 mm Hg. Perfusion was performed at a constant rate (10 ml min−1). A latex balloon filled with saline and connected to a pressure transducer (Gould Statham, Oxnard, CA, USA) via a metal cannula was inserted into left ventricular cavity to measure the isovolumetric contractions of the left ventricle. A second pressure transducer was connected to the aortic cannula to record the perfusion pressure. Drug solutions (0.1 nM–10 μM in DMSO, final concentration 0.1%) were infused into aortic cannula at a rate of 1% of the total flow rate.
Haemodynamics in anaesthetized rats
Male Wistar rats weighing 300–350 g (Harlan Winkelmann) were anaesthetized with thiopental 100 mg kg−1 intraperitoneally (i.p.). A tracheotomy was performed and catheters were inserted into the femoral artery for blood pressure and heart rate measurements (Gould pressure transducer and recorder model RS 3400) and into the femoral vein for test drug administration. The animals were ventilated with room air and their body temperature was controlled. Azaindole 1 was administered intravenously (i.v.) in doses of 0.03–0.1 mg kg−1 in a solution of Transcutol/Cremophor EL/physiological saline (19/10/80=v/v/v). The vehicle Transcutol/Cremophor EL/physiological saline (19/10/80=v/v/v) without test drug was used as control. The volume administered was 1 ml kg−1. Six animals were treated per group.
Haemodynamics in conscious rats
Female conscious normotensive and spontaneously hypertensive rats (Moellegaard, Ejby, Denmark, 220–290 g) were equipped with implantable radiotelemetry, and a data acquisition system (Data Sciences, St Paul, MN, USA) comprising a chronically implantable transducer transmitter unit equipped with a fluid-filled catheter was used. The transmitter was implanted into the peritoneal cavity and the sensing catheter was inserted into the descending aorta. Single doses of azaindole 1 were administered to spontaneously hypertensive rats (1, 3 and 10 mg kg−1) and normotensive rats (3 and 10 mg kg−1), or multiple doses once daily to spontaneously hypertensive rats (10 mg kg−1), as a solution (5 mg ml−1) in Transcutol/Cremophor/H2O (10/20/70=v/v/v) orally by gavage. The volume administered by gavage was 2 ml kg−1 body weight. Each dose was administered to groups of six animals. Animals in the control groups only received the vehicle. Before treatment mean blood pressure and heart rate of treated and untreated control groups was in the range of 131–142 mm Hg and 279–321 beats min−1, respectively.
Haemodynamic variables in anaesthetized dogs
These studies were performed in mongrel dogs of either sex weighing between 20–30 kg. Anaesthesia was induced by thiopental and maintained with isoflurane (0.6–1.2 vol%). Fentanyl (6 μg kg−1 h−1) was used as an analgesic. The dogs were artificially ventilated with N2O/O2 (15 respiratory cycles with 30–40 ml per cycle). Azaindole 1 was dissolved in 1% DMSO, 49.5% polyethylene glycol 400 and 49.5% Transcutol and administered as an i.v. dose of 100 and 300 μg kg−1 (50 μl kg−1, cumulative administration). Administration of vehicle served as a control. The time interval between the two doses was 60 min.
The following parameters were investigated: systemic blood pressure (fluid-filled hollow catheter inserted via the femoral artery into the abdominal aorta and connected to a strain gauge (Braun, Melsungen, Germany), ECG (lead II), cardiac pressures and contractility via a tip catheter (PC350, Millar Instruments, Houston, TX, USA) and coronary blood flow (via a coronary flow probe (Skalar Instruments, Deeft, Netherlands). All signals were amplified with a Gould amplifier system and digitally analysed using the PONEMAH physiology platform (LDS Test&Measurement, Ismaning, Germany).
Pharmacokinetics in mice, rats and dogs
To determine the pharmacokinetics of azaindole 1, blood samples were obtained at 2, 5, 15, 30 min, 1, 2, 3, 5 and 7 h following its i.v. administration to NMRI mice (Harlan Winkelmann) (three animals per time point). In male Wistar rats and female beagle dogs, blood samples were taken at 5, 10, 15, 17, 20, 30, 45 min, 1, 2, 3, 5 and 7 h and at 5, 10, 15, 17, 20, 30, 45 min, 1, 2, 3, 4, 6, 8, 24 and 26 h after infusion, respectively. Time points for blood samples following oral administration were 15, 30 min, 1, 2, 4, 6, 8, 24 h and 15, 30 min, 1, 2, 3, 4, 5, 6, 8, 24, 26 h in rats and dogs, respectively. Oral formulation in rats and dogs was 10% EtOH; 20% Solutol; 70% H2O and 5 % EtOH; 20% polyethylene glycol 400; 75% H2O, respectively. Pharmacokinetics in rats and dogs were determined in three animals using serial bleeding. Animals were conscious throughout the study.
After centrifugation, plasma was precipitated by the addition of acetonitrile. The supernatants were subjected to high performance liquid chromatography performed on a 2300 HTLC system (Cohesive Technologies, Crownhire, UK) equipped with a Lichrochart Purospher Star RP-18e column (55 × 4 mm internal diameter, 3 μM particle size; Merck KGaA, Frankfurt, Germany) at a flow rate of 1 ml min−1. The mobile phase consisted of 10 mM ammonium acetate (pH 3.0) and acetonitrile. A linear gradient from 20 to 80% acetonitrile (v/v) within 2 min was applied. Tandem mass spectrometry was performed on an API 3000 triple-quadruple. Mass spectrometer (PE Sciex) connected to the high performance liquid chromatography system through a TurboIonaspray interface. Limit of quantification was 1 μg l−1. Pharmacokinetic parameters were calculated from plasma concentrations with KINCALC, a validated in-house (Bayer HealthCare, Wuppertal, Germany) calculation programme.
All animal experiments were in compliance with the German guidelines for animal care/husbandry and approved by the local authorities (Regierungspräsidium Düsseldorf).
Statistics
Unless otherwise indicated, the results shown in the present publication are means±s.e.m. from at least three independent experiments performed in duplicates or triplicates. Differences were assessed by one-way analysis of variance followed by the Bonferroni test for comparison of means or by Student's t-test. P<0.05 was considered significant.
Chemicals
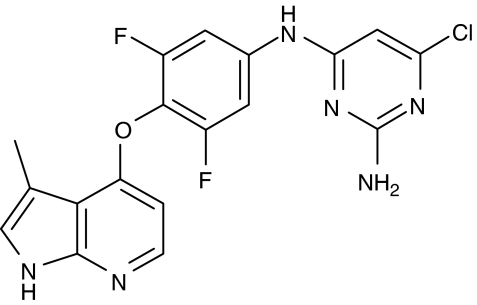
Azaindole 1 (Figure 1) was synthesized as described previously (Schirok et al., 2005). pENTR TOPO was obtained from Invitrogen; Pefabloc, protease inhibitor cocktail Complete from Roche (Mannheim, Germany); nickel-activated chelate sepharose column from GE Amersham Biosciences (Freiburg, Germany); human ROCK-1 and ROCK-2, murine ROCK-2, human ZIP-kinase and human MLCK (aa1425–1771) from Upstate (Charlottesville, VA, USA); ROCK; S6 peptide from Bachem (Bubendorf, Switzerland); ZIP-kinase, long S6 peptide from Upstate; MLCK, MLC peptide from Bachem; paper matts from Wallac (Boston, MA, USA); human MBS (aa654–880) from Upstate; SDS-gel from Amersham Bioscience (Uppsala, Sweden); PHAST System from Amersham Bioscience; thiopental from Nycomed (Munich, Germany); isoflurane (0.6–1.2 vol%, Isoflor) from Abbott (Chicago, IL, USA).
Figure 1.
The chemical structure of the Rho-kinase inhibitor azaindole 1 (6-chloro-N4-{3,5-difluoro-4-[(3-methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy]phenyl}pyrimidin-2,4-diamine).
Results
Azaindole 1 inhibits human ROCK in vitro
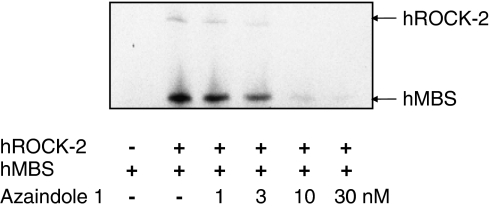
To find ROCK inhibitors with novel structures, we performed a high-throughput-screening using an in-house 400 000 compound library. Chemical optimization of a lead structure resulted in the identification of azaindole 1. The compound is a highly potent inhibitor of human ROCK-1 and ROCK-2 isoenzymes with IC50 values of 0.6 and 1.1 nM, respectively. ROCKs from other species such as murine ROCK-2 or rat ROCK-2 were also potently inhibited by this substance with IC50 values of 2.4 and 0.8 nM, respectively. To demonstrate the effect of azaindole 1 in a kinase assay using a natural substrate, we tested the ROCK-dependent phosphorylation of human MBS in vitro (Kimura et al., 1996). As shown in Figure 2, azaindole 1 concentration dependently reduced ROCK-2-mediated MBS phosphorylation. In the presence of 3 nM azaindole 1, [33P]phosphate incorporation in the substrate was reduced by more than 50%, whereas phosphorylation of MBS was almost blocked at 10 nM of compound azaindole 1. In addition to substrate phosphorylation, autophosphorylation of ROCK-2 in vitro was also concentration-dependently reduced (Figure 2). The results correlate with the inhibitory potency of the phosphorylation of S6 substrate peptide.
Figure 2.
Inhibition of Rho-kinase isoenzymes by azaindole 1 using the physiological substrate MBS (Upstate, aa 654–880). Activity of purified recombinant human ROCK-2 was measured as described in Materials and methods. Arrows denote the autophosphorylated human ROCK-2 and phosphorylated human MBS. MBS, myosin-binding subunit; ROCK, Rho-kinase.
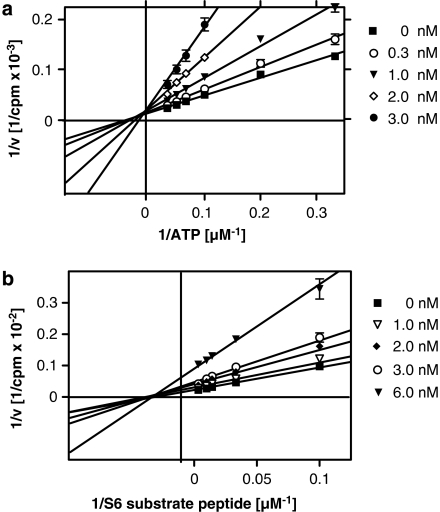
To elucidate the mode of action of azaindole 1 on the enzyme, we performed kinetic analysis experiments. Human ROCK-2 was incubated with different concentrations of ATP or different concentrations of the substrate peptide S6 in the presence of increasing inhibitor concentrations. In the presence of azaindole 1, the apparent Km values for ATP of ROCK-2 increased in a concentration-dependent manner without changes in the Vmax value, suggesting that azaindole 1 interacts with the ATP-binding site of ROCK-2 and interferes with ATP binding in a competitive manner (Figure 3a). In contrast, varying the S6 substrate concentration in the presence of different inhibitor concentrations results in a decrease of Vmax, whereas the apparent Km values of the S6 substrate peptide remained unchanged (Figure 3b). Accordingly, azaindole 1-dependent inhibition of human ROCK-2 is noncompetitive with respect to the S6 substrate binding. Similar results were obtained with human ROCK-1 (data not shown). The Ki values derived from these experiments are 3.7 nM for ROCK-1 and 4.8 nM for ROCK-2.
Figure 3.
Double-reciprocal plots of ROCK-2 activity as a function of ATP (a) or S6 substrate peptide (b) in the presence of increasing azaindole 1 concentrations. ROCK, Rho-kinase.
This observation was supported by measurements of the IC50 values in the presence of increasing ATP concentrations. When the enzyme was assayed with 0.01, 0.1 and 1 mM ATP in the presence of the inhibitor, the IC50s for inhibition of ROCK-2 shifted from 1.1 nM (0.01 mM ATP) to 12.4 (0.1 mM ATP) and 77.2 nM (1 mM ATP). However, even in the presence of 1 mM, the compound was still a potent inhibitor of human ROCK-2 with an IC50 of 77.2 nM. This result suggests that azaindole 1 is a competitive inhibitor of the ATP binding to ROCK-2. When azaindole 1 was preincubated with ROCK-2 for 30 min before the addition of ATP concentrations, the IC50 values were only slightly increased to 2.9 nM in the presence of 0.1 mM ATP and to 4.7 nM in the presence of 1 mM ATP. This result indicates that the binding of azaindole 1 is either irreversible or very slowly reversible.
Selectivity assays for azaindole 1
To exclude the possibility that the observed antagonism of ROCK is due to a nonspecific effect on vasoactive mechanisms, we examined the specificity of the azaindole 1. Firstly, we investigated the effect of azaindole 1 on the kinases involved in smooth muscle contraction. MLCK, the crucial kinase regulating calcium-dependent vasoconstriction, was only moderately inhibited by azaindole 1 with an IC50 of 7.4 μM. In addition, ZIP-kinase, which has been shown to modulate myosin phosphatase activity and to participate in smooth muscle cell contraction by Ca2+ sensitization (Niiro and Ikebe, 2001), was only slightly inhibited by azaindole 1 (IC50=4.1 μM).
The specificity against several other kinases was explored in collaboration with Upstate (Dundee, UK). Azaindole 1 was tested at 10 μM against 112 different kinases. It was inactive against 89 kinases (IC50 >10 μM; data not shown) and showed only a weak activity against an additional 21 different kinases with IC50s in the range of 1–10 μM (data not shown). Only the receptor tyrosine kinases TRK and FLT3 were inhibited, with IC50 values of 252 and 303 nM, respectively.
The selectivity of azaindole 1 against 63 cardiovascular relevant enzymes and receptors was explored in collaboration with MDS Pharma Services (Taipei, Taiwan). The compound inhibited only moderately the L-type calcium channel and the sodium channel with IC50 values of 6.7 and 6.8 μM, respectively. The remaining 60 receptors and enzymes were not significantly affected by azaindole 1 at 10 μM (data not shown).
Docking azaindole 1 in a ROCK-model
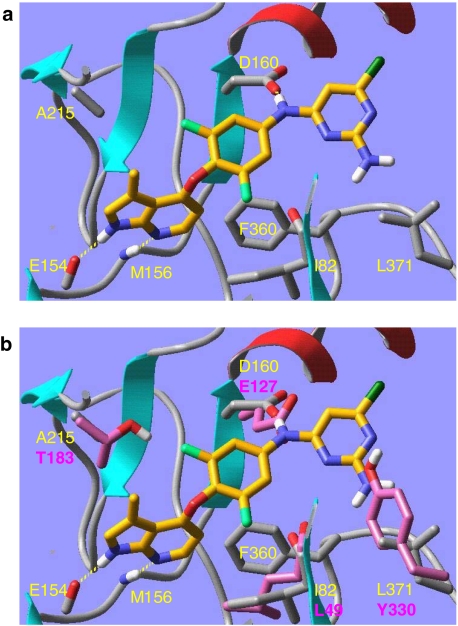
Compared to the other 491 human kinases investigated ROCK (ROCK-1 and ROCK-2) is unique with respect to the combination of the AGC characteristic residue Phe360 (in ROCK-1 corresponds to Phe327 in PKA, which has 37% sequence identity to ROCK) and mutations Ile82 (Leu49 in PKA), Met156 (Val123 in PKA), Ala215 (Thr183 in PKA), and Asp160 (Glu127 in PKA) (Breitenlechner et al., 2003; Takami et al., 2004). These residues are important shape determinants for the ATP-binding cavity of ROCK. Azaindole 1 fits nicely into the X-ray crystal structure of ROCK-1 (co-crystal with fasudil, 2esm.pdb, subunit A (Jacobs et al., 2006)), while it cannot be docked well into PKA (co-crystal with H89, 1ydt.pdb, (Engh et al., 1996)). The inability of azaindole 1 to bind strongly to PKA can be attributed to four key mutations (Figure 4), three of which are part of the residue combination that makes ROCK unique (see above). The finding that azaindole 1 shows a good selectivity profile compared to other human kinases is therefore supported by our molecular modelling studies.
Figure 4.
(a) Azaindole 1 docked and minimized into ROCK-1 (2esm.pdb, subunit a). The inhibitor is shown in tube representation (orange carbon atoms, only polar hydrogen atoms shown). Some key protein residues are labelled yellow and are partly shown in tube representation (grey carbon atoms); the remainder of the protein is shown as a ribbon diagram; (b) same as (a) plus overlayed PKA (1ydt.pdb). Only four key PKA residues are partly shown in tube representation (pink carbon atoms, pink labels). Binding mode of azaindole 1 in the Rho-kinase model. Mutated residues are coloured in magenta. PKA, protein kinase A; ROCK, Rho-kinase.
Azaindole 1 induces vasorelaxation in vitro
We investigated the effect of azaindole 1 on different vascular beds. Azaindole 1 inhibited concentration dependently the phenylephrine-induced contraction of rabbit saphenous artery with an IC50 value of 65 nM. In contrast, the ROCK inhibitor Y-27632 relaxed precontracted saphenous artery with an IC50 of 1.5 μM (data not shown). In the rat heart Langendorff preparation, azaindole 1 reduced the perfusion pressure of the coronary artery in a concentration-dependent manner at concentrations greater than 1 nM. Administration of 10, 100, 1000 nM of azaindole 1 resulted in a reduction of the maximal perfusion pressure of 50, 60, 65%, respectively. No effects on left ventricular pressure, contractility and heart rate were observed (data not shown).
Haemodynamics in anaesthetized rats
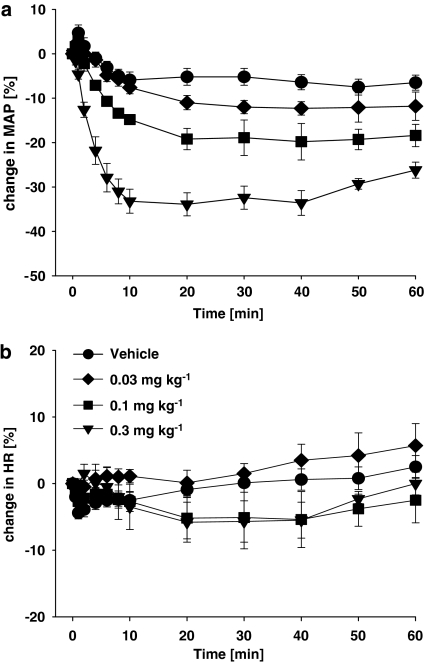
As shown in Figure 5a, azaindole 1 induced a dose-dependent and long-lasting decrease in blood pressure in anaesthetized normotensive rats. i.v. administration of 0.03, 0.1, 0.3 mg kg−1, resulted in a reduction in the maximal blood pressure of 8, 18, 35 mm Hg, respectively. Interestingly, even after the highest dose, no reflex heart rate increase was observed (Figure 5b).
Figure 5.
Effect of i.v.-administered azaindole 1 (0 (vehicle), 0.03, 0.1, 0.3 mg kg−1) on mean arterial blood pressure and heart rate in normotensive anaesthetized rats (n=6–8 per group). Values depicted represent changes in mean arterial blood pressure and heart rate. Mean blood pressure for 0 (vehicle), 0.03, 0.1 and 0.3 mg kg−1 baseline was 133±4, 129±3, 128±4 and 130±4 mm Hg, respectively. Mean heart rate at baseline was 337±7, 332±8, 362±6 and 344±12 beats min−1, respectively.
Haemodynamics in conscious normotensive and hypertensive rats
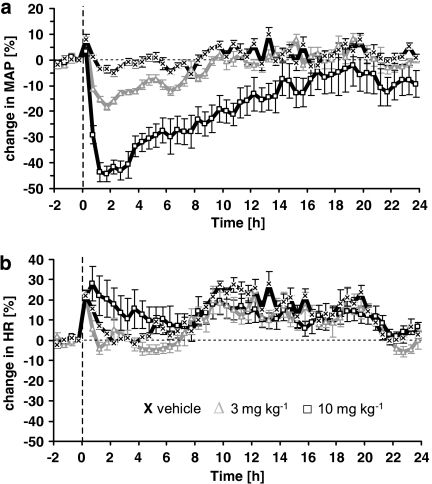
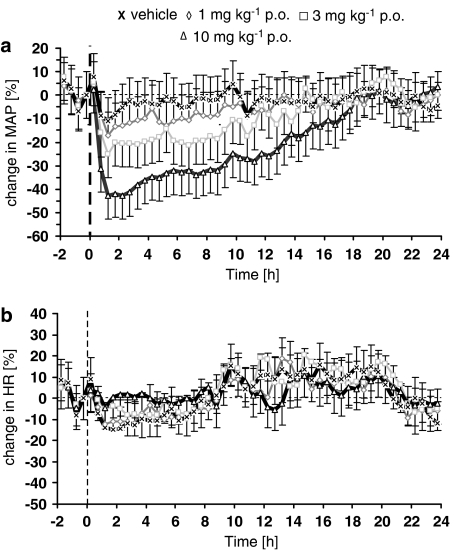
In normotensive rats, azaindole 1 also induced a dose-dependent and persistent decrease in blood pressure (Figure 6a). After oral administration of 3 and 10 mg kg−1, mean blood pressure dropped by 20 mm Hg (13%) and 41 mm Hg (40%), respectively. The effect lasted approximately 10 h after oral application of 3 mg kg−1 and more than 24 h after 10 mg kg−1. A slight and transient increase of heart rate was only observed in rats treated with the highest dose of azaindole 1, 10 mg kg−1 (Figure 6b). In conscious spontaneously hypertensive rats, azaindole 1 induced a dose-dependent and long-lasting decrease in mean blood pressure after oral application (Figure 7a). After 1.0, 3.0 and 10 mg kg−1 oral application, mean blood pressure decreased maximally by 15 (10%) 32 (18%) and 68 mm Hg (40%), respectively. The onset of this lowering effect on blood pressure was fast with the maximum decrease being achieved within the first hour after administration. The effect lasted for about 10 (1.0 mg kg−1), 14 (3.0 mg kg−1) and 17 (10 mg kg−1) h. In the vehicle-treated group, blood pressure remained unchanged. Heart rate was not significantly changed after administration of any of the doses of azaindole 1 (Figure 7b).
Figure 6.
Effect of a single oral treatment with azaindole 1 (0 (vehicle), 3, 10 mg kg−1) on mean arterial blood pressure (a) and heart rate (b) in conscious normotensive rats. The animals were treated at 0 h. Changes in blood pressure and heart rate were recorded over 24 h. Six animals per group were used. Data are given as per cent change from baseline. Mean blood pressure for 0 (vehicle), 3 and 10 mg kg−1at baseline was 97±3, 98±2 and 94±7 mm Hg, respectively. Mean heart rate at baseline was 319±4, 299±12 and 353±19 beats min−1, respectively.
Figure 7.
Effect of a single oral treatment with azaindole 1 (0 (vehicle), 1, 3, 10 mg kg−1) on mean arterial blood pressure (a) and heart rate (b) in conscious spontaneously hypertensive rats. The animals were treated at time=0 h. Changes in blood pressure and heart rate were recorded for 24 h. Six animals per group were used. Data are given as per cent change from baseline. Mean blood pressure for 0 (vehicle), 1, 3 and 10 mg kg−1 at baseline was 150±11, 166±10, 147±13 and 161±13 mm Hg, respectively. Mean heart rate at baseline was 288±13, 299±8, 280±8 and 283±12 beats min−1, respectively.
Multiple dosing in conscious rats
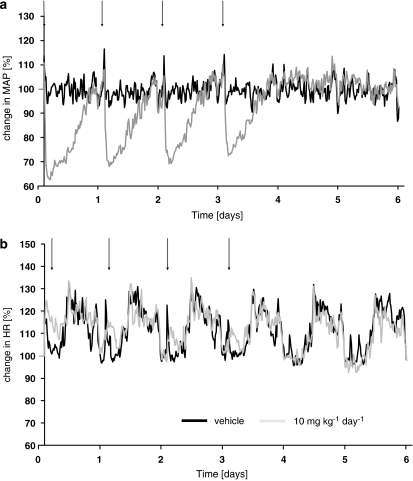
In the single-dose study, 10 mg kg−1 p.o. of azaindole 1 reduced blood pressure for about 17 h. Therefore, we used this dose to investigate the effect of azaindole 1 on blood pressure after multiple dosing. The compound was given orally once daily for 4 days. On day 5, placebo and azaindole 1-treated animals only received the vehicle (Figure 8a). On days 1–4 the maximal decreases in mean arterial blood pressure were by 32, 29, 29 and 25%, respectively, whereas no significant change in heart rate was observed (Figure 8b). In the vehicle-treated group, blood pressure did not change significantly from the initial conditions. Blood pressure returned to baseline levels within 20 h after the final dose of azaindole 1. In the vehicle-treated group, the circadian profile of the heart rate did not change significantly.
Figure 8.
Continuous 24-h profile of mean arterial blood pressure (a) and heart rate (b) in conscious spontaneously hypertensive rats. Effect of azaindole 1 (10 mg kg−1 p.o.) after multiple treatments. Controls received the vehicle. Treatment was started at time=0 h. Arrows denote treatment. Eight animals per group were used. Data are given as per cent change from baseline. Mean arterial blood pressure for vehicle and 10 mg kg−1at baseline was 156±2 and 156±8 mm Hg, respectively. Mean heart rate at baseline was 306±9 and 310±8 beats min−1, respectively.
Haemodynamics in anaesthetized dogs
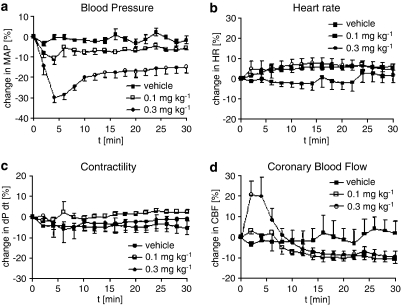
Azaindole 1 was administered as i.v. bolus injections of 0.1 and 0.3 mg kg−1. It decreased mean arterial blood pressure in a dose-related manner (Figure 9). At doses of 0.1 and 0.3 mg kg−1, the maximal blood pressure lowering effect was 10 (10%) and 25 mm Hg (30%), respectively. At all doses tested, azaindole 1 caused only a moderate and dose-independent increase in heart rate. At the doses of 0.1 and 0.3 mg kg−1, heart rate increased maximally by 10–15beats min−1. Despite a marked reduction in blood pressure, no change in cardiac contractility and only a slight, but statistically nonsignificant increase in coronary blood flow was observed.
Figure 9.
Effect of azaindole 1 on mean arterial blood pressure (a), heart rate (b), contractility (c) and coronary blood flow (d) in anaesthetized dogs. Data are given as per cent change from baseline. Five animals per group were used. Mean arterial blood pressure for vehicle, 0.1 and 0.3 mg kg−1 at baseline was respectively 80±6, 79±5 and 77±4 mm Hg. Mean heart rate at baseline was 106±3, 110±4 and 108±6 beats min−1. Contractility at baseline was 1598±153, 1514±100 and 1482±77 mm Hg s−1. Coronary blood flow at baseline was 43±6, 46±7 and 40±8 ml min−1.
Pharmacokinetic profile
The azaindole derivative azaindole 1 is a low to medium clearance drug in concious female NMRI mice, male Wistar rats and female beagle dogs. The clearance decreased with increasing size of the animal species (Table 1). Mice had the highest volume of distribution of 2.2 l kg−1. However, its half-life in dogs was longer than in rats and mice (Table 1). The oral bioavailability amounted approximately to 50% in male Wistar rats and 75% in female beagle dogs. In dogs, the half-life was significantly longer after oral administration compared to that after i.v. administration indicating a slower absorption by the oral route.
Table 1.
In vivo pharmacokinetic parameters of azaindole 1 in mice, rats and dogs
| NMRI mice | Wistar rat | Beagle dog | ||
|---|---|---|---|---|
| i.v. | ||||
| Dose | 0.500 | 0.500 | 0.0500 | |
| AUC (mg h −1) | 0.264 | 0.402 | 0.215 | |
| CL (l h−1 kg−1) | 1.90 | 1.20 | 0.233 | |
| Vss (l kg−1) | 2.20 | 1.60 | 0.832 | |
| t1/2 (h) | 1.50 | 1.24 | 2.50 | |
| p.o | ||||
| Dose | Not determined | 0.910 | 0.100 | |
| AUC (mg h l−1) | 0.352 | 0.315 | ||
| Cmax (mg l−1) | 0.0808 | 0.0430 | ||
| Tmax (h) | 2.00 | 0.794 | ||
| t1/2 (h) | 1.16 | 7.83 | ||
| Bioavailability (%) | 48.0 | 73.2 | ||
Abbreviations: i.v., intravenous; P.O., per oral.
Total plasma concentration observed after oral administration of azaindole 1 correlated well with the reduction in blood pressure (data not shown). From the pharmacological study, AUC and Cmax in spontaneously hypertensive rats following oral administration of 3 mg kg−1 azaindole 1 were 0.774 mg h l−1 and 0.111 mg l−1, respectively. These values are slightly lower than those determined from pharmacokinetic studies (Table 1).
Discussion
In the present study, we describe the biochemical and pharmacological characterizion of the structurally novel ROCK inhibitor azaindole 1. This compound was identified by high-throughput screening of an in-house 400 000 compound library and subsequent chemical optimization of the resulting lead structure using an in vitro ROCK assay. Azaindole 1 is one of the most potent and selective ROCK inhibitors described so far. It inhibits human ROCK-1 and ROCK-2 isoenzymes as well as ROCK-2 from other species in the single digit nanomolar range with almost identical IC50 values, which may be due to the unique and highly conserved active site of ROCKs (Okawa et al., 1996; Amano et al., 2000). Like most of the kinase inhibitors described so far, azaindole 1 acts as competitive inhibitor of ATP binding and as a noncompetitive inhibitor of substrate binding. Accordingly, increasing ATP to physiological relevant millimolar concentrations shifts azaindole 1-mediated ROCK-2 inhibition in vitro to higher IC50 values. When azaindole 1 was preincubated with ROCK-2 for 30 min before the addition of ATP, the IC50 values were only slightly increased. This indicates that the binding of azaindole 1 is either irreversible or very slowly reversible.
Interestingly, although competitive with ATP binding, which implicates a nonselective interaction with the conserved ATP-binding sites of other kinases, azaindole 1 is a highly specific inhibitor of ROCKs. Testing more than 110 kinases in-house and in cooperation with Upstate (UK, Dundee, Scotland) it was found that azaindole 1 weakly affected about 20 kinases with IC50 values in the range of 1–10 μM. Only the receptor tyrosine kinases TRK and FLT3 were inhibited in the submicromolar range. However, azaindole 1 was at least more than 250-fold selective for ROCK. This observation is supported by two recent studies (Breitenlechner et al., 2003; Jacobs et al., 2006), which describe the unique structural features of the active site of ROCKs. Accordingly, docking of azaindole 1 in a model of the catalytic domain of ROCK-1 (Jacobs et al., 2006) shows that the inhibitor interacts with residues at the ligand-binding site, which generates a uniquely shaped inhibitor binding pocket that confers the observed selectivity.
It is well established that the Rho/ROCK pathway plays a central role in smooth muscle cell contraction. Accordingly, inhibition of ROCK by azaindole 1-relaxed phenylephrine-precontracted strips of saphenous artery dose dependently. In addition, azaindole 1 also relaxed coronary arteries in the Langendorff-perfused rat heart model in a dose-dependent manner starting at nanomolar concentrations. The measured potency of the compound in the kinase assay in vitro and in the vasorelaxation experiments as well as the good selectivity profile against kinases and cardiovascular relevant receptors indicate that azaindole 1 acts via ROCK inhibition and exclude a nonspecific interaction with other vasoactive mechanisms such as MLCK, ZIP-kinase (Niiro and Ikebe, 2001) or L-type Ca2+ channels.
Administration of azaindole 1 in different animal models in vivo revealed that it has a profound ability to lower blood pressure. In anaesthetized normotensive rats, the compound induces a long-lasting decrease of blood pressure after i.v. administration from 0.03 to 0.3 mg kg−1 with no reflex heart rate increase. In addition, in conscious spontaneously hypertensive rats, azaindole 1 induced a dose-dependent (1–10 mg kg−1) and long-lasting reduction in the mean blood pressure after oral administration. The onset of this blood pressure lowering effect was fast with the maximum decrease attained within the first hour after administration. The effect lasted for about 10 (1.0 mg kg−1), 14 (3.0 mg kg−1) and 17 (10 mg kg−1) h. A dose-dependent blood pressure reduction after oral administration in spontaneously hypertensive rats has also been observed with Y-27632 (Uehata et al., 1997). However, azaindole 1 was about 30-fold more potent and had distinctly longer duration of action than Y-27632. In addition, azaindole 1 induced a strong dose-dependent and persistent decrease in blood pressure in conscious normotensive rats. The effect lasted approximately 10 h after application of 3 mg kg−1 p.o. and more than 24 h after 10 mg kg−1 p.o. In contrast, Y-27632 lowered blood pressure in normotensive rats only transiently with a threshold dose of 30 mg kg−1 (Uehata et al., 1997). The profound haemodynamic effects of azaindole 1 in the different rat models may be due to the improved potency and the reasonable pharmacokinetic profile of this compound. Interestingly, in conscious normotensive and hypertensive rats heart rate was only slightly and transiently increased at the highest dose. Recently, the vasodilator effects of two structurally different ROCK inhibitors were described (Doe et al., 2007). Both ROCK inhibitors are potent and selective compounds (Doe et al., 2007; Goodman et al., 2007; Stavenger et al., 2007), that show comparable strong blood pressure lowering effects in different animal models of hypertension. As observed for azaindole 1, only moderate and transient increases of heart rate were observed. The reason for the absence of profound reflex tachycardia is unknown and further studies are required to elucidate this effect. Nonetheless, this observation underlines the potential use of ROCK inhibitors for the treatment of cardiovascular diseases like hypertension, where a distinct increase in heart rate is an unwanted side effect. Likewise angiotensin II receptor antagonists also show such favourable haemodynamic profiles with no reflex tachycardia in vivo (Stasch et al., 1997).
Treatment of patients with cardiovascular disorders such as hypertension or angina requires the repeated administration of drugs to achieve constant plasma levels of the substance. In addition, the development of tolerance, as observed for organic nitrates, limits the therapeutic value of a drug. The pharmacokinetic profile of azaindole 1 shows that it has a low to medium clearance in all species investigated (NMRI mice, Wistar rats and beagle dogs). The oral bioavailability amounted to approximately 50% in male Wistar rats and 75% in female beagle dogs. In the single-dose study, 10 mg kg−1 p.o. of azaindole 1 reduced blood pressure for about 17 h. Therefore, we used this dose to investigate the effect of azaindole 1 on blood pressure following multiple dosing in conscious spontaneously hypertensive rats. After oral administration of azaindole 1 once daily, mean arterial blood pressure was decreased significantly. As already observed in animals treated once daily, no significant increase in heart rate was observed. However, the maximal blood pressure lowering effect was gradually reduced over the 4-day observation period. At the moment, it is unclear whether multiple administration of azaindole 1 results in the development of tachyphylaxis, or whether this effect reflects variations in blood pressure due to the development of steady-state plasma levels of azaindole 1. The blood pressure decrease was fully reversible after withdrawal of the compound. Interestingly, rebound hypertension was not observed.
The haemodynamic profile of azaindole 1 was also investigated in anaesthetized dogs. The substance dose relatedly decreased mean arterial blood pressure after i.v. bolus injections of 0.1 and 0.3 mg kg−1. Despite a marked blood pressure decrease, reflex tachycardia was only moderate. No decrease in cardiac contractility was observed. Although azaindole 1 efficiently lowered perfusion pressure in coronary arteries of Langendorff-perfused rat hearts, only a slight but nonsignificant increase in coronary blood flow in the dog was observed. The reason of this discrepancy is unknown and may be due to species differences and/or varying expression levels of ROCK in coronary arteries.
In summary, the results show that azaindole 1 is a potent and highly specific ROCK inhibitor. It is also an ATP-competitive kinase inhibitor that has the ability to induce vasorelaxation in vitro as well as in several in vivo models. Azaindole 1 is therefore a valuable pharmacological tool to elucidate further the physiological and pathophysiological role of ROCK and offers a novel therapeutic approach for treating cardiovascular diseases.
Abbreviations
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulphonate
- DMSO
dimethyl sulphoxide
- IMAC
immobilized metal affinity chromatography
- MBS
myosin-binding subunit
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- ROCK
Rho-kinase
- SHR
spontaneously hypertensive rats
- S6 peptide
RRLSSLRA
- TCEP
Tris(2-carboxyethyl)phosphine hydrochloride
- Y-27632
(+)-R-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide dihydrochloride
- ZIP-kinase
zipper-interacting protein kinase
Conflicts of interest
The authors are employees at Bayer HealthCare with exeption of Professor Ehmke, Hamburg.
References
- Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- Breitenlechner C, Gassel M, Hidaka H, Kinzel V, Huber R, Engh RA, et al. Protein kinase A in complex with Rho-kinase inhibitors Y-27632, fasudil, and H-1152P: structural basis for selectivity. Structure. 2003;11:1595–1607. doi: 10.1016/j.str.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Christ G, Wingard C. Calcium sensitization as a pharmacological target in vascular smooth muscle regulation. Curr Opin Investig Drugs. 2005;9:920–933. [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe C, Bentley R, Behm D, Lafferty R, Stavenger R, Jung D, et al. Novel Rho-kinase inhibitors with anti-inflammatory and vasodilatory activities. J Pharmacol Exp Ther. 2007;320:89–98. doi: 10.1124/jpet.106.110635. [DOI] [PubMed] [Google Scholar]
- Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J Biol Chem. 1996;271:26157–26164. doi: 10.1074/jbc.271.42.26157. [DOI] [PubMed] [Google Scholar]
- Goodman K, Cui H, Dowdell S, Gaitanopoulos DE, Ivy RL, Sehon CA, et al. Development of dihydropyridine indazole amides as selective Rho-kinase inhibitors. J Med Chem. 2007;50:6–9. doi: 10.1021/jm0609014. [DOI] [PubMed] [Google Scholar]
- Gong MC, Iizuka K, Nixon G, Browne JP, Hall A, Ecclestone JF, et al. Role of guanine nucleotide-binding proteins—ras-family or trimeric proteins or both—in Ca2+ sensitization of smooth muscle. Proc Natl Acad Sci USA. 1996;93:1340–1345. doi: 10.1073/pnas.93.3.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Hayakawa K, Swenson L, Bellon SM, Fleming M, Taslimi P, et al. The structure of dimeric ROCK1 reveals the mechanism for ligand selectivity. J Biol Chem. 2006;281:260–268. doi: 10.1074/jbc.M508847200. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukuta Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Masumoto A, Hirooka Y, Shimokawa H, Hironoga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A. Suppression of coronary artery spasm by Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME. In hypertension, the kidney is not always the heart of the matter. J Clin Invest. 2005;115:840–844. doi: 10.1172/JCI24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagumo H, Sasaki Y, Ono Y, Okamoto H, Seto M, Takura M. Rho-kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am J Physiol Cell Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- Niiro N, Ikebe M. Zipper-interacting protein kinase induces Ca2+ free smooth muscle contraction via myosin light chain phosphorylation. J Biol Chem. 2001;276:29567–29574. doi: 10.1074/jbc.M102753200. [DOI] [PubMed] [Google Scholar]
- Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Ono-Saito N, Niki I, Hidaka H. H-Series protein kinase inhibitors and potential clinical applications. Pharmacol Ther. 1999;82:123–131. doi: 10.1016/s0163-7258(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Schirok H, Radtke M, Mittendorf J, Kast R, Stasch JP, Gnoth MJ, et al. Preparation of heteroaryloxy-substituted phenylamino pyrimidines as Rho-kinase inhibitors. Publication No: WO 2,005,097790 (2005) Chem Abstr. 2005;153:405919. [Google Scholar]
- Seasholtz TM, Wessel J, Rao F, Rana BK, Khandrika S, Kennedy BP, et al. Rho-kinase polymorphism influences blood pressure and systemic cascular resistance in human twins: role of heredity. Hypertension. 2006;47:937–947. doi: 10.1161/01.HYP.0000217364.45622.f0. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Hiramori K, Linuma H, Hosoda S, Kishida H, Osada H, et al. Anti-anginal effect of fasudil, a rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002;40:751–762. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Morishige K, Miyata K, Kandabashi T, Eto Y, Ikegabi I, et al. Long-term inhibition of Rho-kinase induces a regression of arteriosclerotic lesions in a porcine model in vivo. Cardiovasc Res. 2001;51:169–177. doi: 10.1016/s0008-6363(01)00291-7. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, Yamawaki T, et al. Rho-kinase mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc Res. 1999;43:1029–1039. doi: 10.1016/s0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]
- Stasch JP, Knorr A, Hirth-Dietrich C, Kramer T, Huebsch W, Dressel J, et al. Long-term blockade of the angiotensin II receptor in renin transgenic rats, salt-loaded Dahl rats, and stroke-prone spontanteously hypertensive rats. Drug Res. 1997;47:1016–1023. [PubMed] [Google Scholar]
- Stavenger RA, Cui H, Dowdell SH, Franz RG, Gaitanopoulos DE, Goodman KB, et al. Discovery of aminofurazan-azabenzimidazoles as inhibitors of Rho-kinase with high kinase selectivity and antihypertensive activity. J Med Chem. 2007;50:2–5. doi: 10.1021/jm060873p. [DOI] [PubMed] [Google Scholar]
- Tachibana E, Harada T, Shibuya M, Saito K, Takayasu M, Suzuki Y, et al. Intra-arterial infusion of fasudil for treating vasospasm following subarachnoid haemorrhage. Acta Neurochir. 1999;141:13–19. doi: 10.1007/s007010050260. [DOI] [PubMed] [Google Scholar]
- Takami A, Iwakubo M, Okada Y, Kawata T, Odai H, Takahashi N, et al. Design and synthesis of Rho-kinase inhibitors (I) Bioorg Med Chem. 2004;12:2115–2137. doi: 10.1016/j.bmc.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Tamura M, Nakao H, Yoshizaki H, Shiratsuchi M, Shigyo H, Yamada H, et al. Development of specific Rho-kinase inhibitors and their clinical application. Biophys Biochem Acta. 2005;1754:245–252. doi: 10.1016/j.bbapap.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]