Abstract
Background and purpose:
Both parasympathetic tone and atrial tachycardia (AT) remodelling of ion channels play important roles in atrial fibrillation (AF) pathophysiology. Different muscarinic cholinergic receptor (mAChR) subtypes (M2, M3, M4) in atrial cardiomyocytes are coupled to distinct K+-currents (called IKM2, IKM3, IKM4, respectively). Pulmonary veins (PVs) are important in AF and differential cholinergic current responses are a potential underlying mechanism. This study investigated AT-induced remodelling of mAChR subtypes and K+-currents in left-atrial (LA) and PV cardiomyocytes.
Experimental approach:
Receptor expression was assayed by western blot. IKM2, IKM3 and IKM4 were recorded with whole-cell patch-clamp in LA and PV cardiomyocytes of nonpaced control dogs and dogs after 7 days of AT-pacing (400 bpm).
Key results:
Current densities of IKM2, IKM3 and IKM4 were significantly reduced by AT-pacing in LA and PV cardiomyocytes. PV cardiomyocyte current–voltage relations were similar to LA for all three cholinergic currents, both in control and AT remodelling. Membrane-protein expression levels corresponding to M2, M3 and M4 subtypes were decreased significantly (by about 50%) after AT pacing. Agonist concentration–response relations for all three currents were unaffected by AT pacing.
Conclusions and implications:
AT downregulated all three mAChR-coupled K+-current subtypes, along with corresponding mAChR protein expression. These changes in cholinergic receptor-coupled function may play a role in AF pathophysiology. Cholinergic receptor-coupled K+-currents in PV cardiomyocytes were similar to those in LA under control and AT-pacing conditions, suggesting that differential cholinergic current properties do not explain the role of PVs in AF.
Keywords: muscarinic acetylcholine receptor (mAChR), M2 receptor, M3 receptor, M4 receptor, ion channels, atrial fibrillation, ionic remodelling
Introduction
Both parasympathetic tone and remodelling of atrial ion channel expression play an important role in the pathophysiology of atrial fibrillation (AF) (Schauerte et al., 2000; Nattel, 2002). Muscarinic cholinergic receptors (mAChRs) mediate parasympathetic control of cardiac function, including electrical activity and mechanical contraction. The major mediator of mAChR regulation of cardiac function is the activation of G-protein-coupled ion currents, of which the single most important is the inwardly rectifying K+-current coupled to M2 receptors (Mark and Herlitze, 2000). Intact M2 receptor-coupled K+-channel subunits are essential for cholinergic AF in animal models (Kovoor et al., 2001). In addition to the well-known presence of the M2 receptor and its corresponding K+-current (which we will refer to as IKM2 (M2 receptor-mediated inward rectifier K+ currents)) in mammalian hearts, recent studies indicate the presence in atrial myocytes of various other mAChR subtypes coupled to distinct K+-currents: IKM3 (M3 receptor-mediated inward rectifier K+ currents) coupled to the M3 receptor, typically activated by choline and IKM4 (M4 receptor-mediated inward rectifier K+ currents) coupled to the M4 receptor, typically activated by 4-aminopyridine, 4AP (Navarro-Polanco and Sanchez-Chapula, 1997; Shi et al., 1999a, 1999b, 1999c, 2003; Wang et al., 1999, 2001, 2004). The very rapid atrial firing caused by AF is known to remodel atrial electrical properties in a way that promotes AF occurrence and maintenance (Wijffels et al., 1995). AF-induced remodelling is indistinguishable from the remodelling caused by any form of very rapid atrial tachycardia (AT) (Nattel, 2002). AF and AT reduce cholinergic agonist-induced IKM2 in human hearts and in animal models (Dobrev et al., 2001; Ehrlich et al., 2004; Cha et al., 2006), and enhance constitutive IKM2 activity (Ehrlich et al., 2004; Dobrev et al., 2005; Cha et al., 2006). The effect of AT on the expression of other mAChR subtypes and their associated currents is presently unknown.
The myocardial sleeves surrounding pulmonary veins (PVs) are an important source of ectopic activity that initiates and may maintain AF in humans (Haissaguerre et al., 1998). Cardiomyocytes from the PV myocardial sleeves have a discrete distribution of transmembrane ion currents and ion channel subunit proteins, associated with specific action potential properties (Ehrlich et al., 2003; Melnyk et al., 2005). PVs are thought to play a role in vagally mediated AF (Schauerte et al., 2001; Takahashi et al., 2006). Ablation lesions around the PVs, used to treat AF, diminish the left atrial response to parasympathetic stimulation, and this effect may contribute to the ability of such lesions to prevent AF recurrence (Pappone et al., 2004; Razavi et al., 2005). AT produces significant remodelling of the electrophysiological properties of PV cardiomyocyte (Cha et al., 2005). Thus, differential responses of mAChR-coupled ion currents in PVs, whether as an intrinsic property or in response to AT remodelling, may contribute to their arrhythmogenic role in AF. Very little is presently known about mAChR-coupled currents in PV cardiomyocytes. The present study was designed to address two specific issues: (1) the nature of AT-induced remodelling of cardiac mAChRs and their corresponding K+ currents (IKM2–4); (2) the properties of such currents in PV cardiomyocytes (compared to left atrium) in the absence and presence of AT remodelling.
Methods
The animal model was prepared as previously described in detail (Shinagawa et al., 2002). All animal care procedures followed Canadian Council on Animal Care and National Institutes of Health guidelines and were approved by the Animal Research Ethics Committee of the Montreal Heart Institute. A total of 30 adult mongrel dogs (20–36 kg) were divided into control (n=15 dogs) and atrial tachypaced (ATP) (n=15 dogs) groups. In ATP dogs, atrioventricular block was created by radiofrequency ablation, the right ventricle was paced at 80 bpm and the right atrium was paced at 400 bpm for 1 week. On study days, dogs were anaesthetized (morphine 2 mg kg−1 s.c.; α-chloralose 120 mg kg−1 i.v. load, 29.25 mg kg−1 h−1) and mechanically ventilated. Open chest study was performed as previously described in detail (Cha et al., 2004). Briefly, atrial effective refractory period (ERP) was measured at the left atrial appendage with 15 basic (S1) stimuli, followed by premature (S2) stimuli with 5-ms decrements (ERP=longest S1S2 failing to capture). AF (defined as an irregular atrial rhythm >400 bpm) was induced by burst pacing. Mean AF duration was determined in each dog on the basis of 10 inductions for AF <5 min and 5 inductions for 5- to 30-min AF. AF >30 min was considered sustained: cardioversion was not performed, and electrophysiological assessment was terminated. At the end of each study, haemodynamic variables were measured with a fluid-filled catheter and pressure transducer.
Cell preparation
Hearts and adjacent lung tissues were quickly excised through a median thoracotomy and immersed in oxygenated Tyrode's solution at room temperature. A left atrial-PV preparation was dissected and then perfused at ∼10 ml min−1 via the left circumflex coronary artery for cardiomyocyte isolation, with previously detailed methods (Ehrlich et al., 2004). Briefly, the preparation was perfused with Ca2+-free Tyrode's solution containing collagenase (110 U ml−1 CLS II collagenase; Worthington Biochemical, Freehold, NJ, USA) and 0.1% bovine serum albumin (Sigma Chemicals, St Louis, MO, USA) for around 40 min. The dispersed cells were stored in a high-K+ storage solution.
Voltage-clamp technique
Currents were recorded with the whole-cell patch-clamp technique at 36±0.5°C as described previously (Ehrlich et al., 2004). Borosilicate glass electrodes with tip resistances between 1.5 and 3.0 MΩ were used to record whole-cell currents. All junction potentials were zeroed before formation of gigaohm seals. The compensated series resistance and capacitive time constant averaged 3.3±0.2 MΩ and 277±79 μs, respectively. Atrial cell capacitance averaged 98±8 pF for control cells and 102±9 pF for cells from tachypaced dogs. Original recordings are presented as current amplitude, but mean data are shown as current density (pA pF−1) to control for varying cell size.
To record separately K+-currents coupled to each subtype of mAChR, other mAChR subtypes were inhibited with subtype-selective antagonists as described previously (Shi et al., 2004a): pirenzepine (100 nM, an M1 blocker), methoctramine (20 nM, an M2 blocker), 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP) (2 nM, an M3 blocker) and tropicamide (200 nM, an M4 blocker). IKM2 was induced by 1 μM carbachol in the presence of antagonists towards the M1, M3 and M4 receptors, and measured as atropine-sensitive current. Choline (10 mM) was used to activate IKM3 in the presence of antagonists towards the M1, M2 and M4 receptors. 4-AP (1 mM) was used to activate IKM4 in the presence of antagonists towards the M1, M2, M3 receptors. Recordings of IKM2, IKM3 and IKM4 were all conducted with E-4031 (N-(4-((1-(2-(6-methyl-2-pyridinyl)ethyl)-4-piperidinyl)carbonyl)phenyl)-methanesulphonamide dihydrochloride dehydrate, 5 μM) and HMR 1556 ([(3R,4S)-(+)-N-[3-hydroxy-2,2-dimethyl-6-(4,4,4-trifluorobutoxy)chroman-4-yl]-N-methylmethanesulphonamide], 1 μM) in the bathing solution to block IKr (rapid component of delayed-rectifier K+ current) and IKs (slow component of delayed-rectifier K+ current), respectively. Sodium current contamination was avoided by the use of a holding potential of −50 mV for all current recordings. Cadmium chloride (200 μM) was used to inhibit Ca2+ current as well as Ca2+-activated chloride current.
Action potential recordings
Action potential (AP) recordings were obtained with fine-tipped standard borosilicate glass microelectrodes in intact multicellular coronary artery-perfused right atrial preparations. The preparations were simultaneously superfused at 10 ml min−1 and perfused via the left circumflex coronary artery with oxygenated bath solution at 36±0.5°C. The bath solution contained (mM): NaCl 120, KCl 4, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, CaCl2 1.25 and dextrose 5 (95% O2–5% CO2, pH 7.4). Floating microelectrodes (resistances 10–20 MΩ when filled with 3-M KCl) connected to a high-input impedance amplifier were used to record APs at 2 Hz in the presence and absence of acetylcholine and various mAChR blockers. Multiple recordings were obtained under each condition in each preparation (with approximately equal numbers of cells recorded for each condition in each preparation), and all recordings from all preparations were used to obtain mean values for that condition.
Solutions and drugs
Normal Tyrode's solution contained (mM): NaCl 136, KCl 5.4, CaCl2 2, MgCl2 1, NaH2PO4 0.33, glucose 10 and HEPES 5 (pH adjusted to 7.4 with NaOH). The cell storage solution contained (mM): KCl 20, KH2PO4 10, glucose 25, potassium glutamate 70, β-hydroxybutyric acid 10, taurine 20, EGTA 10, albumin 0.1% and mannitol 40 (pH adjusted to 7.4 with KOH). The nominally Ca2+-free Tyrode's solution used for the cell isolation procedure was prepared by omitting CaCl2 from the normal Tyrode's solution. The standard internal (pipette) solution contained (mM): potassium aspartate 110, KCl 20, MgCl2 1, Mg-ATP 5, GTP 0.1, EGTA 10, phosphocreatine 5, HEPES 10 (pH adjusted to 7.3 with KOH). Unless otherwise specified, chemicals were obtained from Sigma Chemicals.
Stock solutions of carbachol (1 mM), choline (5 M), 4-AP (1 M), atropine (1 mM), pirenzepine (1 mM), methoctramine (1 mM), 4-DAMP (1 mM), tropicamide (1 mM), cadmium chloride (200 mM) and E-4031 (5 mM, Calbiochem, CA, USA) were prepared with distilled water. Stock solution of HMR 1556 (1 mM, a kind gift from Sanofi-Aventis Pharmaceuticals, Frankfurt, Germany) was made with dimethyl sulphoxide (DMSO).
Western blot analysis
Dog left atrial tissue samples were directly frozen in liquid nitrogen following excision and further reduced to powder with a mortar. Powdered tissue was then resuspended in a cold (4°C) extraction buffer containing in mM: 10 Tris (pH 7.4), 250 sucrose and Complete EDTA-free Protease Inhibitor Cocktail (Roche Applied Science) to avoid protein degradation. The sample mixture was homogenized mechanically, incubated for 20 min on ice and then centrifuged for 10 min at 1000 × g to remove debris and nuclei. The supernatant was then collected and the pellet homogenized again. The latter procedure was repeated three times to increase the efficiency of protein extraction. Collected supernatants were finally centrifuged at 100 000 × g using an Optima LE-80K ultracentrifuge (Beckman, Instruments, Fullerton, CA, USA). The pellet corresponding to the membrane fraction was resuspended in 1% Triton-X100 cold extraction buffer and stored at −80°C.
Membrane proteins (60 μg) were fractionated by SDS polyacrylamide gel electrophoresis (7.5% polyacrylamide gels) and transferred to polyvinyl difluoride (PVDF) membranes. After transfer, membranes were blotted overnight with anti-M2 (1:2000), anti-M3 (1:1000) or anti-M4 (1:1000) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The next day, membranes were incubated for 2 h with the secondary antibody (goat anti-rabbit IgG, 1:10 000, Jackson Immuno Research Laboratories, West Grove, PA, USA). Bands were visualized with enhanced chemiluminescence. All immunoblot band intensity measurements were normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band intensity of the same sample (anti-GAPDH 1:100 000, RDI). Samples from each heart were run separately, with an independent determination obtained for each dog studied.
Data analysis and statistics
Clampfit 6.0 (Axon) and GraphPad Prism 3.0 were used for data analysis. Group data are expressed as mean±s.e.m. Two-way analysis of variance (ANOVA) and Bonferroni-adjusted t-tests (in the case of significant inter-group differences by ANOVA) were used for statistical comparisons of current–voltage relations. Non-paired t-tests were applied for inter-group comparisons of AF duration and western blot analysis. One-way ANOVA and Kruskal-Wallis tests were used for comparisons of action potential duration (APD) in the presence of acetylcholine and various mAChR antagonists in multicellular preparations. Nonlinear regression was used to compare agonist concentration–response curves of each mAChR-coupled current between atrial cardiomyocytes of control and ATP dogs. A two-tailed P<0.05 was taken to indicate a statistically significant difference.
Results
Haemodynamic indices and in vivo electrophysiological measurements
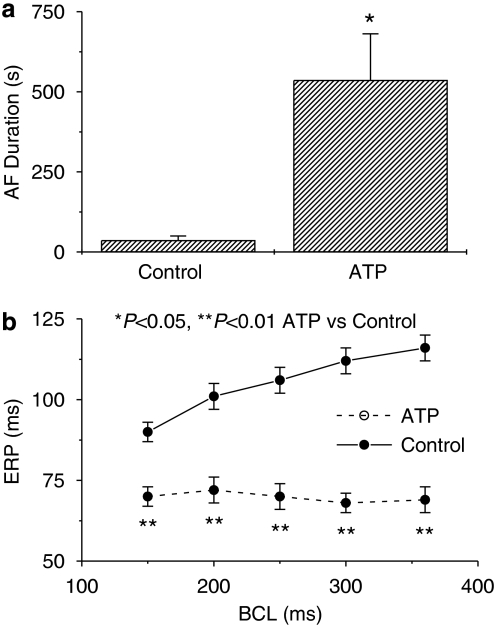
There was no difference in haemodynamics between control and ATP dogs (Table 1). AF duration was significantly prolonged in dogs with AT remodelling compared to controls (Figure 1a, P<0.05). Tachypacing significantly decreased atrial ERP by ∼30% from control at all cycle lengths and suppressed ERP rate adaptation, expressing the classical electrophysiological changes caused by AT remodelling (Wijffels et al., 1995; Nattel, 2002).
Table 1.
Haemodynamics at open chest study
| Control | ATP | |
|---|---|---|
| Systolic BP (mmHg) | 119±9 | 122±7 |
| Diastolic BP (mmHg) | 63±5 | 68±4 |
| LVSP (mmHg) | 109±7 | 112±7 |
| LVEDP (mmHg) | 3.6±0.7 | 4.5±0.6 |
| LAP (mmHg) | 4.8±0.7 | 4.1±0.4 |
| RAP (mmHg) | 4.7±0.7 | 3.1±0.5 |
Abbreviations: ATP, atrial tachypacing; BP, blood pressure; LAP, LA mean pressure; LVEDP, LV end-diastolic pressure; LVSP, LV systolic pressure; RAP, RA mean pressure.
Figure 1.
Mean±s.e.m. values for atrial fibrillation (AF) duration (a) and effective refractory period (ERP) (b) in control and atrial tachypaced (ATP) dogs.
Atrial tachypacing effects on IKM2
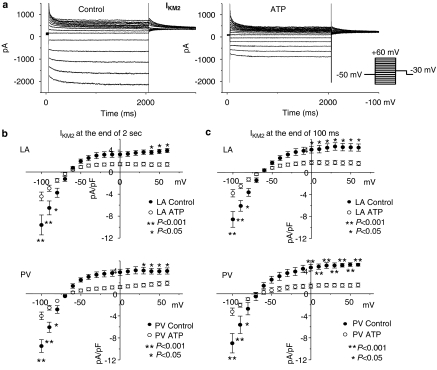
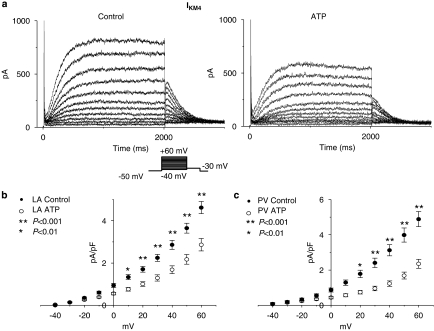
Representative IKM2 recordings after the application of carbachol are illustrated in Figure 2a. Since IKM2 shows some time-dependence, the amplitude of IKM2 was measured in two ways: the magnitude at 100 ms after the onset of voltage steps and that at the end of the 2 s voltage steps. IKM2 was significantly smaller (Figure 2b) in both left atrial (LA, top panels) and PV (bottom panels) cardiomyocytes from ATP dogs vs control dogs over a wide range of test potentials. For example, IKM2 at −100 mV was −9.6±1.8 pA/pF in control (n=9 cells) vs −4.4±1.8 pA pF−1 in tachypaced left atria (n=11 cells, P<0.001). Overall, tachypacing reduced IKM2 by 61±1%. IKM2 was similar in LA vs PV cardiomyocytes under control conditions and after atrial tachypacing. For example, under control conditions IKM2 at −100 mV averaged −9.6±1.8 pA pF−1 in left atrial cells vs −9.5±1.1 pA pF−1 in PV cells from control dogs (n=9, 10 cells, respectively; P=NS for left atrium vs PV) and −4.4±0.8 pA pF−1 in left atrial cells vs −4.0±0.7 pA pF−1 in PV cells from ATP dogs (n=11, 10 cells, respectively; P=NS for left atrium vs PV).
Figure 2.
Atrial tachypacing effects on IKM2. (a) Representative IKM2 recordings obtained with protocol shown in inset, in left atrial control and tachypaced (ATP) cardiomyocytes. (b, c) Current density–voltage relationships of IKM2 in control and ATP cardiomyocytes isolated from left atria (LA) and PVs measured at 2 s (b) and 100 ms (c) after the onset of voltages steps (n=9, 11, 10 and 10 cells in left atrial control, left atrial ATP, PV control and PV ATP, respectively. Data are mean±s.e.m. *P<0.05, **P<0.001). IKM2, M2 receptor-mediated inward rectifier K+ currents; PV, pulmonary vein.
Atrial tachypacing effects on IKM3
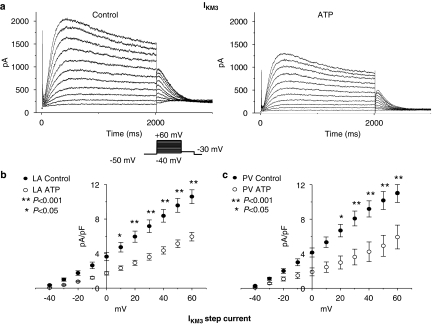
IKM3 was elicited by depolarizing voltage steps in the presence of 10 mM choline in the bath solution and the step-current amplitude was measured at the end of 2 s pulses. Representative IKM3 recordings are illustrated in Figure 3a. The current showed typical rapid activation and time-dependent relaxation with large tail currents on repolarization (Shi et al., 1999a, 1999b, 1999c, 2003; Wang et al., 2001). The currents were reduced in cells from tachypaced dogs. Mean data for step currents are shown in Figure 3b. IKM3 was significantly reduced by tachypacing in both LA and PV cardiomyocytes, with a mean reduction of 51±1%.
Figure 3.
Atrial tachypacing effects on IKM3. (a) Representative IKM3 recordings obtained with protocol shown in inset in left atrial (LA) control and tachypaced (ATP) cardiomyocytes. (b, c) Mean±s.e.m. current density-voltage relationships of IKM3 in control and ATP cardiomyocytes isolated from left atrium and PV (n=17, 21, 16, and 10 cells in left atrial control, left atrial ATP, PV control and PV ATP, respectively. *P<0.05, **P<0.001). IKM3, M3 receptor-mediated inward rectifier K+ currents; PV, pulmonary vein.
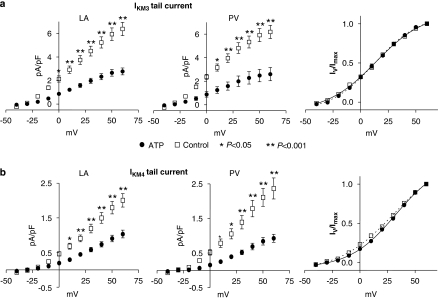
Atrial tachypacing also significantly reduced IKM3 tail currents (tail current–voltage relations shown in Figure 4a, left and middle panels), but did not affect the voltage dependence of activation as reflected by the Boltzmann relation of tail currents normalized to the maximum tail current (Figure 4a, right panel). Overall, the mean V1/2 of activation averaged 15.9±2.4 mV for left atrial and 13.2±1.4 mV for PV cells of control dogs and 15.0±1.8 mV and 11.7±1.9 mV for left atrial and PV cells of tachypaced dogs (P=NS for tachypaced vs control, left atrial vs PV). Current activation and deactivation kinetics were similarly not affected: for example, activation time-constants at +40 mV averaged 183±13, 172±18, 183±12 and 197±14 ms for left atrial control, PV control, left atrial cells and PV cells from tachypaced dogs, respectively (P=NS). Similarly, deactivation kinetics at −30 mV after a depolarizing pulse to +40 mV averaged 268±27, 276±25, 260±19 and 280±22 ms for left atrial control, PV control, left cells and PV cells from tachypaced dogs, respectively (P=NS). As for IKM2, there was no significant difference in IKM3 in PVs vs left atrial cells under either control conditions or after tachypacing. For example, under control conditions IKM3 step current at +60 mV averaged 10.6±0.8 pA pF−1 in left atrial cells vs 11.0±1.1 pA pF−1 in PV cells from control dogs (n=17, 16 cells, respectively; P=NS) and 6.0±0.5 pA pF−1 in left atrial cells vs 5.9±1.3 pA pF−1 in PV cells from ATP dogs (n=21, 10 cells, respectively; P=NS).
Figure 4.
(a) Atrial tachypacing effects on IKM3 tail current–voltage relations in left atrial (LA) and PV cells. Mean±s.e.m. tail current densities are shown as a function of test potential for control and tachypaced (ATP) dog cells. Right panel: mean±s.e.m. tail currents normalized to value at most positive step potential and Boltzmann fits. (b) Atrial tachypacing effects on IKM4 tail current–voltage relations in left atrial and PV cells. Mean±s.e.m. tail current densities are shown as a function of test potential for control and tachypaced dog cells. Right panel: mean±s.e.m. tail currents normalized to value at most positive step potential and Boltzmann fits. (*P<0.05, **P<0.001). IKM4, M4 receptor-mediated inward rectifier K+ currents; PV, pulmonary vein.
Atrial tachypacing effects on IKM4
IKM4 was induced by 1 mM 4-AP in the bath solution and the step-current was measured at the end of 2 s voltage pulses. Representative IKM4 recordings are illustrated in Figure 5a and have typical characteristics (Shi et al., 2003). IKM4 step current was significantly reduced by atrial tachypacing (Figure 5b), with a mean overall reduction of 48±2%. Atrial tachypacing also significantly reduced IKM4 tail currents (IKM4 tail current–voltage relations shown in Figure 4b, left and middle panels), but did not affect the voltage-dependence of activation as reflected by the Boltzmann relation of tail currents normalized to the maximum tail current (Figure 4b, right panel). IKM4 tail currents did not fully saturate over the voltage range studied. Overall, the mean V1/2 of IKM4 activation averaged 29.7±3.4 mV for left atrial and 30.4±2.1 mV for PV cells of control dogs and 31.0±2.2 and 33.8±3.7 mV for left atrial and PV cells of tachypaced dogs (P=NS for tachypaced vs control, left atrial vs PV).
Figure 5.
Tachycardia remodelling of IKM4. (a) Representative IKM4 recordings obtained with protocol shown in inset in left atrial control and ATP cardiomyocytes. (b) Mean±s.e.m. current density–voltage relationships of IKM4 in control and ATP cardiomyocytes isolated from left atria (LA) and PVs. (n=24, 23, 14, and 26 cells in LA control, LA ATP, PV control and PV ATP, respectively. *P<0.01, **P<0.001). ATP, atrial tachypaced; IKM4, M4 receptor-mediated inward rectifier K+ currents; PV, pulmonary vein.
IKM4 activation and deactivation kinetics were unaffected by tachypacing and were similar in left atrium and PVs: for example, activation time-constants at +40 mV averaged 243±22, 249±17, 210±12 and 235±17 ms for left atrial control, PV control, left atrial tachypaced and PV cells from tachypaced dogs, respectively (P=NS). Similarly, deactivation kinetics at −30 mV after a depolarizing pulse to +40 mV averaged 225±21, 229±14, 204±11 and 224±19 ms, for left atrial control, PV control, left atrial and PV cells from tachypaced dogs, respectively (P=NS). As for IKM2 and IKM3, there was no significant difference in IKM4 in PVs vs left atrial cells under either control conditions or after tachypacing. For example, under control conditions IKM4 step current at +60 mV averaged 4.6±0.3 pA pF−1 in left atrial cells vs 4.9±0.4 pA pF−1 in PV cells from control dogs (n=24, 14 cells, respectively; P=NS for left atrium vs PV) and 2.9±0.3 pA pF−1 in left atrial cells vs 2.4±0.3 pA pF−1 in PV cells from ATP dogs (n=23, 26 cells, respectively; P=NS for left atrium vs PV).
Atrial tachypacing effects on membrane-protein expression of mAChR subtypes
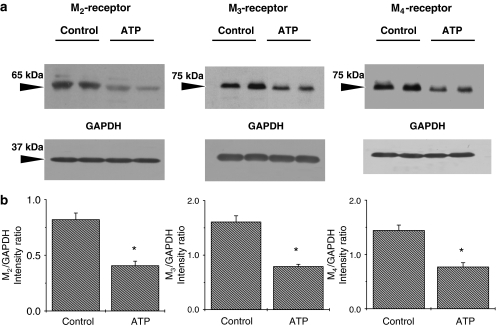
To determine whether the alterations of IKM2, IKM3 and IKM4 density in atrial cardiomyocytes from tachypaced dogs can be explained by alterations in expression levels of the corresponding M2, M3 and M4 receptor proteins, western blot quantification of mAChR subtypes was performed with membrane samples extracted from atria of control and atrial-tachypaced dogs. The antibodies against M2, M3 and M4 receptors identified bands with molecular masses of ∼65, ∼75 and ∼75 kDa, respectively (Figure 6a). Positive controls were used to confirm the location of targeted protein bands for each antibody. Band intensities were determined densitometrically and normalized to corresponding bands for GAPDH. As shown in Figure 6b, the densities of M2, M3 and M4 proteins were significantly decreased, by 50, 49 and 53%, respectively, in tachypaced atria (P<0.05 for each, n=6 dogs for each group).
Figure 6.
Western blot analysis of mAChR subtype expression in the membrane protein samples extracted from control and ATP left atria. (a) Examples of western blot bands recognized by antibodies against M2, M3 or M4 subtypes of mAChRs, as well as corresponding GAPDH bands from the same gels. (b) Mean±s.e.m. protein band densities were expressed as intensity ratios normalized to corresponding GAPDH bands (n=6 independent protein samples, one from each of six different dogs in each group, for each mAChR subtype. *P<0.05). ATP, atrial tachypaced; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mAChR, muscarinic cholinergic receptor.
Agonist concentration–response relationships for IKM2, IKM3 and IKM4
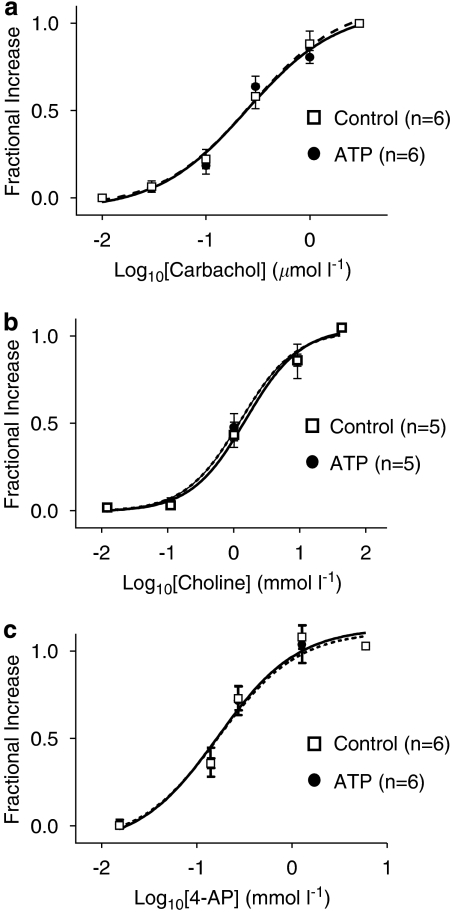
Activation of the various mAChR-coupled K+-channels occurs through different intracellular signalling pathways (Mark and Herlitze, 2000; Shi et al., 2004b; Wang et al., 2004), which could be also altered by atrial tachypacing and contribute to the changes in current responses. These and receptor alterations could alter the agonist concentration–response relations. Therefore, we compared the agonist concentration–response relationships of IKM2, IKM3 and IKM4 between control and tachypaced atrial myocytes. As shown by the normalized concentration–response curves in Figure 7, there are no significant differences in concentration–response relations between control and tachypaced groups. The EC50 for carbachol for IKM2 at −100 mV was 0.28±0.02 μM for control dogs (n=6 cells) vs 0.31±0.08 μM for tachypaced dogs (n=6 cells, P=NS). The EC50 for choline for IKM3 at +60 mV averaged 1.38±0.33 mM for control and 1.71±0.39 mM for tachypaced dogs (n=5, 5 cells, respectively; P=NS). For IKM4, the EC50 for 4-AP at +60 mV was 0.13±0.01 mM for control cells (n=6) and 0.12±0.02 mM for cells from tachypaced dogs (n=6 cells, P=NS).
Figure 7.
Agonist concentration–response relations for IKM2 (a), IKM3 (b) and IKM4 (c) from control and tachypaced (ATP) left atrial cells. Curves are best-fit Boltzmann relations (solid lines are control fits, dashed lines are fits to ATP data).
Potential functional roles of IKM3 and IKM4
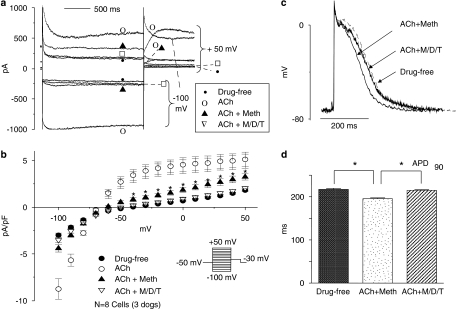
The functional role of IKM3 and IKM4 remains uncertain. To determine whether non-M2 mAChRs can be activated by the endogenous agonist, acetylcholine, we assessed the currents elicited by exposure to acetylcholine (300 nM) in the absence of blockers, in the presence of methoctramine at a concentration (200 nM, 10 times the IC50) that should fully block M2 receptors, and then in the presence of methoctramine plus M3 and M4 antagonists (4-DAMP, 3 nM; tropicamide, 75 nM). As shown in Figure 8a, acetylcholine activated inward and outward currents with inward rectification. Methoctramine returned inward currents to baseline, but the acetylcholine-induced outward current was incompletely reversed, suggesting an M2-receptor-independent outward component. Further application of the M3 and M4 antagonists 4-DAMP and tropicamide suppressed the remaining outward current. The mean results for eight cells, each of which were exposed to all conditions, are shown in Figure 8b. Acetylcholine elicited substantial increases in inward and outward current relative to baseline. Methoctramine returned inward currents to values not significantly different from baseline, but a significant cholinergic outward current remained. The further addition of 4-DAMP and tropicamide returned all currents to values not significantly different from baseline. These results are consistent with the notion that the endogenous vagal neurotransmitter acetylcholine can induce a non-M2 AChR-mediated outward current in atrial myocytes.
Figure 8.
Effects of mAChR subtype-selective antagonists on acetylcholine (ACh)-induced currents and action potential changes in atrial myocytes. (a) Currents elicited in one atrial cardiomyocyte by 2 s depolarizing steps to +50 mV and hyperpolarizing steps to −100 mV under control conditions (Baseline) and then in the presence of following: (1) ACh alone (300 nM)—ACh; (2) ACh (300 nM) plus methoctramine (Meth, 200 nM)—ACh+Meth; (3) ACh (300 nM) plus Meth (200 nM), 4-DAMP (3 nM) and tropicamide (75 nM)—ACh+M/D/T. (b) Mean±s.e.m. current density–voltage relations corresponding to a series of experiments of the type illustrated in (a) (n=8 cells). (c) Representative examples of APs under ACh (300 nM) plus Meth (Meth, 200 nM)—Ach+Meth; and under ACh (300 nM) plus Meth (200 nM), 4-DAMP (3 nM) and tropicamide (75 nM)—ACh+M/D/T. (d) Mean±s.e.m. APD90 of atrial myocytes at baseline, under ACh+Meth and under ACh+M/D/T (total N=37–40 cells for each condition from three preparations, one per dog, from three dogs, APD90=AP duration to 90% repolarization, *P<0.05 for ACh+Meth versus baseline and versus ACh+M/D/T, one-way ANOVA). ANOVA, analysis of variance; APD, action potential duration; 4-DAMP, 4-diphenylacetoxy-N-methylpiperidine methiodide; mAChR, muscarinic cholinergic receptor.
To study the role of non-M2 mAChR-activated currents in action potential repolarization, we recorded multiple APs in three multicellular canine right atrial preparations (one per dog from each of three dogs) before and after the addition of acetylcholine (300 nM), acetylcholine plus methoctramine (200 nM) and then after the further addition of 4-DAMP (3 nM) and tropicamide (75 nM). Examples of typical AP recordings from one preparation before drug perfusion (drug-free baseline), after the addition of acetylcholine plus methoctramine (ACh+Meth) and after the further addition of 4-DAMP and tropicamide (ACh+M/D/T) are shown in Figure 8c. In the presence of methoctramine, acetylcholine reduced APD90 modestly but significantly (Figure 8d). The addition of M3 and M4 blockers returned the APD90 to control values. These results suggest that non-M2 AChR-mediated currents may contribute to the repolarization-accelerating effects of acetylcholine in canine atrial tissues.
Discussion
The major findings of the present study are: (1) the current densities of IKM2, IKM3 and IKM4 in atrial and PVs myocytes are reduced by atrial tachypacing; (2) the membrane protein densities of the M2, M3 and M4 receptor subtypes are decreased by tachypacing, to an extent comparable to the changes in corresponding current densities; (3) agonist concentration–response relations for all three receptor-coupled K+-channel systems are not affected by tachypacing; (4) there are no detectable differences in muscarinic cholinergic receptor-coupled K+ currents between left atrial and PV cardiomyocytes.
Cardiac mAChR-coupled K+-currents
Multiple subtypes of mAChRs have been shown in mammalian hearts, including those of man (Shi et al., 1999b; Wang et al., 2001; Willmy-Matthes et al., 2003). These studies have provided functional, pharmacological and molecular evidence suggesting that IKM2, IKM3 and IKM4 represent currents that are coupled to the three corresponding subtypes of mAChRs. APD is strikingly shortened by choline, an effect reversible by washout or the application of 4-DAMP, an M3 receptor blocker (Shi et al., 1999b, 2003). 4-AP also produces resting membrane potential hyperpolarization and action potential shortening, consistent with enhanced K+ conductance (Navarro-Polanco and Sanchez-Chapula, 1997).
The various mAChR subtypes in the heart appear to be linked to different effector systems by varying signal transduction mechanisms. IKM2 is carried by G-protein-gated K+-channel subunits, with a conductance determined by interactions between M2 receptors and K+ channels coupled directly via Pertussis toxin-sensitive G protein complexes (Reuveny et al., 1994; Wickman and Clapham, 1995; Yamada et al., 1998). In contrast, IKM3 appears to be Gαq protein-coupled, responsive to signals transduced by both Gα-subunits and Gβγ dimers (Shi et al., 2004b).
Remodelling of cardiac mAChR-coupled K+-current function
IKM2 has previously been shown to be downregulated by AT/AF in both humans and animal models (Dobrev et al., 2001; Ehrlich et al., 2004). IKM2 is also reduced by congestive heart failure (Koumi et al., 1994; Shi et al., 2004a). AT causes complex remodelling of components of the IKM2 system. Kir3.4 expression decreases (Ehrlich et al., 2004), but that of Kir3.1 does not (Ehrlich et al., 2004). In AF patients, there is evidence for decreased expression of Kir3.1 and 3.4 (Brundel et al., 2001; Dobrev et al., 2001). There is also previous evidence for downregulation of Gαi, M2 receptors and increased constitutive activity with AT remodelling (Ehrlich et al., 2004). In the setting of ventricular tachycardia-induced heart failure, the current densities of IKM2 and IKM4 are reduced, whereas IKM3 density is enhanced (Shi et al., 2004a). As in our study, changes in receptor expression paralleled those in corresponding currents. To our knowledge, there are no data in the previous literature regarding AT-induced alterations in the M3- and M4-coupled K+-current or corresponding receptor systems in the heart.
The specific K+-channel subunits that carry the delayed-rectifier currents IKM3 and IKM4 are unknown. The corresponding currents are not suppressed by IKr or IKs blockers (Shi et al., 1999b; Benavides-Haro et al., 2003), suggesting that they are not due to cholinergic modulation of classical cardiac delayed-rectifier currents. On the other hand, tertiapin, a selective IKACh blocker, inhibits both inward and delayed rectifier currents induced by bethanechol in feline atrial myocytes (Benavides-Haro et al., 2003). It is thus possible that IKACh channels carry the various forms of K+-current activated by cholinergic stimulation, with different receptors and their respective coupling systems producing different modes of voltage- and time-dependent gating. In that case, downregulation of Kir3.1 and/or 3.4 subunits may contribute to the IKM2, IKM3 and IKM4 reductions that we saw, in addition to receptor downregulation.
The functional importance of the changes in muscarinic receptor-coupled K+-channel systems in AT remodelling is unclear. Action potential abbreviation induced by cholinergic-receptor stimulation is known to be attenuated in patients with AF (Dobrev et al., 2001) and it is most likely that decreases in muscarinic-receptor-coupled K+-current activation is the underlying mechanism. On the other hand, AT enhances a constitutive component of IKACh observed in the absence of cholinergic stimulation, in a fashion that accelerates repolarization and promotes atrial tachyarrhythmias (Cha et al., 2006). The detailed mechanisms underlying these changes are still unknown, but may involve a transition from receptor-activated to receptor-independent function of IKACh channels that favours atrial arrhythmogenesis.
Role of PVs in AF and potential participation of cholinergic K+-current systems
PVs are important in the pathophysiology of AF (Haissaguerre et al., 1998). There is evidence that PVs may be more prone to acetylcholine-induced arrhythmias than other atrial regions (Po et al., 2005, 2006; Patterson et al., 2006). In addition, vagal excitation has been suggested to play a role in enhancing firing rates of PV foci in AF (Takahashi et al., 2006). A plausible mechanism for enhanced PV sensitivity to cholinergic arrhythmias could be altered cholinergic receptor subtype-specific responses, either under control conditions or in the presence of atrial remodelling. Our results exclude this possibility, by showing no significant differences between the PV and left atrial cardiomyocyte density–voltage relations for IKM2, IKM3 and IKM4, both in normal dogs and in dogs subjected to AT remodelling.
Novel findings and potential importance
To our knowledge, the present study is the first comprehensive analysis of the changes in various muscarinic subtype-coupled K+-currents and muscarinic subtype receptor expression associated with AT remodelling. Given the importance of AT remodelling (Wijffels et al., 1995; Nattel, 2002) and of the cholinergic system (Dobrev et al., 2001, 2005; Cha et al., 2006) in AF, our findings provide new pharmacological information relevant to the determinants of this important arrhythmia. In addition, we provide novel information about muscarinic cholinergic-receptor-coupled currents in PVs compared to left atrium, both in normal and AF-related paradigms.
Parasympathetic stimulation shortens APD, increases ERP heterogeneity and enhances AF vulnerability and persistence (Allessie et al., 1984; Liu and Nattel, 1997; Kneller et al., 2002). Atrial parasympathetic system modification by radiofrequency catheter ablation suppresses vagally mediated AF (Schauerte et al., 2000; Pappone et al., 2004), which suggests that a decrease in response to parasymparathetic stimulation might have an antiarrhythmic effect. Thus, the reduction of IKM2, IKM3 and IKM4 that we observed may be an intrinsic protective mechanism to counteract the AF-promoting consequences of AT remodelling.
Potential limitations
Our studies were performed in normal and atrial-tachycardia-remodelled preparations. However, our dogs did not show spontaneously occurring AF, so extrapolation to patients with spontaneous AF onset should be cautious. We isolated cells from the cardiomyocyte sleeves around the PVs. We cannot exclude the possibility that there may be PV cardiomyocytes distal to the visible cardiomyocyte sleeves that have properties different from those that we studied.
We observed tachycardia-induced decreases in mAChR expression for all three subtypes studied, along with reductions in the amplitudes of the associated currents. While it is tempting to attribute the current reductions to downregulation of the associated receptors, it is possible that downregulation of the current-carrying ion channel subunits is responsible. It is also possible that receptors and channel subunits are colocalized in macroscopic complexes that are downregulated. Resolution of these issues is technically challenging and beyond the scope of the present paper, but would be interesting to pursue in further studies.
We used a pharmacological approach to dissecting out a contribution of non-M2 AChR-activated currents to acetylcholine's electrophysiological actions in dog atrium. The blockers we used are the most selective ones available to us, but none of them are perfectly specific. Thus, although our findings suggest a role for M3/M4-coupled currents, they do not prove it.
The extrapolation of these findings in canine models to clinical AF should be cautious. For example, results published in abstract form suggest that the contribution of non-M2 receptors to IKACh is increased in atrial samples from chronic AF patients (Dobrev et al., 2002). In the present studies, there was no apparent difference in the extent of remodelling of M2 vs non-M2 receptors and coupled currents. Patient samples present a variety of complexities, including the effects of varying heart disease, other clinical conditions, concomitant drugs, anaesthetic agents, and so on, whereas in the present study the effect of rapid atrial rate per se was isolated. Further analysis of changes in AChR-coupled currents and signal transduction systems in samples from patients with various AF-associated cardiac conditions would be of interest.
IKACh desensitizes with biexponential time constants of ∼1.5 and 150 s (Zang et al., 1993). We were interested in steady-state currents at physiological temperatures and therefore did not use a fast-perfusion system for solution changes. Steady-state concentrations following solution changes take several minutes with the system that we used, and perhaps as a result, we did not observe significant desensitization of cholinergic-induced currents once we began measurements after bath equilibration.
Acknowledgments
We thank Chantal St Cyr, Chantal Maltais and Nathalie L'Heureux for expert technical assistance, and France Thériault for secretarial help with the manuscript. Funding was provided by the Canadian Institutes of Health Research and the Quebec Heart and Stroke Foundation.
Abbreviations
- 4-AP
4-aminopyridine
- 4-DAMP
4-diphenylacetoxy-N-methylpiperidine methiodide
- AF
atrial fibrillation
- APD
action potential duration
- E-4031
N-(4-((1-(2-(6-methyl-2-pyridinyl)ethyl)-4-piperidinyl)carbonyl)phenyl)-methanesulphonamide dihydrochloride dehydrate
- ERP
effective refractory period
- HMR 1556
[(3R,4S)-(+)-N-[3-hydroxy-2,2-dimethyl-6-(4,4,4-trifluorobutoxy)chroman-4-yl]-N-methylmethanesulphonamide]
- IKM2
M2 receptor-mediated inward rectifier K+ currents
- IKM3
M3 receptor-mediated delayed rectifier K+ current
- IKM4
4-aminopyridine-induced M4 receptor-mediated delayed rectifier K+ current
- IKr
rapid component of delayed-rectifier K+ current
- IKs
slow component of delayed-rectifier K+ current
- LA
left atrium
- mAChR
muscarinic acetylcholine receptor
- PTX
Pertussis toxin
- PV
pulmonary vein myocardial sleeves
Conflict of interest
The only relevant potential interest is that Dr Nattel and Dr Hébert are listed as co-inventors of intellectual property owned jointly by the Montreal Heart Institute and Université de Montréal entitled ‘Acetylcholine-dependent current as a novel ionic target for AF'.
References
- Allessie MA, Lammers WJ, Bonke IM, Hollen J. Intra-atrial reentry as a mechanism for atrial flutter induced by acetylcholine and rapid pacing in the dog. Circulation. 1984;70:123–135. doi: 10.1161/01.cir.70.1.123. [DOI] [PubMed] [Google Scholar]
- Benavides-Haro DE, Navarro-Polanco RA, Sanchez-Chapula JA. The cholinomimetic agent bethanechol activates IK(ACh) in feline atrial myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:309–315. doi: 10.1007/s00210-003-0789-1. [DOI] [PubMed] [Google Scholar]
- Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, et al. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–690. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L, Nattel S. Kir3-based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113:1730–1737. doi: 10.1161/CIRCULATIONAHA.105.561738. [DOI] [PubMed] [Google Scholar]
- Cha TJ, Ehrlich JR, Zhang L, Chartier D, Leung TK, Nattel S. Atrial tachycardia remodeling of pulmonary vein cardiomyocytes: comparison with left atrium and potential relation to arrhythmogenesis. Circulation. 2005;111:728–735. doi: 10.1161/01.CIR.0000155240.05251.D0. [DOI] [PubMed] [Google Scholar]
- Cha TJ, Ehrlich JR, Zhang L, Nattel S. Atrial ionic remodeling induced by atrial tachycardia in the presence of congestive heart failure. Circulation. 2004;110:1520–1526. doi: 10.1161/01.CIR.0000142052.03565.87. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, et al. The G protein-gated potassium current IK,ACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Graf E, Wettwer E, Himmel HM, Hala O, Doerfel C, et al. Molecular basis of downregulation of G-protein-coupled inward rectifying K(+) current (I(K,ACh) in chronic human atrial fibrillation: decrease in GIRK4 mRNA correlates with reduced I(K,ACh) and muscarinic receptor-mediated shortening of action potentials. Circulation. 2001;104:2551–2557. doi: 10.1161/hc4601.099466. [DOI] [PubMed] [Google Scholar]
- Dobrev D, Knuschke D, Richter F, Wettwer E, Christ T, Knaut M, et al. Functional identification of m1 and m3 muscarinic acetylcholine receptors in human atrial myocytes: influence of chronic atrial fibrillation Circulation 2002106Suppl. II-154(Abstract) [Google Scholar]
- Ehrlich JR, Cha TJ, Zhang L, Chartier D, Melnyk P, Hohnloser SH, et al. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: action potential and ionic current properties. J Physiol. 2003;551:801–813. doi: 10.1113/jphysiol.2003.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich JR, Cha TJ, Zhang L, Chartier D, Villeneuve L, Hebert TE, et al. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J Physiol. 2004;557:583–597. doi: 10.1113/jphysiol.2004.061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ, Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res. 2002;90:E73–E87. doi: 10.1161/01.res.0000019783.88094.ba. [DOI] [PubMed] [Google Scholar]
- Koumi S, Arentzen CE, Backer CL, Wasserstrom JA. Alterations in muscarinic K+ channel response to acetylcholine and to G protein-mediated activation in atrial myocytes isolated from failing human hearts. Circulation. 1994;90:2213–2224. doi: 10.1161/01.cir.90.5.2213. [DOI] [PubMed] [Google Scholar]
- Kovoor P, Wickman K, Maguire CT, Pu W, Gehrmann J, Berul CI, et al. Evaluation of the role of IKACh in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol. 2001;37:2136–2143. doi: 10.1016/s0735-1097(01)01304-3. [DOI] [PubMed] [Google Scholar]
- Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997;273:H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5830–5836. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- Melnyk P, Ehrlich JR, Pourrier M, Villeneuve L, Cha TJ, Nattel S. Comparison of ion channel distribution and expression in cardiomyocytes of canine pulmonary veins versus left atrium. Cardiovasc Res. 2005;65:104–116. doi: 10.1016/j.cardiores.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- Navarro-Polanco RA, Sanchez-Chapula JA. 4-aminopyridine activates potassium currents by activation of a muscarinic receptor in feline atrial myocytes. J Physiol. 1997;498 Part 3:663–678. doi: 10.1113/jphysiol.1997.sp021891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–1206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Po SS, Li Y, Tang D, Liu H, Geng N, Jackman WM, et al. Rapid and stable re-entry within the pulmonary vein as a mechanism initiating paroxysmal atrial fibrillation. J Am Coll Cardiol. 2005;45:1871–1877. doi: 10.1016/j.jacc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- Po SS, Scherlag BJ, Yamanashi WS, Edwards J, Zhou J, Wu R, et al. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm. 2006;3:201–208. doi: 10.1016/j.hrthm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Razavi M, Zhang S, Yang D, Sanders RA, Kar B, Delapasse S, et al. Effects of pulmonary vein ablation on regional atrial vagal innervation and vulnerability to atrial fibrillation in dogs. J Cardiovasc Electrophysiol. 2005;16:879–884. doi: 10.1111/j.1540-8167.2005.50048.x. [DOI] [PubMed] [Google Scholar]
- Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhi JA, Lefkowitz RJ, et al. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Schauerte P, Scherlag BJ, Patterson E, Scherlag MA, Matsudaria K, Nakagawa H, et al. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol. 2001;12:592–599. doi: 10.1046/j.1540-8167.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, et al. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000;102:2774–2780. doi: 10.1161/01.cir.102.22.2774. [DOI] [PubMed] [Google Scholar]
- Shi H, Wang H, Li D, Nattel S, Wang Z. Differential alterations of receptor densities of three muscarinic acetylcholine receptor subtypes and current densities of the corresponding K+ channels in canine atria with atrial fibrillation induced by experimental congestive heart failure. Cell Physiol Biochem. 2004a;14:31–40. doi: 10.1159/000076924. [DOI] [PubMed] [Google Scholar]
- Shi H, Wang H, Lu Y, Yang B, Wang Z. Choline modulates cardiac membrane repolarization by activating an M3 muscarinic receptor and its coupled K+ channel. J Membr Biol. 1999a;169:55–64. doi: 10.1007/pl00005901. [DOI] [PubMed] [Google Scholar]
- Shi H, Wang H, Wang Z. Identification and characterization of multiple subtypes of muscarinic acetylcholine receptors and their physiological functions in canine hearts. Mol Pharmacol. 1999b;55:497–507. [PubMed] [Google Scholar]
- Shi H, Wang H, Wang Z. M3 muscarinic receptor activation of a delayed rectifier potassium current in canine atrial myocytes. Life Sci. 1999c;64:PL251–PL257. doi: 10.1016/s0024-3205(99)00142-3. [DOI] [PubMed] [Google Scholar]
- Shi H, Wang H, Yang B, Xu D, Wang Z. The M3 receptor-mediated K+ current (IKM3), a G(q) protein-coupled K(+) channel. J Biol Chem. 2004b;279:21774–21778. doi: 10.1074/jbc.C400100200. [DOI] [PubMed] [Google Scholar]
- Shi H, Yang B, Xu D, Wang H, Wang Z. Electrophysiological characterization of cardiac muscarinic acetylcholine receptors: different subtypes mediate different potassium currents. Cell Physiol Biochem. 2003;13:59–74. doi: 10.1159/000070250. [DOI] [PubMed] [Google Scholar]
- Shinagawa K, Li D, Leung TK, Nattel S. Consequences of atrial tachycardia-induced remodeling depend on the preexisting atrial substrate. Circulation. 2002;105:251–257. doi: 10.1161/hc0202.102014. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Jais P, Hocini M, Sanders P, Rotter M, Rostock T, et al. Shortening of fibrillatory cycle length in the pulmonary vein during vagal excitation. J Am Coll Cardiol. 2006;47:774–780. doi: 10.1016/j.jacc.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Wang H, Han H, Zhang L, Shi H, Schram G, Nattel S, et al. Expression of multiple subtypes of muscarinic receptors and cellular distribution in the human heart. Mol Pharmacol. 2001;59:1029–1036. doi: 10.1124/mol.59.5.1029. [DOI] [PubMed] [Google Scholar]
- Wang H, Shi H, Lu Y, Yang B, Wang Z. Pilocarpine modulates the cellular electrical properties of mammalian hearts by activating a cardiac M3 receptor and a K+ current. Br J Pharmacol. 1999;126:1725–1734. doi: 10.1038/sj.bjp.0702486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shi H, Wang H. Functional M3 muscarinic acetylcholine receptors in mammalian hearts. Br J Pharmacol. 2004;142:395–408. doi: 10.1038/sj.bjp.0705787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- Willmy-Matthes P, Leineweber K, Wangemann T, Silber RE, Brodde OE. Existence of functional M3-muscarinic receptors in the human heart. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:316–319. doi: 10.1007/s00210-003-0796-2. [DOI] [PubMed] [Google Scholar]
- Yamada M, Inanobe A, Kurachi Y. G protein regulation of potassium ion channels. Pharmacol Rev. 1998;50:723–760. [PubMed] [Google Scholar]
- Zang WJ, Yu XJ, Honjo H, Kirby MS, Boyett MR. On the role of G protein activation and phosphorylation in desensitization to acetylcholine in guinea-pig atrial cells. J Physiol. 1993;464:649–679. doi: 10.1113/jphysiol.1993.sp019656. [DOI] [PMC free article] [PubMed] [Google Scholar]