Abstract
Background and purpose:
The present experiments were designed to study the contribution of oxygen-derived free radicals to endothelium-dependent contractions in femoral arteries of rats with streptozotocin-induced diabetes.
Experimental approach:
Rings with and without endothelium were suspended in organ chambers for isometric tension recording. The production of oxygen-derived free radicals in the endothelium was measured with 2′,7′-dichlorodihydrofluorescein diacetate using confocal microscopy. The presence of protein was measured by western blotting.
Key results:
In the presence of L-NAME, the calcium ionophore A23187 induced larger endothelium-dependent contractions in femoral arteries from diabetic rats. Tiron, catalase, deferoxamine and MnTMPyP, but not superoxide dismutase reduced the response, suggesting that oxygen-derived free radicals are involved in the endothelium-dependent contraction. In the presence of L-NAME, A23187 increased the fluorescence signal in femoral arteries from streptozotocin-treated, but not in those from control rats, confirming that the production of oxygen-derived free radicals contributes to the enhanced endothelium-dependent contractions in diabetes. Exogenous H2O2 caused contractions in femoral arterial rings without endothelium which were reduced by deferoxamine, indicating that hydroxyl radicals contract vascular smooth muscle and thus could be an endothelium-derived contracting factor in diabetes. The reduced presence of Mn-SOD and the decreased activity of catalase in femoral arteries from streptozotocin-treated rats demonstrated the presence of a redox abnormality in arteries from rats with diabetes.
Conclusions and implications:
These findings suggest that the redox abnormality resulting from diabetes increases oxidative stress which facilitates and/or causes endothelium-dependent contractions.
Keywords: endothelium-dependent contractions, endothelium-derived contracting factor, oxidative stress, oxygen-derived free radicals, streptozotocin-induced diabetes
Introduction
The augmented release of endothelium-derived contracting factor, as well as decreased nitric oxide-mediated relaxation, is a hallmark of endothelial dysfunction (Cohen, 2002; Vanhoutte et al., 2005). This contracting factor is derived from endothelial cyclooxygenase and activates thromboxane prostanoid receptors of vascular smooth muscle (Lüscher and Vanhoutte, 1986; Auch-Schwelk et al., 1990; Yang et al., 2004b; Vanhoutte et al., 2005). Endothelium- and cyclooxygenase-dependent contractions are prominent in the aorta of the spontaneously hypertensive rat (Lüscher and Vanhoutte, 1986; Yang et al., 2004a, 2004b; Tang et al., 2007), the abdominal aorta of the diabetic rabbit (Tesfamariam et al., 1989) and the femoral artery of the diabetic rat (Shi et al., 2007). The endothelium-derived contracting factor can be prostacyclin, thromboxane A2, endoperoxides, prostaglandin E2, hydroxyl radicals or superoxide anions depending on the animal model, the preparation and the agonist used (Katusic and Vanhoutte, 1989; Tesfamariam et al., 1989; Auch-Schwelk et al., 1990; Yang et al., 2004a, 2004b; Gluais et al., 2005, 2006; Shi et al., 2007). Oxygen-derived free radicals facilitate endothelium-dependent contractions, and antioxidants effectively decreased them in the aorta of spontaneously hypertensive rats (Tesfamariam and Cohen, 1989; Yang et al., 2002, 2003b; Tang et al., 2007). These findings indicate that oxygen-derived free radicals and oxidative stress are directly involved in the production of endothelium-derived contracting factor.
Oxidative stress is increased in diabetic patients and animals (De Vriese et al., 2000; Alp et al., 2003; Maritim et al., 2003; Taniguchi et al., 2005). This is due to the increased production of free radicals and/or impaired antioxidant defences. Mechanisms underlying the increased oxidative stress in diabetes include activation of transcription factors, protein kinase C, glucose oxidation and augmented production of advanced glycated end products (Bucala et al., 1991; Alp et al., 2003; Cosentino et al., 2003; Wong et al., 2003; Sheu et al., 2005; Ho et al., 2006). The evidence suggesting a reduced antioxidant defence in arteries from animals with streptozotocin (STZ)-induced diabetes includes the reduced activity of superoxide dismutase (SOD) and catalase, and a decreased presence or bioavailability of glutathione and nitric oxide (Mekinova et al., 1995; Aragno et al., 1999; Kedziora-Kornatowska et al., 2000; Rauscher et al., 2001; Maritim et al., 2003).
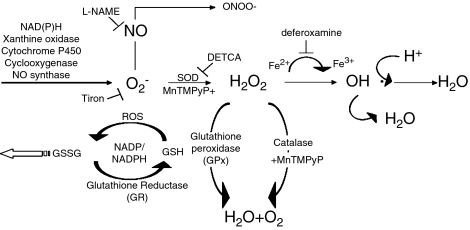
The effect of STZ-induced diabetes on nitric oxide-mediated vasodilatation is still controversial; it has been reported as normal in the aorta (Head et al., 1987; Harris and MacLeod, 1988; Mulhern and Docherty, 1989), mesenteric artery (Harris and MacLeod, 1988) and femoral artery (Wigg et al., 2001), as blunted in aorta (Pieper et al., 1997; Vallejo et al., 2000), mesenteric artery (Heygate et al., 1995; Vallejo et al., 2000; Shi et al., 2007) and femoral artery (Shi et al., 2006) or augmented in aorta (Altan et al., 1989). The production of vasoconstrictor prostaglandins is augmented in diabetic animals (aorta (Tesfamariam et al., 1989), renal artery (Pflueger et al., 1999) and femoral artery (Shi et al., 2007)). Previous work from our laboratory has demonstrated an augmented endothelium- and cyclooxygenase-dependent contraction in the femoral artery of rats with STZ-induced diabetes. Furthermore, the nature of the cyclooxygenase products involved varies in the course of the disease (Shi et al., 2007). Taken together, these findings prompt the hypothesis that oxygen-derived free radicals play a key role in the occurrence of endothelium-dependent contractions in this model of diabetes. Therefore, the present experiments were designed to study the contributions of reactive oxygen species to, and the effects of antioxidants (Figure 1) on, endothelium-dependent contractions of femoral arteries of rats with STZ-induced diabetes.
Figure 1.
Formation of oxygen-derived free radicals. Superoxide anions (O2−) can be generated from molecular oxygen, or by the action of xanthine oxidase, or of various enzymes. O2− can be scavenged by NO. It can also be converted to highly reactive oxygen species as peroxynitrite (ONOO−) when combined with NO, or H2O2 by SOD. H2O2 can be transformed to hydroxyl radicals by ferrous ions or converted to H2O by catalase and glutathione. Tiron scavenges O2− inside cells. DETCA inhibits SOD. Deferoxamine is an iron chelator that scavenges hydroxyl radicals. L-NAME inhibits NO synthase. MnTMPyP mimics the combined effect of SOD and catalase. DETCA, diethyldithiocarbamic acid; GSH, glutathione; GSSG, glutathione disulphide; H2O2, hydrogen peroxide; L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; MnTMPyP, Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride; NO, nitric acid; ROS, reactive oxygen species; SOD, superoxide dismutase; tiron, 4,5-dihydroxy-1,3-benzenedisulphonic acid.
Methods
Experimental animals
All animal procedures and protocols were approved by the institutional animal care committee.
All experiments used the isolated femoral arteries of Sprague–Dawley rats. Diabetes was induced in 16-week-old male rats (450–600 g) by a single administration of STZ (55 mg kg−1, injected in the tail vein) dissolved in citric acid–trisodium citrate (0.2 mM) buffer (pH 4.0–4.5). Seventy-two hours later, after overnight starvation, rat tail blood samples were obtained and the glucose concentration was measured using a one-touch glucometer (LifeScan Inc., Milpitas, CA, USA). Induction of diabetes was considered successful when the glucose level was higher than 16.6 mM (day 0). Non-diabetic control rats were injected with vehicle solution alone and kept under identical conditions. The animals were housed in the Laboratory Animal Unit of the University of Hong Kong, fed with regular chow and given free access to water. Twelve weeks later (day 84), they were anaesthetized with intraperitoneal pentobarbitone sodium (70 mg kg−1), anticoagulated with heparin (0.5 U kg−1) and exsanguinated. Non-fasting glucose level was measured on the day of death. Control rats with blood glucose higher than 11.1 mM were excluded from the study.
Functional studies in isolated tissues
Preparation of the arteries
The femoral arteries were dissected free, excised and placed into ice-cold modified Krebs–Ringer solution of the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25.0, KH2PO4 1.18, calcium disodium EDTA 0.026 and glucose 11.1 (control solution). The blood vessels were cut into rings (1.5–2 mm in length). When arterial rings without endothelium were needed, the arteries were perfused with 1 ml of saponin solution (1 mg ml−1, diluted with Krebs–Ringer solution; Samata et al., 1986) before the rings were cut. The rings were suspended in organ chambers containing control solution (37 °C) aerated with 95% O2 and 5% CO2. They were connected to a force transducer (Powerlab Model ML785 and ML119). Changes in isometric tension were recorded. The rings were stretched progressively to their optimal resting tension (1.0 g) determined in preliminary experiments and were allowed to equilibrate for 90 min before experimentation. Changes in tension were expressed as a percentage of the reference contraction to 60 mM KCl, obtained at the beginning of the experiment.
To study endothelium-dependent contractions, all experiments were performed under quiescent conditions in the presence of L-NAME (Nω-nitro-L-arginine methyl ester hydrochloride, 3 × 10−4 M; Auch-Schwelk et al., 1992; Yang et al., 2004a; Tang et al., 2005). The drugs were incubated in the organ chamber for 30 min before the administration of the calcium ionophore A23187 in a cumulative manner.
Concentration–response curves to hydrogen peroxide were obtained in a cumulative manner in rings without endothelium.
Western blotting
Femoral arteries were dissected free and cleaned of connective tissue. To remove the remaining blood cells, arteries were rinsed three times with Krebs–Ringer solution. Blood vessels were homogenized at 4 °C in lysis buffer (Tris–HCl 10 mM, NaCl 150 mM, EDTA 1 mM, sodium pyrophosphate 25 mM, β-glycophosphate 1 mM, sodium orthovanadate 1 mM, leupeptin 2.1 μM, aprotinin 1 mg ml−1, phenylmethylsulphonyl fluoride 1 mM and Triton X-100 1%) and incubated on ice for 10 min. Samples were then centrifuged at 3000 g for 10 min at 4 °C, and the supernatant was collected. Protein concentrations were determined using the Bradford assay. Lysates were used immediately or stored at −70 °C.
In all immunoblot experiments, equal amounts of protein were loaded in each lane of SDS–polyacrylamide gel electrophoresis gels. Molecular masses of immunoreactive bands were determined by loading biotinylated molecular mass standards (Bio-Rad Laboratories, Hercules, CA, USA). After electrophoresis, the proteins were transferred to nitrocellulose membranes (Bio-Rad Laboratories) and blocked with 5% non-fat dry milk in Tris-buffered saline (Tris–HCl 20 mM, NaCl 140 mM, pH 7.4) for an hour. Membranes were then incubated with the suggested dilution of corresponding primary antibodies overnight at room temperature, followed by three 10 min wash in 0.1% Tween-20–Tris-buffered saline. Blots were incubated with secondary antibodies at 1:1000 dilutions for 2 h at room temperature membrane, and were then washed three times for 10 min in Tween-20–Tris-buffered saline solution. Membranes were then incubated with enhanced chemiluminescence reagent (Amersham Biosciences, Buchinghamshire, UK) for 1 min and exposed to X-ray films (Fuji Super RX Medical X-ray films; Fuji Photo Film, Dusseldorf, Germany) for the recommended optimal time, depending on the signal strength. Software for electrophoresis analysis (Multi-analyst; Bio-Rad Laboratories) was used for densitometric analysis of immunoreactive bands. The presence of protein was normalized to β-actin and expressed as percentage of control.
Fluorescence studies
Reactive oxygen species were measured with DCF (2′,7′-dichlorodihydrofluorescein diacetate). Non-ionized DCF is membrane permeable and, therefore, diffuses readily into cells (Nasr-Esfahani et al., 1990). The fluorescence experiments were performed in a dark room. Rings of femoral artery were loaded with DCF solution (10−5 M) for 15 min followed by incubation with tested agents for 30 min. After rinsing three times in control solution, the rings were opened longitudinally and placed with the endothelial layer down on a microscope stage. Reactive oxygen species were measured using a confocal laser scanning microscope. The intensity of the production was recorded after excitation at 488 nm and emission at 510 nm, using the appropriate software (Laser Sharp 2000, Bio-Rad Cell Science Division, Hemel Hempstead, UK). The production of reactive oxygen species measured from nine randomly related fields was averaged in the endothelium and the vascular smooth muscle of each preparation (Supplementary Information). To correct for nonspecific changes in fluorescence, the background signal was subtracted from the measurements and only the changes induced by A23187 are reported and discussed.
Enzymatic activities
Femoral arteries were dissected and cleaned free of connective tissue. The preparations were homogenized at 4 °C in lysis buffer (50 mM potassium phosphate containing 1 mM EDTA). The samples were centrifuged at 10 000 g for 10 min at 4 °C, and the supernatant was collected. Protein concentrations were determined using the Bradford assay. Lysates were used immediately or stored at −70 °C. The level of glutathione and catalase activity was measured following the instructions of the manufacturers.
Data analysis
Data are presented as means±s.e.m.; n refers to the number of rats. Concentration–response curves are shown as such or as area under the curve. Statistical analysis was done by one-way analysis of variance followed with post hoc comparison using the Bonferroni test, or by two-way analysis of variance, as appropriate (Prism version 3a, GraphPad Software, San Diego, CA, USA). Differences were considered to be statistically significant when P was less than 0.05.
Drugs
A23187, anti-β-actin monoclonal antibody, catalase, deferoxamine mesylate salt, DCF, DETCA (diethyldithiocarbamic acid), indomethacin, L-NAME, saponin, SC19220 (2-acetylhydrazide 10 (11 H)-carboxylic acid 8-chloro-dibenz [b,f] [1,4] oxazepine-10 (11 H)-carboxylic acid), STZ, SOD, tiron (4,5-dihydroxy-1,3-benzenedisulphonic acid) were purchased from Sigma Chemical Company (St Louis, MO, USA). Anti-catalase polyclonal antibodies were purchased from the Abcam Company (Cambridge, UK). Anti-Mn-SOD antibodies were purchased from Bethyl Laboratories Inc. (Montgomery, TX, USA). Anti-CuZn-SOD antibodies were purchased from BioVision (Mountain View, CA, USA). Glutathione and catalase commercial kits were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Heparin was purchased from LEO Pharm (Ballerup, Denmark). MnTMPyP (Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride) was purchased from Calbiochem (San Diego, CA, USA). Hydrogen peroxide (30%) was purchased from Merck (Darmstadt, Germany). Secondary antibodies (ECL anti-rabbit immunoglobulin G and ECL anti-mouse immunoglobulin G) were obtained from Amersham Biosciences. S18886 (terutroban, (3-[(6-amino-(4-chlorobenzenesulphonyl)-2-methyl-5,6,7,8-tetrahydronapht]-1-yl) propionic acid) was a kind gift of the Institut de Recherche Servier (Suresnes, France). Drug concentrations are given as final molar concentrations in the bath solution.
Results
General condition of animals
At the beginning of the study, the body weights were comparable in the control and STZ-treated groups. Twelve weeks after the injection of STZ, control rats gained weight, while the STZ-treated rats lost weight significantly. The glucose level in the STZ-treated group was significantly higher than that in the controls (Table 1).
Table 1.
Body weight, glucose level in control and streptozotocin (STZ; 55 mg kg−1)-treated rats
| Control rats | STZ-treated rats | |
|---|---|---|
| Body weight (day 0) (g) | 545.9±7.7 | 530.9±6.2 |
| Body weight (day 84) (g) | 705.5±11.9* | 415.6±7.7*,# |
| Glucose level (day 0) (fasting; mM) | 5.50±0.01 | 21.31±0.05# |
| Glucose level (day 84) (w/o fasting; mM) | 10.31±0.30 | 29.81±0.50# |
Data shown as means±s.e.m.; n=10–18.
Statistically significant differences with that on day 0 (P<0.05).
Statistically significant differences with control rats on the same day (P<0.05).
Endothelium-dependent contractions in arterial rings
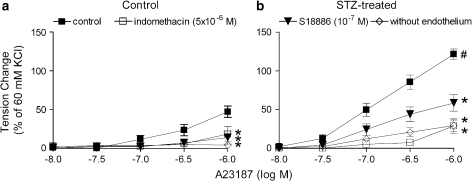
In the presence of L-NAME (3 × 10−4 M), the calcium ionophore A23187 induced a concentration-dependent contraction in rings with endothelium, which was not observed in preparations without endothelium. The endothelium-dependent response was significantly larger in arteries from STZ-treated rats than those from control rats. Indomethacin (5 × 10−6 M) and S18886 (10−7 M) significantly reduced the response (Figure 2).
Figure 2.
Contraction to A23187 in femoral arteries from control (a) and STZ-treated (b) rats in the presence of L-NAME. Data are expressed as percentage of the control contraction to 60 mM KCl and are shown as means±s.e. mean; n=7. *Significant differences (P<0.05) from ring with endothelium under control conditions; #significant differences (P<0.05) from control rats. L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; STZ, streptozotocin.
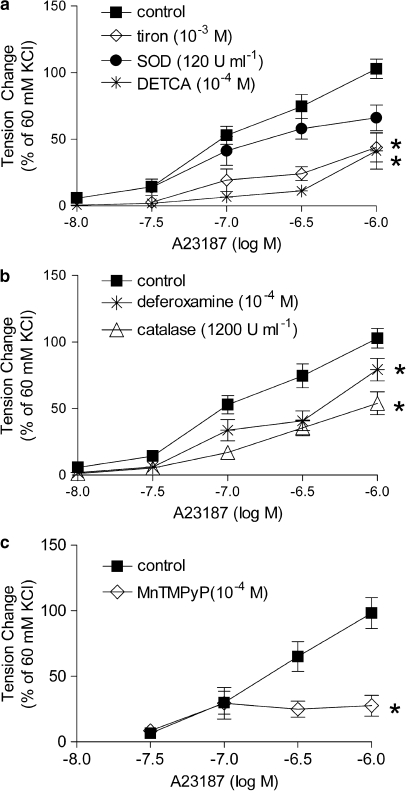
In the presence of L-NAME, tiron (10−3 M) significantly decreased the endothelium-dependent contraction to A23187, while SOD (120 U ml−1) did not significantly affect it (Figure 3a). DETCA (10−4 M), catalase (1200 U ml−1) or deferoxamine (10−4 M) alone significantly inhibited the endothelium-dependent contraction to A23187 (Figure 3b). MnTMPyP (10−4 M) also significantly reduced the response to the calcium ionophore (Figure 3c).
Figure 3.
Effect of antioxidants on concentration-dependent contractions to A23187 in femoral arteries with endothelium from STZ-treated rats in the presence of L-NAME. (a) Tiron and SOD, n=8–10, (b) deferoxamine, catalase and DETCA; n=12–20 and (c) MnTMPyP; n=8. Data are expressed as percentage of the reference contraction to 60 mM KCl and are shown as means±s.e. mean; *Significant difference (P<0.05) from the controls (rings with endothelium). DETCA, diethyldithiocarbamic acid; L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; MnTMPyP, Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride; SOD, superoxide dismutase; STZ, streptozotocin.
Exogenous hydrogen peroxide
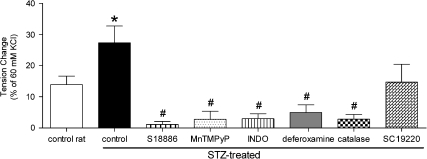
In rings without endothelium of femoral arteries from control and STZ-treated rats, hydrogen peroxide produced concentration-dependent contractions, which were maximal at 3 × 10−5 M.
In arteries from STZ-treated rats, the maximal response to hydrogen peroxide was statistically significantly greater than those from control rats (Figure 4) and the concentration–response curve was shifted significantly to the left (effector concentration for half-maximum response (−log M): −4.87±0.20 and −5.22±0.12; for control and diabetic rats, respectively). Catalase, deferoxamine, MnTMPyP, indomethacin and S18886, but not SC19220, prevented the contractions to hydrogen peroxide (Figure 4).
Figure 4.
Maximal contractions to hydrogen peroxide (3 × 10−5 M) in rings without endothelium of femoral arteries from control and STZ-treated rats. Effect of catalase, deferoxamine, MnTMPyP, INDO, S18886 and SC19220 on the response. Data are expressed as percentage of the reference contraction to 60 mM KCl and are shown as means±s.e. mean; n=9. *Significant difference from the rings from control rats; #statistically significant difference from the rings from STZ-treated rats under control conditions (P<0.05). INDO, indomethacin; MnTMPyP, Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride; S18886, terutroban, (3-[(6-amino-(4-chlorobenzensulphonyl)-2-methyl-5,6,7,8-tetrahydronapht]-1-yl) propionic acid; SC19220, 2-acetylhydrazide 10 (11 H)-carboxylic acid 8-chloro-dibenz [b,f] [1,4] oxazepine-10 (11 H)-carboxylic acid; STZ, streptozotocin.
Confocal microscopy
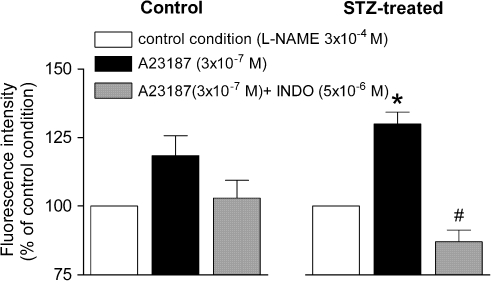
All experiments were performed in the presence of L-NAME 3 × 10−4 M. In arteries from STZ-treated rats, the calcium ionophore A23187 (3 × 10−7 M) increased the fluorescence intensity in the endothelial layer. This increase was reduced significantly by indomethacin (Figure 5), but not by S18886 (data not shown). In control preparations, A23187 had no significant effect (Figure 5).
Figure 5.
Changes in fluorescence intensity in femoral arteries from control (left) and STZ (right)-treated rats in response to A23187 (3 × 10−7 M) and effects of indomethacin on the response. Data are expressed as percentage fluorescence value to arteries under control conditions and are shown as means±s.e. mean; n=6–7. *Significant difference (P<0.05) from the arteries under control conditions; #significant difference (P<0.05) from the arteries stimulated with A23187 in the absence of indomethacin. INDO, indomethacin; STZ, streptozotocin.
In the vascular smooth muscle layers from control and STZ-treated rats, the calcium ionophore A23187 did not significantly affect the fluorescence signal, in the presence of L-NAME (data not shown).
Superoxide dismutase and catalase
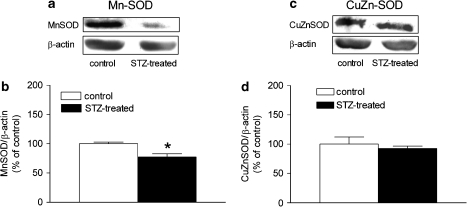
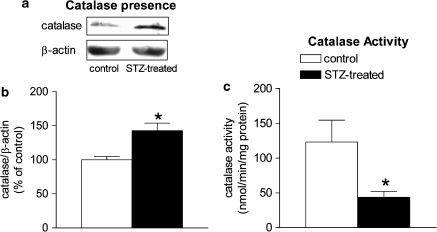
The protein level of manganese-SOD (Mn-SOD) was reduced in the femoral arteries from STZ-treated rats, whereas the protein level of copper–zinc-SOD (CuZn-SOD) was comparable (Figure 6). The expression of catalase was significantly greater in the STZ-treated group than that in the controls (Figures 7a and b).
Figure 6.
Presence of Mn-SOD (a, b) and copper–zinc-SOD (c, d) in femoral arteries with endothelium from control and STZ-treated rats. (a and c) Representative western blots demonstrating the presence of proteins and (b and d) graphic representation of the data, normalized to β-actin, presented as percentage of control and shown as means±s.e. mean; n=6. *Significant difference from controls (P<0.05). CuZn-SOD, copper–zinc-SOD; Mn-SOD, manganese-superoxide dismutase; STZ, streptozotocin.
Figure 7.
Catalase protein presence and activity in femoral arteries with endothelium from control and STZ-treated rats. (a) Western blot demonstrating the presence of catalase. (b) Graphic representation of the data, normalized to β-actin, presented as percentage of control and shown as means±s.e.m.; n=6. *Significant difference from controls (P<0.05). (c) Catalase activity in femoral arteries with endothelium from control and STZ-treated rats. Data are normalized to the protein level and are shown as means±s.e.m.; n=6. *Significant difference from controls (P<0.05). STZ, streptozotocin.
Antioxidant enzymes
The activity of catalase was reduced in femoral arteries from the STZ-treated group (Figure 7c), while the total glutathione level was comparable in the two groups (data not shown).
Discussion
Oxidative stress results from the imbalance between an increased generation of oxygen-derived free radicals and an impaired antioxidant defence. It increases under pathological conditions and along with the course of certain diseases. Oxygen-derived free radicals play an important role in the occurrence of endothelium-dependent contractions in the basilar artery of the dog and the aorta of the spontaneously hypertensive rat (Katusic and Vanhoutte, 1989; Katusic et al., 1993; Yang et al., 2003a, 2004a; Tang et al., 2007). Previous studies from our laboratory demonstrate that the calcium ionophore A23187 induces greater endothelium-dependent contractions in the femoral artery of diabetic rats than those of normoglycaemic animal (Shi et al., 2007), exemplifying the endothelial dysfunction caused by diabetes (Pieper and Gross, 1988; Heygate et al., 1995; Makimattila et al., 1996; Pieper et al., 1997; Vallejo et al., 2000). This prompted the investigation of the role of oxygen-derived free radicals in the endothelium-derived contracting factor-mediated responses of the femoral artery of rats with STZ-induced diabetes. Because acetylcholine, the most commonly used agonist to evoke such responses in the rat aorta (Katusic et al., 1993; Yang et al., 2003b; Tang et al., 2005), does not cause such responses in the rat femoral artery (Shi et al., 2007), the calcium ionophore A23187 was selected for the present experiments.
The present functional studies demonstrate that tiron, a cell-permeable non-enzymatic scavenger of superoxide anion (Malendowicz, 1972), reduced the endothelium-dependent response to A23187 while SOD, which cannot permeate into cells, had no effect. This observation resembles the findings obtained in the aorta of the spontaneously hypertensive rat (Auch-Schwelk et al., 1989; Yang et al., 2002). These observations support the concept that the superoxide anions inside endothelial cells are the primary oxidative contributor to endothelium-dependent contractions. Treatment with DETCA (Greenstock and Miller, 1975), an inhibitor of endogenous CuZn-SOD, reduced the endothelium-dependent response to A23187. Since DETCA is supposed to cause intracellular accumulation of superoxide anions (Omar et al., 1991; Pagano et al., 1993), this finding suggests that superoxide anions per se do not mediate the endothelium-dependent response, as they do in the canine basilar artery (Katusic et al., 1993). Rather, superoxide anions are the source of other oxygen-derived free radicals in the endothelial cells. Catalase, a scavenger of hydrogen peroxide (for example, Katusic and Vanhoutte, 1989) diminished the endothelium-dependent contractions to A23187, supporting a key role of hydrogen peroxide in the occurrence of the endothelium-dependent contractions evoked by the calcium ionophore. The role of hydrogen peroxide was confirmed by the experiments with MnTMPyP, since this SOD/catalase mimetic (Pasternack et al., 1986) nearly abolished the response. Ferrous ions catalyse interactions between hydrogen peroxide and superoxide anions, producing hydroxyl radicals. Deferoxamine, an iron chelator that scavenges hydroxyl radicals (for example, Auch-Schwelk et al., 1989), reduced the response to A23187, indicating that hydroxyl radicals are also involved in endothelium-dependent contractions in the femoral artery of rats with STZ-induced diabetes. Taken together, the findings of the present study strongly suggest that an increase in intracellular concentration of Ca2+ leads to the generation of superoxide anions, which are converted by SOD to hydrogen peroxide that is further transformed to hydroxyl radicals, responsible in part for the endothelium-dependent contractions observed in arteries of the diabetic rats.
Thus, the present findings indicate that hydrogen peroxide, which can diffuse freely within the tissues since it is not charged (Wolin, 2000), plays an important role in the occurrence of endothelium-dependent contractions. In line with such a role, exogenous hydrogen peroxide produced moderate contractions in rings without endothelium. Since this contraction is prevented by catalase, MnTMPyP and deferoxamine, the mediators of the response are hydroxyl radicals but not hydrogen peroxide per se. The maximal level of contraction generated by exogenous H2O2, and thus presumably hydroxyl radicals, is comparable to the deferoxamine-sensitive part of the endothelium-dependent contraction to the calcium ionophore. A similar conclusion has been reached in the aorta of spontaneously hypertensive rats (Yang et al., 2002). The inhibitory effect of S18886, but not SC19220, indicates that ultimately hydroxyl radicals must cause activation of thromboxane prostanoid receptors on the vascular smooth muscle (Yang et al., 2002). The hyper-responsiveness of the femoral artery from the diabetic rat can be explained in part by the hyper-responsiveness of the thromboxane prostanoid receptors of its vascular smooth muscle (Shi et al., 2007). The fact that indomethacin, the inhibitor of cyclooxygenase, inhibited the response suggests that the hydroxyl radicals activate the production of vasoconstrictor metabolites of arachidonic acid, presumably endoperoxides or thromboxane A2, in vascular smooth muscle (Auch-Schwelk et al., 1989; Yang et al., 2002, 2003b; Wolin, 2004). These data reached in functional studies with exogenous hydrogen peroxide suggest that this peroxide, at least, could be the precursor of endothelium-derived contracting factor in the femoral artery of rats with diabetes.
The measurement of oxidative stress by means of fluorescence under confocal microscopy revealed that in the presence of L-NAME, A23187, at a concentration that induced submaximal endothelium-dependent contractions in the functional studies, augmented the release of reactive oxygen species in the endothelial layer, but not in the vascular smooth muscle layer. S18886 (Simonet et al., 1997), a selective blocker of thromboxane prostanoid receptors, did not reduce the fluorescence in the preparation with endothelium. These findings concur with the results in the vascular functional study, confirming the contribution of oxygen-derived free radicals in the endothelium-dependent contractions. Indomethacin reduced this production of reactive oxygen species, as it inhibits the endothelium-dependent contractions observed in organ chamber experiments, indicating that the release of reactive oxygen free radicals evoked by A23187 depends upon endothelial cyclooxygenase. This interpretation is consistent with that reached in the aortic endothelium of the spontaneously hypertensive rat (Tang et al., 2007). Cyclooxygenase, the rate-limiting enzyme in the production of endothelium-derived contracting factor, is upregulated in the aorta of spontaneously hypertensive rats and the femoral artery of diabetic rats (Ge et al., 1995, 1999; Shi et al., 2007). Therefore, the increased generation of reactive oxygen species with A23187 in arteries from STZ-treated but not control rats, in accordance with the augmented endothelium-dependent response in the organ chambers, can be attributed to the enhanced presence of endothelial cyclooxygenase. Thus, the experiments performed under confocal microscopy strongly suggest that oxygen-derived free radicals play a major role in endothelium-dependent contractions in the femoral artery of diabetic rats.
The fact that the potentiating effect of oxygen-derived free radicals on endothelium-dependent contractions is observed only in preparations from STZ-treated but not control rats resembles observations in the aorta of normotensive and spontaneously hypertensive rats (Yang et al., 2003b), suggesting that the redox abnormality is seen only under certain disease conditions.
In the present experiments, the protein expression of Mn-SOD, which is confined to the mitochondria, was decreased in the arteries from the STZ-treated rats, whereas the protein expression of CuZn-SOD, which mainly metabolizes superoxide anion in the cytosol, was comparable, suggesting that the anti-oxidative ability is reduced in the mitochondria in diabetes (Sheu et al., 2005). Both catalase and glutathione take part in the conversion of hydrogen peroxide to water (Wolin, 2000). The net glutathione level was comparable in the two groups, suggesting that a reduction in glutathione level is not a contributing factor to the endothelial dysfunction in diabetes. However, the activity of catalase was decreased in the femoral arteries from STZ-treated rats despite an increased protein presence of the enzyme. Catalase is not efficient in eliminating low levels of hydrogen peroxide because it is difficult to saturate with hydrogen peroxide and its catalytic activity requires the interaction of two hydrogen peroxide molecules with a single active site, which is less likely to occur at low hydrogen peroxide concentrations (Halliwell and Gutteridge, 2000; Rhee et al., 2005). This further suggests that the accumulation of hydrogen peroxide in these arteries can be mainly attributed to a reduced activity of catalase. This interpretation is consistent with the augmented DCF fluorescence observed. Taken together, the present findings thus confirm an increased generation of reactive oxygen species, in particular hydrogen peroxide, in diabetes (Karasu, 1999; Pandolfi et al., 2003).
In summary, the present series of experiments demonstrated a link between oxidative stress and the occurrence of endothelium-dependent contractions in femoral arteries of diabetic rats. The oxygen-derived free radicals potentiated and/or mediated the occurrence of endothelium-dependent contractions by facilitating the production of vasoconstrictor prostanoids.
External data objects
Acknowledgments
This study was supported in part by Research Grants Council Grant HKU 7524 and by the Research Centre of Heart, Brain, Hormonal and Healthy Aging (HBHA) of the University of Hong Kong.
Abbreviations
- DCF
2′,7′-dichlorodihydrofluorescein diacetate
- DETCA
diethyldithiocarbamic acid
- L-NAME
Nω-nitro-L-arginine methyl ester hydrochloride
- MnTMPyP
Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachloride
- S18886
terutroban, 3-[(6-amino-(4-chlorobenzenesulphonyl)-2-methyl-5,6,7,8-tetrahydronapht]-1-yl) propionic acid
- SC19220
2-acetylhydrazide 10 (11 H)-carboxylic acid 8-chloro-dibenz [b,f] [1,4] oxazepine-10 (11 H)-carboxylic acid
- SOD
superoxide dismutase
- STZ
streptozotocin
- Tiron
4,5-dihydroxy-1,3-benzenedisulphonic acid
- TP receptor
thromboxane prostanoid receptor
Conflict of interest
The authors state no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, Jefferson A, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altan VM, Karasu C, Ozuari A. The effects of type-1 and type-2 diabetes on endothelium-dependent relaxation in rat aorta. Pharmacol Biochem Behav. 1989;33:519–522. doi: 10.1016/0091-3057(89)90379-1. [DOI] [PubMed] [Google Scholar]
- Aragno M, Tamagno E, Gatto V, Brignardello E, Parola S, Danni O, et al. Dehydroepiandrosterone protects tissues of streptozotocin-treated rats against oxidative stress. Free Radic Biol Med. 1999;26:1467–1474. doi: 10.1016/s0891-5849(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–864. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Thromboxane A2 receptor antagonists inhibit endothelium-dependent contractions. Hypertension. 1990;15:699–703. doi: 10.1161/01.hyp.15.6.699. [DOI] [PubMed] [Google Scholar]
- Auch-Schwelk W, Katusic ZS, Vanhoutte PM. Nitric oxide inactivates endothelium-derived contracting factor in the rat aorta. Hypertension. 1992;19:442–445. doi: 10.1161/01.hyp.19.5.442. [DOI] [PubMed] [Google Scholar]
- Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA. Does EDCF contribute to diabetic endothelial cell dysfunction? Dialogues Cardiovasc Med. 2002;7:225–231. [Google Scholar]
- Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, et al. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- Ge T, Vanhoutte PM, Boulanger CM. Increased response to prostaglandin H2 precedes changes in PGH synthase-1 expression in the SHR aorta. Zhongguo Yao Li Xue Bao. 1999;20:1087–1092. [PubMed] [Google Scholar]
- Gluais P, Lonchampt M, Morrow JD, Vanhoutte PM, Feletou M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol. 2005;146:834–845. doi: 10.1038/sj.bjp.0706390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Paysant J, Badier-Commander C, Verbeuren T, Vanhoutte PM, Feletou M. In SHR aorta, calcium ionophore A-23187 releases prostacyclin and thromboxane A2 as endothelium-derived contracting factors. Am J Physiol Heart Circ Physiol. 2006;291:H2255–H2264. doi: 10.1152/ajpheart.01115.2005. [DOI] [PubMed] [Google Scholar]
- Greenstock CL, Miller RW. The oxidation of tiron by superoxide anion. Kinetics of the reaction in aqueous solution in chloroplasts. Biochim Biophys Acta. 1975;396:11–16. doi: 10.1016/0005-2728(75)90184-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine 2000Oxford University Press: New York, NY; 3rd edn. [Google Scholar]
- Harris KH, MacLeod KM. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur J Pharmacol. 1988;153:55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- Head RJ, Longhurst PA, Panek RL, Stitzel RE. A contrasting effect of the diabetic state upon the contractile responses of aortic preparations from the rat and rabbit. Br J Pharmacol. 1987;91:275–286. doi: 10.1111/j.1476-5381.1987.tb10282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heygate KM, Lawrence IG, Bennett MA, Thurston H. Impaired endothelium-dependent relaxation in isolated resistance arteries of spontaneously diabetic rats. Br J Pharmacol. 1995;116:3251–3259. doi: 10.1111/j.1476-5381.1995.tb15132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho FM, Lin WW, Chen BC, Chao CM, Yang CR, Lin LY, et al. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell Signal. 2006;18:391–399. doi: 10.1016/j.cellsig.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Karasu C. Increased activity of H2O2 in aorta isolated from chronically streptozotocin-diabetic rats: effects of antioxidant enzymes and enzymes inhibitors. Free Radic Biol Med. 1999;27:16–27. doi: 10.1016/s0891-5849(99)00028-3. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Schugel J, Cosentino F, Vanhoutte PM. Endothelium-dependent contractions to oxygen-derived free radicals in the canine basilar artery. Am J Physiol. 1993;64:H859–H864. doi: 10.1152/ajpheart.1993.264.3.H859. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–H37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- Kedziora-Kornatowska KZ, Luciak M, Paszkowski J. Lipid peroxidation and activities of antioxidant enzymes in the diabetic kidney: effect of treatment with angiotensin convertase inhibitors. IUBMB Life. 2000;49:303–307. doi: 10.1080/15216540050033177. [DOI] [PubMed] [Google Scholar]
- Lüscher TF, Vanhoutte PM. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986;8:344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- Makimattila S, Virkamaki A, Groop PH, Cockcroft J, Utriainen T, Fagerudd J, et al. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- Malendowicz L. The application of tiron (sodium salt of pyrocatechin-3,5-disulphonic acid) as a lead complexing factor in the histochemical reaction for detection of ATPase. Folia Histochem Cytochem (Krakow) 1972;10:227–236. [PubMed] [Google Scholar]
- Maritim AC, Sanders RA, Watkins JB., III Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- Mekinova D, Chorvathova V, Volkovova K, Staruchova M, Grancicova E, Klvanova J, et al. Effect of intake of exogenous vitamins C, E and beta-carotene on the antioxidative status in kidneys of rats with streptozotocin-induced diabetes. Nahrung. 1995;39:257–261. doi: 10.1002/food.19950390402. [DOI] [PubMed] [Google Scholar]
- Mulhern M, Docherty JR. Effects of experimental diabetes on the responsiveness of rat aorta. Br J Pharmacol. 1989;97:1007–1012. doi: 10.1111/j.1476-5381.1989.tb12555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr-Esfahani MH, Aitken JR, Johnson MH. Hydrogen peroxide levels in mouse oocytes and early cleavage stage embryos developed in vitro or in vivo. Development. 1990;109:501–507. doi: 10.1242/dev.109.2.501. [DOI] [PubMed] [Google Scholar]
- Omar HA, Cherry PD, Mortelliti MP, Burke-Wolin T, Wolin MS. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ Res. 1991;69:601–608. doi: 10.1161/01.res.69.3.601. [DOI] [PubMed] [Google Scholar]
- Pagano PJ, Tornheim K, Cohen RA. Superoxide anion production by rabbit thoracic aorta: effect of endothelium-derived nitric oxide. Am J Physiol. 1993;265:H707–H712. doi: 10.1152/ajpheart.1993.265.2.H707. [DOI] [PubMed] [Google Scholar]
- Pandolfi A, Grilli A, Cilli C, Patruno A, Giaccari A, Di Silvestre S, et al. Phenotype modulation in cultures of vascular smooth muscle cells from diabetic rats: association with increased nitric oxide synthase expression and superoxide anion generation. J Cell Physiol. 2003;196:378–385. doi: 10.1002/jcp.10325. [DOI] [PubMed] [Google Scholar]
- Pasternack RF, Sidney D, Hunt PA, Snowden EA, Gibbs EJ. Interactions of water soluble porphyrins with Z-poly(dG-dC) Nucleic Acids Res. 1986;14:3927–3943. doi: 10.1093/nar/14.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflueger AC, Gross JM, Knox FG. Adenosine-induced renal vasoconstriction in diabetes mellitus rats: role of prostaglandins. Am J Physiol. 1999;277:R1410–R1417. doi: 10.1152/ajpregu.1999.277.5.R1410. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Gross GJ. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1988;255:H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Langenstroer P, Siebeneich W. Diabetic-induced endothelial dysfunction in rat aorta: role of hydroxyl radicals. Cardiovasc Res. 1997;34:145–156. doi: 10.1016/s0008-6363(96)00237-4. [DOI] [PubMed] [Google Scholar]
- Rauscher FM, Sanders RA, Watkins JB., III Effects of isoeugenol on oxidative stress pathways in normal and streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2001;15:159–164. doi: 10.1002/jbt.13. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Yang KS, Kang SW, Woo HA, Chang TS. Controlled elimination of intracellular H(2)O(2): regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid Redox Signal. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- Samata K, Kimura T, Satoh S, Watanabe H. Chemical removal of the endothelium by saponin in the isolated dog femoral artery. Eur J Pharmacol. 1986;128:85–91. doi: 10.1016/0014-2999(86)90561-3. [DOI] [PubMed] [Google Scholar]
- Sheu ML, Ho FM, Yang RS, Chao KF, Lin WW, Lin-Shiau SY, et al. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005;25:539–545. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
- Shi Y, Feletou M, Ku DD, Man RYK, Vanhoutte PM. The calcium ionophore A23187 induces endothelium-dependent contractions in femoral arteries from rats with streptozotocin-induced diabetes. Br J Pharmacol. 2007;150:624–632. doi: 10.1038/sj.bjp.0706999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ku DD, Man RY, Vanhoutte PM. Augmented endothelium-derived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. J Pharmacol Exp Ther. 2006;318:276–281. doi: 10.1124/jpet.105.099739. [DOI] [PubMed] [Google Scholar]
- Simonet S, Descombes JJ, Vallez MO, Dubuffet T, Lavielle G, Verbeuren TJ. S 18886, a new thromboxane (TP)-receptor antagonist is the active isomer of S 18204 in all species, except in the guinea-pig. Adv Exp Med Biol. 1997;433:173–176. doi: 10.1007/978-1-4899-1810-9_35. [DOI] [PubMed] [Google Scholar]
- Tang EH, Feletou M, Huang Y, Man RY, Vanhoutte PM. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am J Physiol Heart Circ Physiol. 2005;289:H2434–H2440. doi: 10.1152/ajpheart.00568.2005. [DOI] [PubMed] [Google Scholar]
- Tang EH, Leung FP, Huang Y, Feletou M, So KF, Man RYK, et al. Calcium and reactive oxygen species increase in endothelial cells in response to releases of endothelium-derived contracting factor. Br J Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N, Takahashi M, Sakiyama H, Park YS, Asahi M, Misonou Y, et al. A common pathway of intracellular reactive oxygen species production by glycoxidative and nitroxidative stress in vascular endothelial cells and smooth muscle cells. Ann NY Acad Sci. 2005;1043:521–528. doi: 10.1196/annals.1333.059. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Cohen RA. Contractions to oxygen-derived free radicals are augmented in aorta of the spontaneously hypertensive rat. Hypertension. 1989;13:859–864. doi: 10.1161/01.hyp.13.6.859. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Jakubowski JA, Cohen RA. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989;257:H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Vallejo S, Angulo J, Peiro C, Sanchez-Ferrer A, Cercas E, Llergo JL, et al. Prevention of endothelial dysfunction in streptozotocin-induced diabetic rats by gliclazide treatment. J Diabetes Complications. 2000;14:224–233. doi: 10.1016/s1056-8727(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigg SJ, Tare M, Tonta MA, O'Brien RC, Meredith IT, Parkington HC. Comparison of effects of diabetes mellitus on an EDHF-dependent and an EDHF-independent artery. Am J Physiol Heart Circ Physiol. 2001;281:H232–H240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]
- Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol. 2000;20:1430–1442. doi: 10.1161/01.atv.20.6.1430. [DOI] [PubMed] [Google Scholar]
- Wolin MS. Subcellular localization of Nox-containing oxidases provides unique insight into their role in vascular oxidant signaling. Arterioscler Thromb Vasc Biol. 2004;24:625–627. doi: 10.1161/01.ATV.0000117201.14603.5d. [DOI] [PubMed] [Google Scholar]
- Wong RK, Pettit AI, Quinn PA, Jennings SC, Davies JE, Ng LL. Advanced glycation end products stimulate an enhanced neutrophil respiratory burst mediated through the activation of cytosolic phospholipase A2 and generation of arachidonic acid. Circulation. 2003;108:1858–1864. doi: 10.1161/01.CIR.0000089372.64585.3B. [DOI] [PubMed] [Google Scholar]
- Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, et al. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Feletou M, Levens N, Zhang JN, Vanhoutte PM. A diffusible substance(s) mediates endothelium-dependent contractions in the aorta of SHR. Hypertension. 2003a;41:143–148. doi: 10.1161/01.hyp.0000047651.45322.16. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Nitric oxide and inactivation of the endothelium-dependent contracting factor released by acetylcholine in spontaneously hypertensive rat. J Cardiovasc Pharmacol. 2004a;43:815–820. doi: 10.1097/00005344-200406000-00011. [DOI] [PubMed] [Google Scholar]
- Yang D, Gluais P, Zhang JN, Vanhoutte PM, Feletou M. Endothelium-dependent contractions to acetylcholine, ATP and the calcium ionophore A 23187 in aortas from spontaneously hypertensive and normotensive rats. Fundam Clin Pharmacol. 2004b;18:321–326. doi: 10.1111/j.1472-8206.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Yang D, Levens N, Zhang JN, Vanhoutte PM, Feletou M. Specific potentiation of endothelium-dependent contractions in SHR by tetrahydrobiopterin. Hypertension. 2003b;41:136–142. doi: 10.1161/01.hyp.0000047669.93078.a7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.