Abstract
Background and purpose:
Ototoxicity is a known adverse effect of cisplatin (CDDP). Since apoptosis is involved in the development of some pathological conditions associated with the administration of anticancer drugs, we examined, using immunohistochemical and electrophysiological techniques, the apoptotic changes in the cochlea of Sprague-Dawley (SD) rats after an injection of CDDP (5 mgkg-1 body weight).
Experimental approach:
Luciferase assays were used to determine the different caspase activities and ATP levels in protein extracts of whole cochleae. The expression of several apoptotic-related proteins was measured by means of Western blotting. These analyses were performed 2, 7 and 30 days after the CDDP injection. The auditory brain stem response was obtained before and at the different times after the injection of CDDP, before the animals were killed.
Key results:
CDDP significantly increased the levels of caspase-3/7 activity and active caspase-3 protein expression and caspase-3 immunofluorescence staining, caspase-9 activity, and Bax protein expression but decreased Bcl-2 protein expression within the rat cochleae. Threshold shifts were significantly elevated 2 days after CDDP treatment.
Conclusions and implications:
These findings support the hypothesis that cisplatin-related apoptosis evokes an intrinsic pathway of pro-apoptotic signalling within the rat cochleae. Thus, selective inhibition of the sequence of events involved in the intrinsic apoptotic pathway could provide a strategy to minimize cisplatin-induced ototoxicity.
Keywords: cisplatin, ototoxicity, apoptosis, caspase-3, caspase-9
Introduction
Cisplatin (cis-diamminedichloroplatinum II; CDDP) is a first-generation platinum-containing anticancer drug, known to be effective against a variety of solid tumours. Its anticancer effect is obtained by several mechanisms, including formation of DNA adducts and production of reactive oxygen species (ROS). Second-generation platinum-derived drugs have comparable anticancer effects to cisplatin and although they have several side effects such as myelosuppression, they induce less nephrotoxicity and ototoxicity than CDDP.
Ototoxicity has been observed in up to 36% of patients receiving cisplatin. These patients present with tinnitus and/or hearing loss in high frequencies; but cisplatin can also affect the speech frequencies (Nagy et al., 1999). Although the dose of cisplatin given is an important factor in achieving optimal antineoplastic effects (Bohm et al., 1999), high doses can cause serious side effects, for example, peripheral neuropathies, renal insufficiency and sensorineural hearing loss. Hearing impairment is dose related, cumulative, bilateral and usually permanent. Initially, it is characterized by a high-frequency deficit, but in patients receiving repeated doses of cisplatin, this progressively extends towards frequencies that are important for speech perception. Cochlear damage is one of the most common reasons for interrupting chemotherapy with this drug.
The most prominent change seen in the cochlea after cisplatin administration consists of loss of outer hair cells (OHCs), initially in the third row of OHC stereocilia bundles at basal turn (tip links, disarrayed or fused, decreased in number). Several studies have demonstrated morphological damage in the supporting cells (Estrem et al., 1981; Anniko and Sobin, 1986; Watanabe and Yagi, 2000). Supporting cells (Deiter's cells) appeared more sensitive than OHCs, and the alteration of the supporting cell ultrastructure preceded detectable changes in OHCs (Ramírez-Camacho et al., 2004).
Programmed cell death or apoptosis has been associated with the administration of several anticancer drugs, including CDDP (Lee et al., 2004; Matsuhashi et al., 2005). This active form of cell death is characterized by morphological changes such as cell shrinkage, chromatin condensation and fragmentation of DNA, which often requires the synthesis of new proteins called caspases (cysteine-requiring aspartate proteases).
The cytotoxic effects of CDDP are due to coordinative bonds between the atom of platinum and DNA and formation intrastrand DNA crosslinks (McKeage, 1995). Apoptotic death of OHCs has emerged as a final common pathway in response to ischaemia, loss of trophic factor support and ototoxins such as aminoglycoside and cisplatin (Huang et al., 2000). However, the intracellular pathways involved in the stimulus recognition, signal transduction and execution phases of OHCs apoptosis following cisplatin exposure remain unknown. Likewise, inner ear cells are also injured by the generation of free radicals and antioxidant depletion; they cause oxidative injury in the cochlea of the rat (Husain et al., 2001). The two major intracellular apoptosis pathways are the cell-surface death receptor or extrinsic pathway, that is, Fas and Tumor necrosis factor receptor superfamily member 1 (TNFR1)-mediated apoptosis, (involving procaspase-8 interactions) (Ashkenazi and Dixit, 1998), and the mitochondrion-initiated or intrinsic pathway, regulated by the members of the B-cell lymphoma 2 (Bcl-2) family (involving procaspase-9 interactions) (Harris and Thompson, 2000). Once activated, both caspases-8 and caspase-9 participate in a cascade that culminates in the activation of caspase-3 that cleaves several substrates, resulting in chromosomal DNA fragmentation and the cellular morphological changes characteristic of apoptosis (Goldstein et al., 2000). Cross-talk has been demonstrated between the two procaspase pathways (Li et al., 1998).
The aim of this study was to explore the effects of cisplatin on the apoptosis of rat cochlear cells, and thereby, to characterize the possible proapoptotic signalling pathways involved, to design strategies to minimize cisplatin-induced ototoxicity.
Methods
Animals
Thirty-six female Sprague–Dawley rats from Harlan (Harlan Iberica, Barcelona, Spain) weighing between 180 and 280 g were used in the present study. After transportation, the animals were maintained in the central animal laboratory for at least 1 week. All animals were housed in plastic cages with water and food available ad libitum, and maintained on a 12-h light/dark cycle. Rats with inner ear infection were not used. Cisplatin 5 mg kg−1 in 1.0 ml normal solution (0.9% NaCl) was injected intraperitoneally. Animals were then observed for poor food and water intake and divided into three groups as follows: (1) (n=10) rats were killed 2 days after CDDP administration, (2) (n=10) animals were killed 7 days after CDDP treatment and (3) (n=10) were killed 30 days after CDDP injection. In the control group (n=6), only 10 ml kg−1 NaCl 0.9% w/v was injected; animals were killed at 2 days (n=2), at 7 days (n=2) and at 30 days (n=2).
Auditory brainstem response measurements
Preyer's reflex and otoscopy were used to confirm that middle ears were normal. Evoked potentials were recorded before drug administration and before the animals were killed. Rats were anaesthetized with an intraperitoneal injection of ketamine 100 mg kg−1, diazepam 0.1 mg kg−1 and atropine 0.02–0.04 mg kg−1. Subdermal platinum-alloy needle electrodes were attached, with the active lead at the vertex and referred to a second electrode located at the tip of the nose. The ground electrode was placed on the arm muscles. Auditory brainstem response (ABR) stimuli were generated using a Medelec Synergy System (Oxford Instruments Medical Systems, Surrey, UK); 10-ms tone burst stimuli (8 kHz) were delivered monaurally through a hollow rat ear bar. Tone bursts (rise–fall time 2 ms, duration 10 ms) were delivered at the rate of 20 s−1, with increasing intensity from 10 to 80 dB sound–pressure level in 5-dB steps; 1500 trials were averaged to assure an adequate brain response. The lowest response that clearly demonstrated a reproducible waveform was interpreted as the threshold response.
Preparation of tissues
After fixation by intralabyrinthine perfusion of 4% paraformaldehyde (pH 7.4), cochleae were removed. Right cochleae were used for the immunohistochemical study, and then were incubated in the same fixative overnight. Decalcification was performed in ethylenediamine tetraacetic acid solution (10% in phosphate-buffered saline) for 3–4 weeks. Subsequently, the tissues were embedded in paraffin.
Structure examination
Each paraffin-embedded specimen was sectioned at a thickness of 5 μm. The paraffin was removed by immersion in graded series of ethanol (from 100 to 70 °C), and then each section was stained with a haematoxylin–eosin solution and observed with an Olympus BX-51 microscope (Olympus, Tokyo, Japan).
Immunofluorescence examination
Although this study does not constitute a main objective of the present investigation, preliminary results may support and implicate the role of caspase-3 in the toxic effect of CDDP on the different cell populations of the inner ear. For immunofluorescence analysis, paraffin was removed from the sections before they were incubated with an antibody against active caspase-3 protein. Paraffin was washed from the cochlear sections, as indicated above, before they were embedded into a citrate buffer (0.1 M citric acid plus 0.1 M sodium citrate in bi-distilled water) and heated in a microwave. Specimens were subsequently washed and incubated with phosphate buffered saline 0.1 M solution (vol/vol)+0.3% (vol/vol) Tween-20 and 5% bovine serum albumin for 60 min at 37 °C. After that, sections were incubated with caspase-3 antibody Ab13847 (dilution 1/500) diluted in phosphate buffered saline+1% bovine serum albumin for 60 min at 37 °C. Secondary antibody Alexa Fluor (R) 546 (dilution 1/1500) was added for 45 min at 37 °C. Specimens were embedded in Moviol 4–88 supplemented with 300 nM of 49, 6-diamidino-2-phenylindole. Sections were observed with an Olympus BX-51 epifluorescence microscope. All tissue sections were counterstained with haematoxylin–eosin.
Western blotting and protein content
Extraction of protein homogenates and western blotting were performed as described previously (Nevado et al., 2006). Briefly, protein from rat cochleae was extracted in lysis buffer containing 10 mM Tris pH 7.4, 1% sodium dodecyl sulphate, 10 mM orthovanadate, 2 mM phenylmethylsulphonyl fluoride and 12.5 μg ml−1 aprotinin. Total protein extracts were diluted 3:1 in 4 × Laemmli's buffer and boiled for 5 min. Proteins were loaded equally (10 μg per lane), separated on 8% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gels and transferred onto a nitrocellulose membrane. After being blocked overnight at 4 °C in 0.2% Tween-20 and 5% non-fat dry milk, the membrane was incubated for 1 h at room temperature, with a polyclonal antibody against either Bcl-2 (Ab7973, dilution 1/200), or monomeric Bcl-2-associated X protein (Bax) (Ab16837, dilution 1/200), or active caspase-3 proteins (Ab13847, dilution 1/200), followed by incubation for 45 min with the respective anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (dilution 1/10 000). Immunoreactive bands were detected using an enhanced chemiluminiscence detection kit and quantified using Chemi-Imager 5.5 software from AlphaInnotec (San Leandro, CA, USA). The data from the densitometric analysis are representative of the mean results from four experiments.
Measurements of activity of different caspases, and quantification of ATP levels
Left cochleae were used to analyse different apoptosis-related caspase activities. Thus, protein extracts of whole cochlea were obtained by using mammalian protein extraction reagent, with the addition of Complete mini protease inhibitor cocktail (Boehringer Mannheim GmbH, Mannheim, Germany), according to the manufacturer's instructions. The homogenates were treated for 3 min with a homogenizer ultra-Turrax T8 (IKA, Staufen, Germany) followed by 1 min on ice. This treatment was repeated twice. The lysate was cleared by centrifugation at 16 100 g for 10 min at 4 °C to pellet the cell debris, and the supernatant was used or immediately stored at −80 °C. The protein concentration was determined by the BCA protein assay. For the quantification of different caspase activities, different luminescent assays were used according to the manufacturer's instructions; each assay provides a specific pro-luminescent substrate, which contains tetrapeptide sequences, DEVD (for caspase-3/7), LETD (for caspase-8) and LEHD (for caspase-9) that have been shown to be selective for caspase-3/7, caspase-8 and caspase-9, respectively (Thornberry et al., 2000). Luminescence is proportional to the amount of respective caspase activities present, and 10 μg proteins were utilized to normalize.
The CellTiter-Glo reagent was used to measure ATP in whole cell protein extracts by following the manufacturer's protocol, and 10 μg of protein were utilized to normalize the results.
Measurement of total superoxide dismutase activity
To analyse the antioxidant enzyme activity in cisplatin-induced ototoxicity, total superoxide dismutase (SOD) activity was measured in whole rat cochlear extracts by using an SOD activity kit, a colorimetric method. This kit measures all the three types of SOD (Cu/Zn-, Mn- and Fe-SOD), and utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine in these tissue homogenates. SOD activity is standardized using the cytochrome c- and xanthine oxidase-coupled assay. One unit of activity is defined as the activity of enzyme required to inhibit the production of formazan by 50%.
Ethics
The study was carried out in accordance with the guidelines for research involving animals (the Spanish Animal Care and Use Committee), and was approved by the Clinical Research and Ethics Committee of Hospital Universitario Puerta de Hierro (Exp. PI050673, 28 June 2005).
Statistical analyses
Results are expressed as mean±s.e.mean. The statistical analysis was carried out using the Stat-View statistics programme (Abacus Concepts Inc., Berkeley, CA, USA). Differences between the means or in the variance were evaluated using the factorial analysis of variance, followed by Fisher's protected least-significance-test, with the level of significance set at P⩽0.05. The sample size and statistical power of the study (95%) were calculated using the C4 Study Design Pack program (GSK Biometry Department, Madrid, Spain).
Materials
The following materials were used in the experiments: cisplatin (Ferra Farma, S.A., Barcelona, Spain), Ab13847 (AbCam, Cambridge, UK), Alexa Fluor (R) 546 (Molecular Probes/Invitrogen Ltd, Paisley, UK), Moviol 4–88 (Hoechst Pharmaceuticals, Frankfurt, Germany), nitrocellulose membrane (Bio-Rad Laboratories, Madrid, Spain), polyclonal and secondary antibodies (AbCam), enhanced chemiluminiscence detection kit (Amersham, Arlington Hills, IL, USA), mammalian protein extraction reagent (Pierce, Rockford, IL, USA), Complete mini protease inhibitor cocktail (Boehringer Mannheim GmbH), Bicinchoninic acid (BCA) protein assay (Pierce), luminescent assays (Caspase-Glo-3/7; Caspase-Glo-8 and Caspase-Glo-9; Promega, Madison, WI, USA), CellTiter-Glo reagent (Promega) and SOD activity kit (Cayman Chemical Company, Ann Arbor, MI, USA).
Results
Threshold shifts of ABR
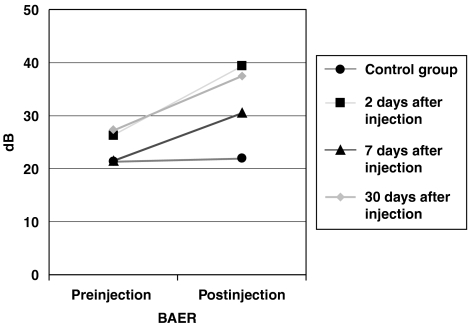
The ABR thresholds were elevated in animals from all groups after CDDP treatment (Figure 1). ABR thresholds from the 2-day treatment group were elevated from 21.5±7 (mean±s.d.) to 30.5±5.9 dB. At 7 days, ABR examination showed an increase from 26.2±6.7 to 39.3±16.1 dB. In the 30-day group ABR was elevated from 27.2±8.5 to 37.5±14 dB. However, this increase was only significant in the 2-day group (P⩽0.05). No changes in the threshold were apparent in the control group.
Figure 1.
Threshold shifts of ABR. The ABR threshold shift was significantly elevated only in the animals killed 2 days after cisplatin (CDDP) administration. Animals from the 7-day and 30-day groups showed a non-significant increase in the ABR threshold shift. ABR, auditory brainstem response; CDDP, cis-diamminedichloroplatinum II.
Cisplatin-related apoptosis in the rat cochleae
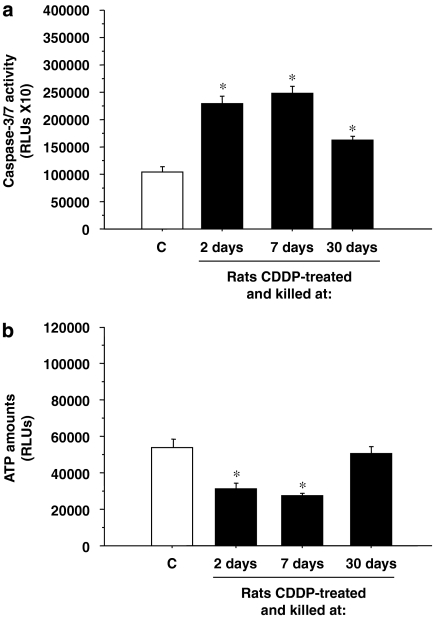
We monitored caspase-3/7 activities as an early indicator of apoptotic cell death in whole-protein extracts from rat cochleae, at different times after CDDP injection. The results presented in Figure 2a show that caspase-3/7 activities in the protein extracts clearly increased with the CDDP treatment. Thus, rats from both the 2-day and 7-day groups showed increased caspase-3/7 activity compared with the control groups (P⩽0.001). However, rats from the 30-day group showed a decreased caspase-3/7 activity compared with the 7-day and 3-day groups, although the difference from the control group was still statistically significant (P⩽0.001). In addition, ATP levels present in these extracts clearly decreased with time (Figure 2b). Again in the 30-day group the ATP was no longer decreased, but had almost returned to control levels.
Figure 2.
Cisplatin-related apoptosis in rat cochleae. Caspase-3/7 activity (a) and ATP levels (b) were measured in whole cochlea protein extracts of rats killed 2, 7 or 30 days after CDDP injection. Results are expressed as RLUs (relative light units); *P⩽0.05 vs control group. CDDP, cis-diamminedichloroplatinum II.
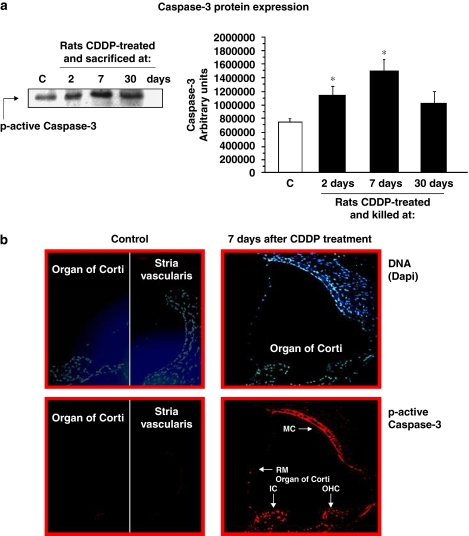
We also analysed ‘active p-17' caspase-3 protein expression by western blotting. A representative western blot is shown in Figure 3a; the results obtained confirmed a higher active caspase-3 protein expression with CDDP treatment. Densitometric analyses of at least four independent experiments confirmed that CDDP treatment increased caspase-3 protein expression in the rat cochleae. The amount of caspase-3 expressed was significantly increased compared with the control group in both the 2-day and 7-day cisplatin-treated groups (Figure 3a). The last group that was killed 30 days after CDDP treatment also showed an increase in the active caspase-3 protein expression, but this was not statistically significant (Figure 3a).
Figure 3.
Active caspase-3 expression. (a) A representative western blot of active p-17 caspase-3 protein expression levels in whole cochlea extracts of rats killed at 2, 7 or 30 days after CDDP injection is shown (left). Densitometric analyses of previous western blots are also shown (right). Results are expressed as arbitrary units; *P⩽0.05 vs control. (b) Immunostaining of active caspase-3 in the nuclei of inner ear cells from rats 7 days after a single dose of cisplatin (5 mg kg−1). Cryosections from the cochleae of exposed animals were stained (see Methods section). Increased nuclear caspase-3 immunostaining is seen in several cell populations after administration of cisplatin. MC, marginal cells of stria vascularis; RM, Reissner's membrane; OHCs, outer hair cells; IC, interdental cells of the spiral limbus. Magnification × 400. CDDP, cis-diamminedichloroplatinum II.
Caspase-3 immunofluorescence staining
We selected the cochlear sections from animals killed at 7 days after CDDP administration, since they presented the highest caspase-3 activity in the whole cochlear extracts. Preliminary results showed caspase-3 activity in different cell populations of the organ of Corti, such as the interdental cells of the spiral limbus, inner sulcus cells, inner and OHCs and Hensen's cells. Likewise, there was a more intense staining for caspase-3 in Reissner's membrane and marginal cells of the stria vascularis, in comparison with animals from the control group (Figure 3b).
Cisplatin-associated cochlear apoptosis in the rat involved an ‘intrinsic'mitochondria-initiated cell-death pathway
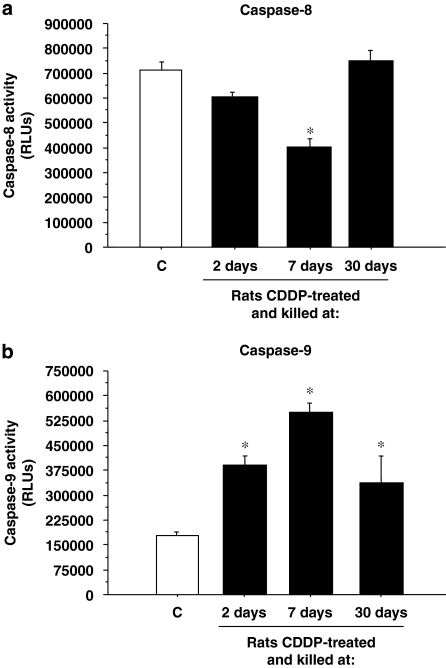
To distinguish the apoptotic signalling pathways activated by CDDP treatment in the rat cochleae, caspase-8 and caspase-9 activities as initiator caspases were determined by specific luminescence assays. Figure 4a shows that only a significant time-dependent decrease in caspase-8 activity was observed in the 7-day group. On the other hand, the group killed 30 days after the treatment showed levels of caspase-8 slightly higher than controls. Likewise, caspase-9 activity was clearly increased by CDDP administration. An increased glow-luminescence was seen in all three treated groups, and this was maximum in the 7-day group (Figure 4b).
Figure 4.
Caspase-8 and caspase-9 activities. Caspase-8 (a) and caspase-9 (b) activities were measured in rat cochlea protein extracts of rats killed at 2, 7 or 30 days after CDDP injection. Results are expressed as RLUs (relative light units); *P⩽0.05 vs control group. CDDP, cis-diamminedichloroplatinum II.
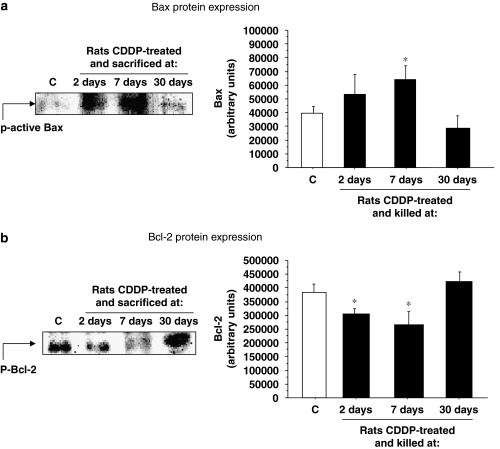
To confirm the involvement of the intrinsic pathway of proapoptotic signalling, we also determined Bcl-2 and Bax protein expressions by western blotting. Bcl-2 is a member of Bcl-2 family of proteins, which normally inhibits cell death by blocking the binding of caspase-9 to the apoptotic protease activating factor 1 complex. Figure 5b shows that Bcl-2 protein expression is higher in protein extracts from control rats (392 434±22 963 arbitrary units), than in the extracts from the rats treated with cisplatin for 2 and 7 days. However, in the 30-day group the expression of this protein was lower than in the control group. In contrast, Bax protein expression (a proapoptotic protein) was clearly increased by CDDP treatment in the 2-day and 7-day groups (Figure 5a). The last group, which was killed at 30 days after CDDP treatment, showed no significant differences from the control group (Figure 5a).
Figure 5.
Bax and Bcl-2 protein expressions. Representative western blots of active Bax (a, left) or Bcl-2 (b, left) protein expressions in whole cochlea extracts of rats killed at 2, 7 or 30 days after CDDP injection are shown. Densitometric analyses of previous western blots are also shown (right). Results are expressed as arbitrary units; *P⩽0.05 vs controls. CDDP, cis-diamminedichloroplatinum II.
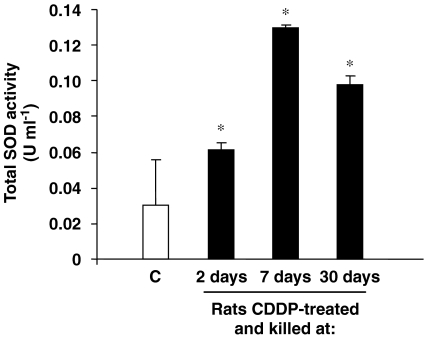
CDDP administration changes the level of total SOD activity inside the cochlea
A significant increase in the total SOD activity in whole cochlear extracts was observed in the animals from the CDDP-treated groups compared with controls (Figure 6). Likewise, differences between CDDP groups were also statistically significant (for 2 days after treatment rats vs either 7-day or 30-day rats; P⩽0.0001 and P=0.0002 respectively; or 7 days after treatment rats vs 30 days; P=0.04).
Figure 6.
CDDP treatment modulates total SOD activity in rat cochleae. Total SOD activity is measured in whole cochlear extracts of rats killed at 2, 7 or 30 days after CDDP injection. Results are expressed as U ml−1. One unit of activity is defined as the activity of enzyme required to inhibit the production of formazan by 50%; *P⩽0.05 vs control group. CDDP, cis-diamminedichloroplatinum II; SOD, superoxide dismutase.
Discussion and conclusions
Although the ototoxic effect of cisplatin has been extensively studied, the cellular mechanisms by which cisplatin induces loss of OHCs, and subsequent degeneration of the organ of Corti remains elusive. Furthermore, the cellular sites of cisplatin uptake and accumulation in the cochlea have not yet been properly identified. It is commonly accepted that binding of cisplatin to DNA results in the formation of cisplatin–DNA adducts that produce severe distortions in the DNA double helix. This antineoplastic effect is mediated by the platination of nucleophilic centres in DNA bases, leading to intra- or inter-strand crosslinking of the bases, to abnormal base pairing or to DNA strand breakage. One important way by which cisplatin–DNA adducts may kill cells is by the induction of programmed cell death or apoptosis (Lee et al., 2004). A high accumulation of platinum–DNA adducts has been observed in the nuclei of marginal cells of the stria vascularis, but not in OHCs that have been discussed as the main target of cisplatin-induced cell damage (Thomas et al., 2006). The augmented adduct levels in the nuclei of marginal cells are not caused by their inability to repair such DNA lesions (Siddik, 2003). When disregarding the impact of repair mechanisms, the level of DNA platination products is directly linked to the intracellular concentration, which can be increased by both excessive influx and decreased export. For the platinum drugs such membrane transport mechanisms are not yet fully understood. The excessive DNA platination in the marginal cells represents the earliest event in short-term cisplatin ototoxicity triggering their functional impairment and apoptotic destruction. Marginal cell damage may lead to an impaired uptake of K+ from the intrastrial space, as well as an impaired K+ secretion into the endolymph with subsequent dysfunction and loss of hair cells. The impairment of K+-recycling process that is essential to the endocochlear potential, is a toxic effect of cisplatin induced by apoptosis of type I spiral ligament fibrocytes (Liang et al., 2005) and by the direct apoptotic effect of cisplatin on the stria vascularis, responsible for the decreased cellular Na+, K+ ATPase and Ca2+ ATPase activities (Cheng et al., 2005).
Apoptosis in the stria vascularis cells on day 3 following CDDP treatment has been suggested by Lee et al. (2004). Morphologically, oedema formation in the stria vascularis was followed by severe atrophy after CDDP treatment. The majority of marginal cells affected by CDDP exhibited expression of cleaved caspase-3, indicating that caspases are involved in the process of apoptotic cell death after CDDP treatment. However, they showed expression of cleaved caspase-9, indicating that apoptosis was initiated by permeabilization of mitochondrial membranes. Our data pointed to the existence of caspase-mediated apoptosis after CDDP treatment within the rat cochlea, by different approaches. Quantification of the ATP levels (showing a significant decreased with CDDP administration) in the whole cochlea protein extracts also supported these results. Decreased levels of ATP have been found in apoptotic cells, according with an increased energy requirement by cells undergoing cell death by apoptosis (Eguchi et al., 1997). Our data also support the involvement of the ‘intrinsic' mitochondrial-initiated cell death-signalling pathway by CDDP in rat cochleae. Thus, cochlear extracts exhibited a progressively higher expression of caspase-9, with a maximum on the seventh day and a decline by the 30th day after CDDP treatment.
Interestingly, we showed the existence of CDDP-dependent caspase-9 activation, relating to a clear modulation of protein expression levels of Bax, involved in the control and execution of the intrinsic mitochondrial cell-death pathway. Our results implicating Bax complement previous studies showing overexpression of Bax and downregulation of Bcl-2 in the organ of Corti of gerbils exposed to cisplatin (Alam et al., 2000). Although previous studies have shown that initial activation of caspase-8 can induce caspase-9 activation (Li et al., 1998), we did not observed activation of caspase-8.
Animals with a long-term survival after the cisplatin injection (30-day group) presented caspases activities, Bcl-2 and Bax protein expression and ATP levels similar to those observed in the control group. A plausible hypothesis could be that once the initial toxic stimulus has occurred, cells develop repair mechanisms and, consequently, the apoptosis ceases. Previous results suggesting that the toxic drug initially affects the stria vascularis and/or the supporting cells (Deiter's and Hensen's), leading to an impaired uptake and secretion of K+ into the endolymph, and an impairment of the metabolic homeostasis of outer and inner hair cells, respectively, that experience structural and functional lesions (Ramírez-Camacho et al., 2006), require a profound re-analysis, since the present study has shown a probable implication of many cell types. The biochemical mechanisms by which cisplatin activates the intrinsic apoptotic pathway remain incompletely understood. Although the interaction of cisplatin with nuclear DNA may have important cellular effects contributing to apoptosis, mitochondria may be the principal and only target of cisplatin (Devarajan et al., 2002; Cardinaal et al., 2004).
Cisplatin may directly lead to the generation of ROS or may induce the release of reactive oxygen molecules normally sequestered within mitochondria that may trigger several mechanisms of apoptosis. ROS-mediated damage could occur as a consequence of antioxidant depletion and increased lipid peroxidation in the cochlea of rats (Rybak et al., 1999). Unpublished data from our investigation show a high ROS activity in cochlear extracts from controls very similar to that observed in animals treated with CDDP. This finding is consistent with the incessant metabolic production of ROS observed in all cell types of the inner ear, independent of exposure to cisplatin (Evans and Halliwell, 1999). Furthermore, compared with basal levels in untreated control cochleae, no additional formation of the DNA oxidation product 8-oxoguanine was detectable after cisplatin treatment in the DNA of either inner ear cell type (Thomas et al., 2006). Our results, however, do not completely rule out the concept of free radical-induced damage to other cell organelles.
Total SOD activity analysis presented a significantly increase of this free-radical scavenger. This finding could be relevant since we used a low CDDP dose (5 mg kg−1) to reproduce the therapeutic administration usually used in humans; in our model, cells will initially increase the antioxidant enzymes to minimize ROS-induced damage. A depletion of SOD was observed in animal models that have used a higher dose (16 mg kg−1) (Rybak et al., 1999). Another emerging problem concerning the use of high doses of cisplatin is the different cell death-induced pattern; at lower doses, the proapoptotic initiator caspases-8 and caspase-9, and the executioner caspase-3 are activated and result in apoptosis. In contrast, higher doses of cisplatin are accompanied by a decline in caspase-3 activity and widespread cell necrosis (Lieberthal et al., 1996).
According to our immunofluorescence analysis, preliminary results after CDDP administration showed that caspase-3 expression was stimulated in diverse cell populations inside the cochlea. Traditionally, the main role in cisplatin-induced ototoxicity has been attributed to OHCs. However, our findings demonstrate that cell types other than OHCs may be implicated. Thus, the interdental cells of the spiral limbus, inner sulcus cells, inner and OHCs and Hensen's cells presented significant caspase-3 expression after CDDP injection. Likewise, Reissner's membrane and marginal cells of the stria vascularis also showed such as expression. These latter findings have also recently been observed in guinea pig cochleae after short-term exposure to cisplatin, by detecting fragments of single-stranded DNA (Watanabe et al., 2002) and guanine–guanine intrastrand crosslinks in DNA (Thomas et al., 2006). These authors have suggested that marginal cells of the stria vascularis have a major role in the early events of cisplatin ototoxicity. The more extensive effects observed in our study could be due to the different times used to analyse the effects of the drug: 7 days (present study) vs 1–3 days (Lee et al., 2004; Thomas et al., 2006), following cisplatin administration. The results from of our study indicate that a profound re-examination of the possible role of the apoptosis of different inner ear cells following cisplatin treatment should be undertaken.
One restrictive factor for the study of the potential therapies used to limit the ototoxicity is the functional and morphological recovery of the organ of Corti after cessation of cisplatin administration (Cardinaal et al., 2000). This finding suggests several hypotheses: an intrinsic regenerative mechanism (Cardinaal et al., 2000), an efficient drug clearance system inside the inner ear and/or a step-by-step damage of the cochlea (Ramírez-Camacho et al., 2004). Thus, the reversal or cessation of the damage to the stria vascularis marginal cells and spiral ligament fibrocytes could prevent injury of the organ of Corti. This hypothesis may have clinical implications, since recovery of cisplatin-induced hearing loss has been reported to occur in some patients. Likewise, the inter-individual variability in susceptibility to the ototoxic effect observed in our animals could also be explained by the previous hypotheses. A variety of antioxidants have been shown to attenuate cisplatin-induced apoptosis in the organ of Corti (Lopez-Gonzalez et al., 2000; Rybak and Kelly, 2003). Thus, several strategies have been suggested to prevent the oxidative stress-induced apoptosis of OHCs that have been exposed to cisplatin: prevention of the formation of ROS either by binding the toxin or reversing the binding of the toxin, inhibition of the lipid peroxidation, addition of exogenous free-radical scavengers and antioxidant enzymes, inhibitors of caspases and gene therapy (to upregulate antiapoptotic gene products such as Bcl-2) (Lopez-Gonzalez et al., 2000; Rybak and Kelly, 2003).
In conclusion and according to our findings, selective inhibition of the sequence of events involved in the intrinsic apoptotic pathway could provide a strategy to minimize cisplatin-induced ototoxicity.
Acknowledgments
This study was supported by Spanish Research Fund (FIS) PI05/0673. JN was a recipient of a Research Contract from ISC III (FIS 99/3077).
Abbreviations
- ABR
auditory brainstem response
- Bax
Bcl-2 associated X protein
- Bcl-2
B-cell lymphoma 2
- CDDP
cis-diamminedichloroplatinum II
- OHCs
outer hair cells
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Conflict of interest
The authors state no conflict of interest.
References
- Alam SA, Ikeda K, Oshima T, Suzuki M, Kawase T, Kikuchi T, et al. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000;141:28–38. doi: 10.1016/s0378-5955(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Anniko M, Sobin A. Cisplatin: evaluation of its ototoxic potential. Am J Otol. 1986;7:276–293. doi: 10.1016/s0196-0709(86)80050-3. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Death receptors: signalling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- Bohm S, Oriana S, Spatti G, Di Re F, Breasciani G, Pirovano C, et al. Dose intensification of platinum compounds with glutathione protection as induction chemotherapy for advanced ovarian carcinoma. Oncology. 1999;57:115–120. doi: 10.1159/000012017. [DOI] [PubMed] [Google Scholar]
- Cardinaal RM, De Groot JC, Huizing EH, Smoorenburg GF, Veldman JE. Ultrastructural changes in the albino guinea pig cochlea at different survival times following cessation of 8-day cisplatin administration. Acta Otolaryngol. 2004;124:144–154. doi: 10.1080/00016480310015164. [DOI] [PubMed] [Google Scholar]
- Cardinaal RM, De Groot JCMJ, Huizing EH, Veldman JE, Smoorenburg GF. Cisplatin-induced ototoxicity: morphological evidence of spontaneous outer hair cell recovery in albino guinea pigs? Hear Res. 2000;44:147–156. doi: 10.1016/s0378-5955(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Cheng PW, Liu SH, Hsu CJ, Lin-Shiau SY. Correlation of increased activities of Na+, K+ ATPase and Ca2+ ATPase with the reversal of cisplatin ototoxicity induced by D-methionine in guinea pigs. Hear Res. 2005;205:102–109. doi: 10.1016/j.heares.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, et al. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- Eguchi Y, Shimizu S, Tsujimoto Y. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 1997;57:1835–1840. [PubMed] [Google Scholar]
- Estrem SA, Babin RW, Ryu JH, Moore KC. Cis-diamminedichloroplatinum (II) ototoxicity in the guinea pig. Otolaryngol Head Neck Surg. 1981;89:638–645. doi: 10.1177/019459988108900424. [DOI] [PubMed] [Google Scholar]
- Evans P, Halliwell B. Free radicals and hearing. Cause, consequence and criteria. Ann N Y Acad Sci. 1999;884:19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Harris MH, Thompson CB. The role of Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7:1182–1191. doi: 10.1038/sj.cdd.4400781. [DOI] [PubMed] [Google Scholar]
- Huang T, Cheng AG, Stupak H, Liu W, Kim A, Staecker H, et al. Oxidative stress-induced apoptosis of cochlear sensory cells: otoprotective strategies. Int J Dev Neurosci. 2000;18:259–270. doi: 10.1016/s0736-5748(99)00094-5. [DOI] [PubMed] [Google Scholar]
- Husain K, Scott RB, Whitworth C, Somani SM, Rybak LP. Dose response of carboplatin-induced hearing loss in rat. Hear Res. 2001;151:71–78. doi: 10.1016/s0300-2977(00)00081-4. [DOI] [PubMed] [Google Scholar]
- Lee JE, Nagakawa T, Kita T, Kim TS, Iguchi F, Endo T, et al. Mechanisms of apoptosis induced by cisplatin in marginal cells in mouse stria vascularis. ORL J Otorhinolaryngol Relat Spec. 2004;66:111–118. doi: 10.1159/000079329. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of Bid by caspase 8 mediated the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Liang F, Schulte BA, Qu C, Hu W, Shen Z. Inhibition of the calcium and voltage-dependent big conductance potassium channel ameliorates cisplatin-induced apoptosis in spiral ligament fibrocytes of the cochlea. Neuroscience. 2005;135:263–271. doi: 10.1016/j.neuroscience.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Triaca V, Levine J. Mechanisms of death induced by cisplatin in proximal tubular epithelial cells: apoptosis vs necrosis. Am J Physiol. 1996;270:700–708. doi: 10.1152/ajprenal.1996.270.4.F700. [DOI] [PubMed] [Google Scholar]
- Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F. Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res. 2000;28:73–80. doi: 10.1034/j.1600-079x.2001.280202.x. [DOI] [PubMed] [Google Scholar]
- Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S. Apoptosis induced by 5-fluorouracil, cisplatin and paclitaxel are associated with p53 gene status in gastric cancer cell lines. Int J Oncol. 2005;26:1563–1567. [PubMed] [Google Scholar]
- McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug Saf. 1995;13:228–244. doi: 10.2165/00002018-199513040-00003. [DOI] [PubMed] [Google Scholar]
- Nagy JL, Adelstein DJ, Newman CW, Rybicki LA, Rice TW, Lavertu P. Cisplatin ototoxicity: the importance of baseline audiometry. Am J Clin Oncol. 1999;28:305–308. doi: 10.1097/00000421-199906000-00020. [DOI] [PubMed] [Google Scholar]
- Nevado J, Sanz R, Casqueiro JC, Ayala A, García-Berrocal JR, Ramirez-Camacho R. Ageing evokes an intrinsic pro-apoptotic signalling pathway in rat's cochleae. Acta Otolaryngol. 2006;126:1134–1139. doi: 10.1080/00016480600672592. [DOI] [PubMed] [Google Scholar]
- Ramírez-Camacho R, García-Berrocal JR, Buján J, Martin-Marero A, Trinidad A. Supporting cells as a target of cisplatin-induced inner ear damage: therapeutic implications. Laryngoscope. 2004;114:533–537. doi: 10.1097/00005537-200403000-00027. [DOI] [PubMed] [Google Scholar]
- Ramírez-Camacho R, García-Berrocal JR, Trinidad A, González-García JA, Verdaguer JM, Ibáñez A, et al. Central role of supporting cells in cochlear homeostasis and pathology. Med Hypotheses. 2006;67:550–555. doi: 10.1016/j.mehy.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Kelly T. Ototoxicity: bioprotective mechanisms. Curr Opin Otolaryngol Head Neck Surg. 2003;11:328–333. doi: 10.1097/00020840-200310000-00004. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Husain K, Whitworth C, Somani SM. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: antioxidant defense system. Toxicol Sciences. 1999;47:195–202. doi: 10.1093/toxsci/47.2.195. [DOI] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Thomas JP, Lautermann J, Liedert B, Seller F, Thomale J. High accumulation of platinum–DNA adducts in strial marginal cells of the cochlea is an early event in cisplatin but not in carboplatin ototoxicity. Mol Pharmacol. 2006;70:23–29. doi: 10.1124/mol.106.022244. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Chapman KT, Nicholson DW. Determination of caspase specificities using a peptide combinatorial library. Meth Enzymol. 2000;322:100–110. doi: 10.1016/s0076-6879(00)22011-9. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Yagi T. Expression of myeloperoxidase in the inner ear of cisplatin-treated guinea pigs. Anticancer Drugs. 2000;11:727–730. doi: 10.1097/00001813-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Watanabe K-I, Jinnouchi K, Hess A, Michel O, Baba S, Yagi T. Carboplatin induces less apoptosis in the cochlea of guinea pigs than cisplatin. Chemotherapy. 2002;48:82–87. doi: 10.1159/000057667. [DOI] [PubMed] [Google Scholar]