Abstract
Background:
The endocannabinoid system functions through two well characterized receptor systems, the CB1 and CB2 receptors. Work by a number of groups in recent years has provided evidence that the system is more complicated and additional receptor types should exist to explain ligand activity in a number of physiological processes.
Experimental approach:
Cells transfected with the human cDNA for GPR55 were tested for their ability to bind and to mediate GTPγS binding by cannabinoid ligands. Using an antibody and peptide blocking approach, the nature of the G-protein coupling was determined and further demonstrated by measuring activity of downstream signalling pathways.
Key results:
We demonstrate that GPR55 binds to and is activated by the cannabinoid ligand CP55940. In addition endocannabinoids including anandamide and virodhamine activate GTPγS binding via GPR55 with nM potencies. Ligands such as cannabidiol and abnormal cannabidiol which exhibit no CB1or CB2 activity and are believed to function at a novel cannabinoid receptor, also showed activity at GPR55. GPR55 couples to Gα13 and can mediate activation of rhoA, cdc42 and rac1.
Conclusions:
These data suggest that GPR55 is a novel cannabinoid receptor, and its ligand profile with respect to CB1 and CB2 described here will permit delineation of its physiological function(s).
Keywords: cannabinoid, GPR55, G-protein-coupled receptor, orphan receptor, rhoA
Introduction
Preparations of Cannabis sativa have been used for medicinal and recreational purposes for at least 4000 years and extracts of C. sativa contain over 60 different pharmacologically active components the most prominent being Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (Mechoulam, 1970a; Mechoulam et al., 1970b; Howlett, 2002). Cannabinoids exert their effects by binding to specific receptors located in the membrane of the cell. Two types of high-affinity cannabinoid receptors have been identified so far by molecular cloning; CB1 receptors (Devane et al., 1988; Matsuda et al., 1990), and CB2 receptors (Munro et al., 1993). Both CB1 and CB2 are coupled to the Gi, G-protein signal transduction pathway. Activation of these cannabinoid receptors leads to inhibition of adenylate cyclase and activation of mitogen-activated protein (MAP) kinase. CB1 receptors can also modulate ion channels, inhibiting N-, and P/R-type calcium channels, stimulating inwardly rectifying potassium channels and enhancing the activation of A-type potassium channels (for recent reviews of cannabinoid signal transduction see Howlett, 2004; Demuth and Molleman, 2006).
Cannabinoid type 1 (CB1) receptors are primarily, but not exclusively expressed in the CNS and are believed to mediate the CNS effects of endogenous (for example, anandamide) and exogenously administered cannabinoids. Peripherally, CB1 receptor expression is found in the pituitary gland, immune cells, reproductive tissues, gastrointestinal tissues, superior cervical ganglion, heart, blood vessels, lung, bladder and adrenal gland (reviewed by Howlett, 2002). Recently, the liver and adipose has been added to the list (Cota et al., 2003; Osei-Hyiaman et al., 2005). CB1 receptors are also located on central and peripheral nerve terminals and when activated, seem to suppress the neuronal release of excitatory and inhibitory transmitters for example, acetylcholine, noradrenaline, dopamine, 5-hydroxytryptamine, γ-amino butyric acid, glutamate and aspartate (Pertwee, 1997, 2001; Ong and Mackie, 1999) adding to the complexity of the physiological responses to the endocannabinoids.
CB2 receptor expression is restricted to the periphery, mainly in immune cells with particularly high levels in B cells and natural killer cells (Galiegue et al., 1995) although it has been reported that the CB2 receptor is expressed in microglia cells of the CNS (Walter et al., 2003) and in brain stem neuronal cells (Van Sickle et al., 2005).
Some studies suggest that endocannabinoids regulate multiple physiological and pathological reproductive functions (Maccarrone et al., 2002) and that endocannabinoids such as 2-arachidonoylglycerol play a role in the progression of the pathophysiology of shock (Cainazzo et al., 2002) and act as immunomodulators (Parolaro et al., 2002). Others have shown that CB2 receptors play a very important role in the stimulation of growth in most haematopoietic lineages (Valk et al., 1997; Derocq et al., 2000). Thus, cannabinoid receptors and endocannabinoids are physiologically or pathophysiologically relevant in a great diversity of tissues and organs like the CNS and cardiovascular, reproductive, endocrine, immune and gastrointestinal systems. Particularly, the CNS and its hypothalamic appetite-regulating control system have attracted much attention over the last ten years and endocannabinoids have classically been shown to play a role in the physiological regulation of food intake (Sofia and Knobloch, 1976; Anderson-Baker et al., 1979; Pacheco et al., 1993; Berry and Mechoulam, 2002; Fride, 2002), effects that are inhibited by the non-endogenous ligand N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-pyrazole-3-carboxamide (SR141716).
A number of endogenous ligands such as anandamide, 2-arachidonoylglycerol, noladin ether, palmitoylethanolamine, virodhamine and oleoylethanolamide (OEA) have been identified, which are believed to modulate the cannabinoid system via the previously identified CB1 and CB2 receptors, or by their action at as-yet unidentified receptors.
In recent years, a number of studies have suggested the existence of additional cannabinoid receptors that function in these processes and these reports have been reviewed by Begg et al. (2005). In this study we show that the orphan G-protein-coupled receptor, GPR55, is a novel cannabinoid receptor with an ability to interact with and be modulated by endogenous, plant and synthetic cannabinoid ligands and to be a candidate for one of the non-CB1/CB2 receptors, described by others.
Methods
Cloning of hGPR55
hGPR55 (EMBL accession no. BC032694) was amplified from human genomic DNA by polymerase chain reaction (PCR) and sub-cloned into mammalian expression plasmids pIRESneo2 and pcDNA3 using standard techniques.
Expression profiling
GPR55 mRNA levels in human and mouse tissues were analysed by quantitative real-time PCR analysis using ABI PRISM 7900 HT sequence detection system (Applied Biosystems, Hercules, CA, USA). Primer/probe sets for hGPR55 were: 5′-TCTACATGATCAACCTGGCAGTCT-3′, 5′-CTGGGACAGGACCATCTTGAA-3′ and 5′-FAM-TGACCTGCTGCTGGTGCTCTCCC-TAMRA-3′, and for mGPR55 were: 5′-CTATCTACATGATCAACTTGGCTGTTT-3′, 5′-TGTGGCAGGACCATCTTGAA-3′ and 5′-FAM-CGATTTACTGCTGGTGCTCTCCCTCCC-TAMRA-3′. To determine relative mRNA levels of GPR55, results were normalized to its content of the mRNA encoding the ribosomal protein 36B4 (used as an internal standard).
Cell transfection and membrane preparation
Human embryonic kidney—HEK293s cells (5 × 106) were seeded in T75 flasks and after 24 h, cells were transiently transfected with 10 μg of relevant plasmid using Lipofectamine Plus (Invitrogen, Carlsbad, CA, USA). Membranes were prepared after 48 h using standard methods and stored at −80 °C. Protein concentration was measured according to the method of Bradford (Bio-rad Laboratories, Foster City, CA, USA) (Bradford, 1976). CB1 and CB2 membranes were commercially available (PerkinElmer).
Radioligand binding assays
Radioligand binding was initiated by the addition of 5 μg of membrane protein to each well of a 96-well plate containing 50 nM [3H]-(−)-cis-3-[2-hydroxy-4-(1,1–dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP55940) (Tocris, Ellisville, Missouri, USA), [3H]-SR141716 (Amersham, Piscataway, NJ, USA) or [3H]-R(+)-[2,3-di-hydro-5-methyl-3-[(morpholinyl)methyl] pyrrolo[1,2,3-de] -1,4-benz-oxazinyl]-(1-naphthalenyl)-methanone-mesylate (WIN55,212-2) (Amersham), sufficient volume of buffer (50 mM Tris–HCl, 5 mM MgCl2, 50 mM NaCl, pH 7.4, 0.1% bovine serum albumin (BSA)) to bring the total volume of each well to 200 μl. Non-specific binding was determined in the presence of 10 μM CP55940 (Tocris), SR141716 and WIN55,212-2 (Tocris). The membranes were incubated at 30 °C for 90 min and the reaction was then terminated by the addition of ice-cold wash buffer (50 mM Tris–HCl, 5 mM MgCl2, 50 mM NaCl, pH 7.4) followed by rapid filtration under vacuum through Printed Filtermat B glass fibre filters (Wallac, Turku, Finland) (0.05% polyethylenimine (PEI)-treated) using a Micro 96 Harvester (Skatron Instruments, Lier, Norway). The filters were dried for 30 min at 50 °C, then a paraffin scintillant pad was melted onto the filters and the bound radioactivity was determined using a 1450 Microbeta Trilux (Wallac) scintillation counter.
[35S]-GTPγS binding assay
[35S]-Guanosine 5′-[γ-35S]-triphosphate (GTPγS) binding assays were conducted at 30 °C for 45 min in membrane buffer (100 mM NaCl, 5 mM, 1 mM EDTA, 50 mM HEPES, pH 7.4) containing 0.025 μg μl−1 of membrane protein with 0.01% BSA (fatty-acid free) (Sigma, St Louis, MO, USA), 10 μM guanosine 5′-diphosphate (GDP) (Sigma), 100 μM dithiothreitol (DTT) (Sigma) and 0.53 nM [35S]-GTPγS (Amersham) in a final volume of 200 μl. Non-specific binding was determined in the presence of 20 μM unlabelled GTPγS (Sigma). The reaction was terminated by addition of ice-cold wash buffer (50 mM Tris–HCl, 5 mM MgCl2, 50 mM NaCl, pH 7.4) followed by rapid filtration under vacuum through Wallac GF/B glass-fibre filters using a cell harvester (Skatron). The filters were left to dry for 30 min at 50 °C, then a paraffin scintillant pad was melted onto the filters and the bound radioactivity was determined using a microbeta scintillation counter (Wallac). Antagonist potency was determined versus an EC80 concentration of CP55940 that was determined empirically on the day of the experiment. Data were fitted using the equation y=A+((B−A)/1+((C/x)∧D))) and the EC50 estimated where A is the non-specific binding, B is the total binding, C is the IC50 and D is the slope.
Peptide and antibody blocking of [35S]-GTPγS binding assays
[35S]-GTPγS binding assays were performed as above with additional pre-incubation of membranes with and without peptides or antibodies for the G-protein subunits Gα13, Gαi and Gαs for 15 min at 30 °C (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Data were analysed using paired t-test (**P<0.05; ***P<0.01).
Pertussis toxin treatment
Cell transfections were conducted as described above with the exception that the cells prior to harvesting were pre-incubated with Pertussis toxin (Sigma) overnight (0.1 μg ml−1 final concentration). The cells were then harvested and membranes were prepared as described above.
Plate-based FLIPR Ca2+ assays
In brief, 1 day before the assay was performed, HEK293 cells expressing GRP55 were plated in 96-well, black-walled, assay plates, at a density of 25 000 cells per well. These plates were then returned to the cell-culture incubator until 1.5 h before the assay when they were removed and the cells were loaded with the Ca2+ reporter dye Fluo4 (Invitrogen) for 1 h in a cell-culture incubator. After this, the plates were placed into a fluorescent imaging plate reader (FLIPR) to monitor fluorescence (λex=488 and λEM=540 nm) before and after the addition of ligands of interest.
Determination of rhoA, rac1 and cdc42 activity
RhoA, rac1 and cdc42 activity was measured according to the manufacturer's instructions (Upstate Biotechnology Inc., Charlottesville, Virginia, USA). HEK293s GPR55-transfected cells were seeded on six-well plates, grown to 80% confluence, and serum-starved for 24 h. Following treatment with selected compounds at 37 °C for 15 min, the cells were washed with phosphate-buffered saline and harvested with 500 μl of lysis buffer provided by the manufacturer, with the addition of a mixture of protease inhibitors (Roche Molecular Biochemicals, Basel, Switzerland). The cell lysates were clarified by centrifugation at 15 000 g for 1 min. For a negative control, cell lysate was incubated with 1 mM GDP for 15 min at 30 °C. The cell lysates were then incubated with 10 μg of GST-RBD-agarose (Rho-binding domain of rhotekin) or GST-PBD-agarose (p21-binding domain of human PAK-1) to precipitate GTP-bound rhoA and GTP-bound rac1 and cdc42, respectively. The beads were then washed three times with lysis buffer and samples were prepared for electrophoresis by adding 1 × sodium dodecyl sulphate loading dye. Samples were boiled for 5 min and resolved by 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis . Bound rhoA, rac1 and cdc42 were detected by western blot using the appropriate polyclonal antibodies specific for rac1, cdc42 (1:1000; Upstate Biotechnology) and rhoA (1:200; Santa Cruz Biotechnology).
Results
Cloning and sequence determination of GPR55
Nucleotide primers designed against the 5′- and 3′-ends of human, mouse and rat gpr55 were used to isolate open reading frames for GPR55 from the three species using genomic DNA. The sequence of the human gene was similar but not identical to that already described (Sawzdargo et al., 1999), however it was consistent with the human genome sequence. The sequence of all clones isolated differed in that there was a nucleotide insertion and deletion at positions 393 and 427 respectively, resulting in a frame shift of the translated sequence, consequently changing 11 amino acids at the predicted junction of intracellular loop 2 and transmembrane helix 4 (Figure 1). Since we could find no evidence for the existence of the previously published sequence, we concluded that the difference originated from a sequencing error by the authors (Sawzdargo et al., 1999).
Figure 1.
Alignment between human (hGPR55), mouse (mGPR55) and rat (rGPR55) GPR55 protein sequences. The putative positions of the transmembrane regions (TM1-7), extracellular loops (EC1-3) and intracellular loops (EC1-3) are shown. The amino-acid differences in the previously published sequence (Sawzdargo et al., 1999) for human GPR55 at the IC2/TM4 boundary are shown above the sequences.
The rat and mouse genes were cloned using a similar approach and their sequences were found to be identical to those found in GenBank (AC119315 (position 129078–130085) AC107707 (position 31198–32181)) demonstrating 75 and 78% identity to the human sequence respectively (Figure 1). Both the rat and mouse sequences are consistent with the human genome sequence in the region of the intracellular loop 2—transmembrane helix 4 region rather than the published sequence (Sawzdargo et al., 1999) containing the insertion and deletion (Figure 1). Despite the low level of identity between the human and rodent forms of GPR55, the genomic linkage confirms that the rodent genes are orthologues of the human gpr55. Phylogenetically, the GPR55 sequence belongs to a cluster of receptors that are either orphans (GPR35, GPR92, P2Y5) or have been recently deorphanized (P2Y9 (Noguchi et al., 2003), GPR40 (Briscoe et al., 2003), GPR41 and GPR43 (Brown et al., 2003)).
Expression profile of GPR55
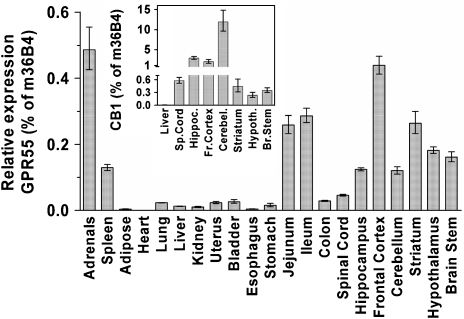
We next investigated the expression pattern of GPR55 in a panel of mouse tissues using quantitative PCR (Figure 2). GPR55 mRNA is found in a number of tissues with the highest mRNA levels detected in the adrenals, parts of the gastrointestinal tract, as well as in the CNS. As seen with CB1 receptors, a broad distribution of GPR55 mRNA is found in brain tissue, however the levels are significantly lower than those for CB1 (Figure 2, inset).
Figure 2.
mRNA expression levels of GPR55 and CB1 receptors in mouse tissues measured by quantitative PCR relative to m36B4. Tissues were dissected from C57BL/6 female mice. Samples from different mice were processed individually in all subsequent steps; RNA preparation, cDNA synthesis and quantitative PCR. Data are mean values±s.e.m. using tissues from eight (GPR55) or four mice (CB1) and presented as per cent of the ubiquitously and homogenously expressed ribosomal protein 36B4.
GPR55 binds and is activated by cannabinoid ligands
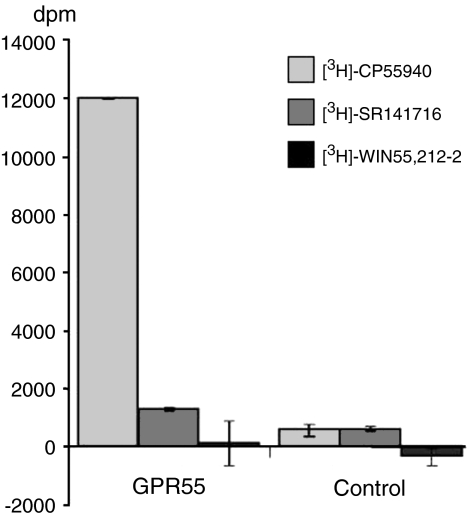
To test the possibility that GPR55 maybe a cannabinoid receptor, we generated an N terminus FLAG-tagged human GPR55 and transiently transfected the plasmid containing the cDNA into HEK293s cells. Cell-surface expression of the recombinant receptor was confirmed using an anti-FLAG antibody (Figure 3). We then examined the ability of the cannabinoid receptor radioligands [3H]-CP55940, [3H]-SR141716 and [3H]-WIN55,212-2 to bind to membranes prepared from the transiently transfected cells. No specific binding was observed using 50 nM of each radioligand in membranes prepared from untransfected HEK293s cells. However, in membranes prepared from HEK293s cells transfected with the FLAG-tagged cDNA for human GPR55, a clear specific binding for [3H]-CP55940 was observed (Figure 4). In addition, a small specific binding for [3H]-SR141716 was seen whereas there was no binding for [3H]-WIN55,212-2 (Figure 4). Subsequent experiments repeated these findings using alternative ligands as unlabelled competitors to confirm specificity. As a consequence of these observations, we generated a HEK293s cell line stably expressing the FLAG-tagged human GPR55. Cell-surface expression was confirmed using an anti-FLAG antibody and this cell line was used for further studies.
Figure 3.
Cell-surface expression of FLAG-tagged hGPR55. Immunofluorescence images of anti-FLAG-stained HEK293s cells transiently transfected with FLAG-hGPR55 (a) or empty vector (Vec.co; (c)). Corresponding phase-contrast images are shown in (b) and (d).
Figure 4.
Radioligand binding to GPR55. Membranes prepared from cells transiently transfected with hGPR55 or vector control were incubated with 50 nM [3H]-CP55940, [3H]-SR141716 or [3H]-WIN55,212-2. Specific binding was determined by the addition of 10 μM unlabelled ligand as competitor. The bars show the specific binding (mean±s.e.m.; n=5) determined for each ligand.
We, next determined whether the interaction of CP55940 with GPR55 had a functional consequence. Since GTPγS has the potential to pick up activation of most heterotrimeric G proteins if the experimental conditions are appropriate, we tested membranes expressing GPR55 using a factorial design strategy with and without 1 μM CP55940 varying GDP, MgCl2, NaCl and saponin. A number of the conditions tested generated an increased GTPγS binding in the GPR55-containing membranes, but not with control membranes, in the presence of CP55940 (data not shown). Using the optimum condition identified (see Methods), we found that CP55940 stimulated GTPγS binding with an EC50 of 5 nM (Figure 5a and Table 1). With this finding we went on to evaluate other cannabinoid ligands for their ability to promote GTPγS binding via GPR55.
Figure 5.
(a) Concentration–response curves for various ligands at GPR55 determined using a GTPγS assay: (a) CP55940 and Δ9-THC; (b) cannabidiol antagonism of O1602 activation; (c) anandamide and WIN55,212-2; (d) O1602 and abnormal cannabidiol. Values shown are mean±s.e.m.; n=5.
Table 1.
Profile of agonist activities of ligands at GPR55, CB1 and CB2 receptors
| Ligand | GPR55 EC50 (nM) GTPγS binding | GPR55 Emax (%) | CB1 EC50 (nM) GTPγS binding | CB1 Emax(%) | CB2 EC50 (nM) GTPγS binding | CB2 Emax(%) |
|---|---|---|---|---|---|---|
| Anandamide | 18±3 | 73±5 | 31±6 | 66±4 | 27±6 | 58±5 |
| Noladin ether | 10±1 | 95±7 | 37±5 | 89±5 | >30 000 | |
| 2-Arachidonoylglycerol | 3±1 | 99±2 | 519±48 | 92±6 | 618±45 | 87±5 |
| Virodhamine | 12±3 | 160±10 | 2920±325 | 75±9 | 381±34 | 91±10 |
| Palmitoylethanolamide | 4±1 | 92±1 | >30 000 | 19 800±2821 | 93±12 | |
| Oleoylethanolamide | 440±145 | 92±3 | >30 000 | >30 000 | ||
| Δ9-THC | 8±1 | 92±5 | 6±1 | 61±5 | 0.4±0.1 | 67±3 |
| Cannabidiol | Antagonist | >30 000 | >30 000 | |||
| Cannabinol | >30 000 | >30 000 | >30 000 | |||
| Abnormal cannabidiol | 2523±579 | 76±17 | >30 000 | >30 000 | ||
| AM281 | >30 000 | Antagonist | Antagonist | |||
| AM251 | 39±3 | 88±4 | Antagonist | Antagonist | ||
| WIN55,212-2 | >30 000 | 18±3 | 101±14 | 1±0.2 | 97±8 | |
| HU210 | 26±7 | 78±3 | 0.2±0.03 | 91±2 | 0.5±0.1 | 99±6 |
| O1602 | 13±2 | 99±4 | >30 000 | >30 000 | ||
| CP55940 | 5±1 | 100±2 | 0.2±0.01 | 100±2 | 0.3±0.01 | 100±4 |
Values shown are the means±s.e.m. derived from five independent experiments.
A number of endogenous cannabinoid ligands have been identified and characterized to date and we therefore examined their effect upon GPR55. The endocannabinoid anandamide stimulated GTPγS binding with an EC50 of 18 nM (Figure 5b and Table 1). The other endocannabinoids, 2 arachidonoylglycerol (2-AG), noladin ether, palmitoylethanolamide, virodhamine and OEA all stimulated GTPγS binding with EC50 values of 3, 10, 4, 12 and 440 nM respectively (Table 1). In parallel experiments, all these compounds generated the expected activities at CB1 and CB2 receptors (Table 1). None of these ligands had any effect when tested under identical conditions against membranes prepared from untransfected cells. Of note is the efficacy of virodhamine which under the assay conditions used is approximately 160% that of the other endocannabinoid ligands, noladin ether and 2-AG and double the efficacy of anandamide.
We next tested Δ9-THC, the psychoactive component of the cannabis plant C. sativa, for its activity at GPR55. Δ9-THC activated GTPγS binding with an EC50 of 8 nM (Figure 5a and Table 1). We also examined the effect of cannabinol, cannabidiol and related compounds. Cannabidiol was without effect as an agonist in the GTPγS assay. However, cannabidiol was able to antagonize the agonist effect of CP55940 with an IC50 of 445 nM (Figure 5b and Table 2). Abnormal cannabidiol functioned as an agonist with an EC50 of 2.5 μM while a similar compound O1602, was significantly more potent at 13 nM. (−) 11-OH-8-Tetrahydrocannabinol-dimethylheptyl (HU210) is a highly potent CB1 agonist and also demonstrated agonist activity at GPR55 with a potency of 26 nM, which is more than a 100-fold less potent than that found in parallel experiments at the CB1 receptor (Table 1). A commonly used tool ligand of the cannabinoid system is WIN55,212-2. Consistent with the demonstrated lack of binding activity of this compound in our initial experiments, we observed no functional activity of WIN55,212-2 as either an agonist or antagonist (Figure 5c and Table 1). Finally, we tested the ability of known antagonists of CB1 receptors for their effect at GPR55. 1-(2,4-Dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide (AM281) was without effect as either an agonist or antagonist whereas 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide (AM251) behaved as an agonist with an EC50 of 39 nM. In all the experiments described above, the data were the same whether the receptor was expressed with or without the FLAG epitope.
Table 2.
Profile of antagonist activities of ligands at GPR55, CB1 and CB2 receptors
| Ligand | GPR55 IC50 (nM) GTPγS binding | CB1 IC50 (nM) GTPγS binding | CB2 IC50 (nM) GTPγS binding |
|---|---|---|---|
| Cannabidiol | 445±67 | >30 000 | >30 000 |
| Cannabinol | >30 000 | >30 000 | >30 000 |
| AM281 | >30 000 | 7±0.6 | 2600±463 |
| AM251 | Agonist | 8±1 | 2915±102 |
| WIN55,212-2 | >30 000 | Agonist | Agonist |
Data obtained using an EC80 concentration of CP55940 as agonist for each receptor. Values shown are the means±s.e.m. derived from five independent experiments.
G-protein coupling of GPR55
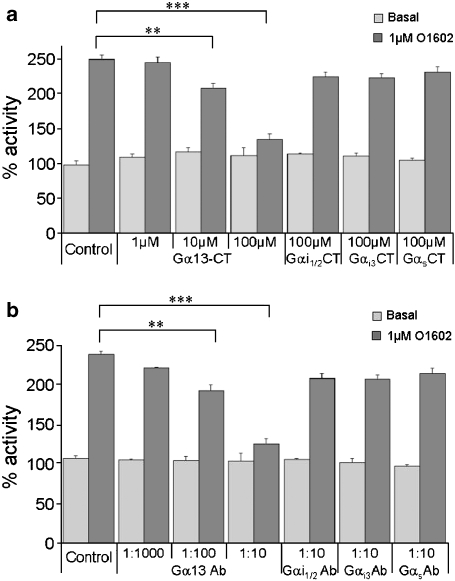
We next investigated the nature of the signalling pathway activated by GPR55 by examining the G-protein coupling. In the first instance, we examined the effect of Pertussis toxin on the ability of GPR55 to mediate GTPγS binding. Membranes prepared from cells treated with toxin were still able to mediate a robust response to compounds shown to be agonists of GPR55 (data not shown), suggesting that Gi G-proteins are not involved downstream of GPR55. We also tested GPR55-expressing HEK293s cells using FLIPR to determine whether there was evidence of a calcium signal that could be indicative of Gq coupling. No agonist-mediated calcium signalling was detected when compared to untransfected cells suggesting that Gq was not coupling to GPR55. To further investigate the G-protein signalling pathway downstream of GPR55 we took an antibody and peptide blocking approach in the GTPγS assay. Peptides equivalent to the last 12 amino acids of Gαi1/2, Gαi3, Gαs and Gα13 were incubated with GPR55-containing membranes for 15 min prior to performing GTPγS assays. The peptides equivalent to Gi1/2, Gi3 and Gs had no effect upon the GTPγS signal consistent with the lack of effect of Pertussis toxin (Figure 6a). However, the G13 peptide dose dependently inhibited GTPγS binding suggesting that this peptide makes a specific interaction with GPR55 and prevents the receptor coupling to and activating G13 (Figure 6a). A similar experiment was then performed using antibodies raised against the C-terminal peptides of the different G proteins. Consistent with the peptide studies anti-Gi1/2, anti-Gi3 and anti-Gαs had no effect upon GTPγS binding mediated by GPR55 (Figure 6b). At the same time, anti-Gα13 prevented GTPγS binding in a dose-dependent manner, demonstrating that the GTPγS signal being measured as a consequence of agonist activity at GPR55 was a result of G13 activation (Figure 6b).
Figure 6.
Mapping G-protein coupling of GPR55. (a) Basal and 1 μM O1602 stimulated GTPγS binding (% activity, mean±s.e.m.) in human GPR55-expressing membranes in the absence and presence of various concentrations of peptides equivalent to the C termini of Gα13, Gαi1/2, Gαi3 and Gαs. (b) Basal and 1 μM stimulated GTPγS binding (% activity, mean±s.e.m.) in human GPR55-expressing membranes in the absence and presence of various dilutions of antibodies that bind to the C termini of Gα13, Gαi1/2, Gαi3 and Gαs. Data were analysed using paired t-test (**P<0.05; ***P<0.01; n=5).
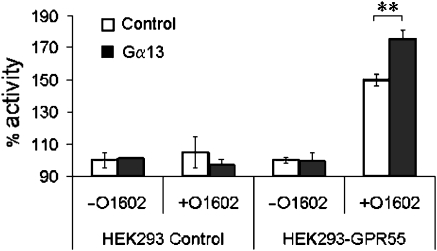
To further demonstrate that the signalling of GPR55 was Gα13–mediated, we performed additional studies. Cells stably expressing human GPR55 were transiently transfected with plasmid DNA containing the human Gα13 gene or with vector control. As shown in Figure 7, while the vector control did not change GTPγS readout, the membranes prepared from the Gα13-transfected cells showed an augmented signal in response to cannabinoid ligands, indicative of increased expression of the coupling G protein.
Figure 7.
Transfection of Gα13 into GPR55-expressing HEK293 cells leads to an increased GTPγS signal via GPR55. Membranes prepared from HEK293s cells and HEK293s-GPR55-expressing cells were transfected with transfected with either control or Gα13-containing plasmids and tested in a GTPγS with and without 1 μM O1602. Membranes containing GPR55 demonstrate a clear increase in GTPγS binding as a result of overexpression of Gα13. Data (mean±s.e.m.) were analysed using paired t-test (**P<0.05; n=5).
Downstream signalling by GPR55
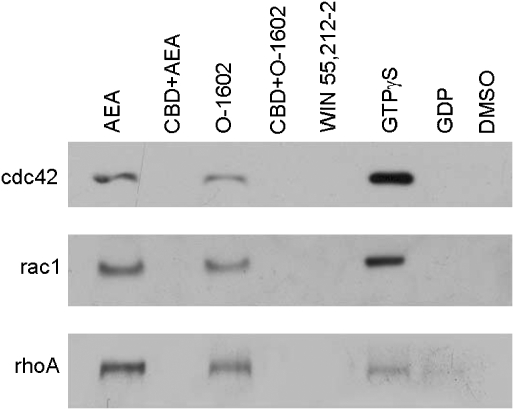
Assuming therefore that GPR55 is Gα13-coupled, it is reasonable to expect that downstream signalling pathways of the G protein will be activated in a GPR55-dependent manner. To this effect, we looked at the activation of rhoA, cdc42 and rac1 in response to various ligands in GPR55-expressing and control HEK293s cells. Figure 8 shows that both anandamide and O1602 but not WIN55,212-2 treatment induced the activation of rhoA, cdc42 and rac1. This effect was blocked by the GPR55 antagonist, cannabidiol.
Figure 8.
Activation of GPR55 leads to activation of rhoA, cdc42 and rac1. Cells transfected with GPR55 demonstrated O1602-(1 μM) and anandamide (1 μM)-mediated activation of the small G proteins rhoA, cdc42 and rac1 while the non-GPR55-activating ligand WIN55,212-2 had no effect. The activation was blocked by cannabidiol (10 μM) while the positive control GTPγS and negative controls (GDP and dimethyl sulphoxide ) generated the expected responses. The blots shown are representative of three independent experiments.
Discussion and conclusions
In recent years, it has been suggested that there are cannabinoid receptors in addition to CB1 and CB2 in brain (Di Marzo et al., 2000; Hajos et al., 2001; Monory et al., 2002), vascular endothelium (Jarai et al., 1999) and vascular smooth muscle (Ho and Hiley, 2003) as well as in the immune system (Kaplan et al., 2003). In this study, we describe that the orphan G-protein-coupled receptor, GPR55, is expressed in these tissues and is liganded by a range of endogenous, plant-derived and synthetic cannabinoid ligands.
GPR55 was specifically bound and activated by the synthetic cannabinoid ligand CP55940 (Table 1). CP55940 interacts with GPR55 at a potency 25-fold lower than at CB1 in the comparable experimental system used here. [3H]-CP55940 has been used in several studies (Zimmer et al., 1999; Buckley et al., 2000) to examine cannabinoid receptor distribution. Because GPR55 binds the radioligand [3H]-CP55940 it may be expected that this radioligand would detect the presence of GPR55, especially in CB1 and CB2 knockout mice, but this has not been the case (Zimmer et al., 1999). We conclude that the lower affinity of CP55940 for GPR55 without suitably adapted conditions may prevent the detection of GPR55. Taken together, these findings imply that the detection of GPR55 using [3H]-CP55940 in CB1 knockout mice should be possible if sufficient concentrations of radioligand are used. We have also shown that WIN55,212-2 does not display any activity towards GPR55. Since WIN55,212-2 has been used to define specific binding of [3H]-CP55940 in some of the studies reported, a specific binding to GPR55 would not be detectable. WIN55,212-2 has however been reported to influence activity at a novel cannabinoid receptor in the CNS (Hajos et al., 2001) and, as WIN55,212-2 does not bind to or induce activity of GPR55, this receptor is not the brain receptor described, pointing to the presence of at least two novel non-CB1/CB2 receptors in the CNS, one of which is GPR55.
Another area of non-CB1/CB2 pharmacology relevant for GPR55 is control of vascular tone. We have shown that WIN55,212-2 is not a ligand for GPR55 while abnormal cannabidiol is an agonist and cannabidiol is an antagonist. WIN55,212-2 has been shown to be without effect at novel CB receptors in the vasculature while abnormal cannabidiol behaves as an agonist and cannabidiol is an antagonist (Jarai et al., 1999). The finding that cannabidiol is an antagonist of GPR55 is interesting since until recently (Thomas et al., 2007) it has not been shown to have any significant effect on CB1 and CB2 receptor signalling (Pertwee, 1997), as confirmed by our studies (Table 1). Clearly, the precise pharmacology of this ligand remains to be determined. In addition, O1602, an analogue of abnormal cannabidiol reported to be active in vaso-relaxation (Jarai et al., 1999) was found by us to be a potent agonist of GPR55. Another aspect of the GPR55 pharmacology consistent with a novel cannabinoid receptor in the vasculature is the potent activation by virodhamine (Ho and Hiley, 2004) which appears to be more selective for GPR55 versus CB1 and CB2 receptors compared with anandamide (Table 1). Taken together, these findings suggest that GPR55 is a prime candidate for a cannabinoid vascular tone-controlling receptor. Other aspects of the GPR55 receptor may seem inconsistent with a role in vascular tone control. HU210, widely used in the study of cannabinoids, has been shown to affect many physiological processes including vascular tone control and this activity has been attributed to its activity at CB1 receptors since no effect is observed in CB1 knockout mice. However, it needs to be considered if appropriate concentrations have been selected to conclusively say that HU210 has no effect through non-CB1-mediated processes (Jarai et al., 1999), since HU210 is more than 100 times less potent at GPR55 than at CB1 receptors (Table 1)
Yet another aspect of non-CB1/CB2 pharmacology that is relevant to GPR55 based on its expression profile, is immune cell function and cell migration. We show that palmitoylethanolamide (PEA) is a potent and selective agonist of GPR55. PEA has been reported to affect inflammatory activities (Lambert et al., 2002) and microglial cell migration (Franklin et al., 2003) and it has been accepted that these effects, at least in part, are via CB2 receptors. Nevertheless, PEA has also been demonstrated to be activating anti-inflammatory activities through peroxisome proliferator-activated receptor α mediation (Lo Verme et al., 2005) and thus such contributions to an anti-inflammatory effect have to be considered. However, PEA activity in microglial-cell migration also overlaps with an activity of abnormal cannabidiol at the so-called abnormal cannabidiol-sensitive receptors in the same cells (Franklin and Stella, 2003), and could be said to advocate GPR55 as a target for its function. (Table 2).
It is also noteworthy that anandamide, the predominant circulating endocannabinoid, activated GPR55 with a potency equivalent to that activating CB1 and CB2 receptors, demonstrating that this ligand has the potential to influence signalling by all three receptors equally. Anandamide has been found to be active at non-CB1/CB2 receptors (Begg et al., 2005) and GPR55 should now be considered a candidate for these receptors. In contrast, PEA, 2-AG and virodhamine show significantly more potent action through GPR55 than through either CB1 or CB2, suggesting that GPR55 is more likely to be the cognate receptor for these ligands.
Most of the reports describing non-CB1/CB2 receptors suggest that several, though not all (for example Vaccani et al. (2005) of these receptors are Gi-coupled, since they appear to be Pertussis toxin sensitive (Begg et al., 2005). In contrast, GPR55 appears to be G13 coupled at least in the recombinant systems we have tested. This observation may be taken to disqualify GPR55 for a role in the Pertussis toxin-sensitive cannabinoid-mediated activities. However, the mechanism of Pertussis toxin action results in preventing Gi G proteins interacting with their receptors. Since Gi G proteins are highly abundant and the levels of G13 are considered to be lower, it should be considered that the Pertussis toxin effect may also be a consequence of G13 being bound and sequestered by Gi-coupled receptors resulting in a dominant-negative effect. Furthermore, we have demonstrated that GPR55 also mediates activation of the small G proteins rhoA, cdc42 and rac1. Such an observation is consistent with the G13 coupling we have described and fits well with the cannabidiol-mediated effects on cell migration that are Pertussis toxin insensitive and described for non-CB1/CB2 cannabinoid receptors in glial cells (Vaccani et al., 2005).
The results presented herein demonstrate that the orphan G-protein-coupled receptor, GPR55, binds a range of endogenous, plant-derived and synthetic cannabinoid ligands. While the data themselves do not, at this stage, point to an unequivocal role for this receptor in any particular cannabinoid function, the comparative ligand profile that we have described provides the tools to start dissecting the functions of GPR55.
Acknowledgments
We thank our colleagues Annika Åstrand, Anna Linblom, Per-Ove Sjöquist, Rita Raddatz, Rosemarie Panetta and Thierry Groblewski for useful discussions and technical assistance. We also acknowledge Patrik Holm for chemical synthesis.
Abbreviations
- 2-AG
2 arachidonoylglycerol
- AM251
1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide
- AM281
1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide
- BSA
bovine serum albumin
- CP55940
(−)-cis-3-[2-hydroxy-4-(1,1–dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- DTT
dithiothreitol
- FLIPR
fluorescent imaging plate reader
- GDP
guanosine 5′-diphosphate
- GTPγS
guanosine 5′-[γ-35S]-triphosphate
- HEK
human embryonic kidney
- HU210
(−) 11-OH-8-tetrahydrocannabinol-dimethylheptyl
- OEA
oleoylethanolamide
- PCR
polymerase chain reaction
- PEA
palmitoylethanolamide
- PEI
polyethylenimine
- SR141716
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-pyrazole-3-carboxamide
- Δ9-THC
Δ9-tetrahydrocannabinol
- WIN55,212-2
R(+)-[2,3-di-hydro-5-methyl-3-[(morpholinyl)methyl] pyrrolo[1,2,3-de] -1,4-benz-oxazinyl]-(1-naphthalenyl)-methanone-mesylate
Conflict of interest
The authors state no conflict of interest.
References
- Anderson-Baker WC, McLaughlin CL, Baile CA. Oral and hypothalamic injections of barbiturates, benzodiazepines and cannabinoids and food intake in rats. Pharmacol Biochem Behav. 1979;11:487–491. doi: 10.1016/0091-3057(79)90030-3. [DOI] [PubMed] [Google Scholar]
- Begg M, Pacher P, Bátkai S, Osei-Hyiaman D, Offertáler L, Ming Mo F, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–145. doi: 10.1016/j.pharmthera.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Berry EM, Mechoulam R. Tetrahydrocannabinol and endocannabinoids in feeding and appetite. Pharmacol Ther. 2002;95:185–190. doi: 10.1016/s0163-7258(02)00257-7. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. [see comment] J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2000;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- Cainazzo MM, Ferrazza G, Mioni C, Bazzani C, Bertolini A, Guarini S. Cannabinoid CB(1) receptor blockade enhances the protective effect of melanocortins in hemorrhagic shock in the rat. Eur J Pharmacol. 2002;441:91–97. doi: 10.1016/s0014-2999(02)01487-5. [DOI] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschop M, Grubler Y, Flachskamm C, Schubert M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. [see comment] J Clin Invest. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Derocq JM, Jbilo O, Bouaboula M, Segui M, Clere C, Casellas P. Genomic and functional changes induced by the activation of the peripheral cannabinoid receptor CB2 in the promyelocytic cells HL-60. Possible involvement of the CB2 receptor in cell differentiation. J Biol Chem. 2000;275:15621–15628. doi: 10.1074/jbc.275.21.15621. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Di Marzo V, Breivogel CS, Tao Q, Bridgen DT, Razdan RK, Zimmer AM, et al. Levels, metabolism, and pharmacological activity of anandamide in CB(1) cannabinoid receptor knockout mice: evidence for non-CB(1), non-CB(2) receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. doi: 10.1046/j.1471-4159.2000.0752434.x. [DOI] [PubMed] [Google Scholar]
- Franklin A, Parmentier-Batteur S, Walter L, Greenberg DA, Stella N. Palmitoylethanolamide increases after focal cerebral ischemia and potentiates microglial cell motility. J Neurosci. 2003;23:7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin A, Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur J Pharmacol. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- Fride E. Endocannabinoids in the central nervous system—an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:221–233. doi: 10.1054/plef.2001.0360. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasorelaxant activities of the putative endocannabinoid virodhamine in rat isolated small mesenteric artery. J Pharm Pharmacol. 2004;56:869–875. doi: 10.1211/0022357023682. [DOI] [PubMed] [Google Scholar]
- Howlett A. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142:1209–1218. doi: 10.1038/sj.bjp.0705881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BL, Rockwell CE, Kaminski NE. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J Pharmacol Exp Ther. 2003;306:1077–1085. doi: 10.1124/jpet.103.051961. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Vandevoorde S, Jonsson KO, Fowler CJ. The palmitoylethanolamide family: a new class of anti-inflammatory agents? Curr Med Chem. 2002;9:663–674. doi: 10.2174/0929867023370707. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Falciglia K, Di Rienzo M, Finazzi-Agro A. Endocannabinoids, hormone-cytokine networks and human fertility. Prostaglandins Leukot Essent Fatty Acids. 2002;66:309–317. doi: 10.1054/plef.2001.0354. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. [see comment] Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Marihuana chemistry. Science. 1970a;168:1159–1166. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Shani A, Edery H, Grunfeld Y. Chemical basis of hashish activity. Science. 1970b;169:611–612. doi: 10.1126/science.169.3945.611. [DOI] [PubMed] [Google Scholar]
- Monory K, Tzavara ET, Lexime J, Ledent C, Parmentier M, Borsodi A, et al. Novel, not adenylyl cyclase-coupled cannabinoid binding site in cerebellum of mice. Biochem Biophys Res Commun. 2002;292:231–235. doi: 10.1006/bbrc.2002.6635. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. [see comment] Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience. 1999;92:1177–1191. doi: 10.1016/s0306-4522(99)00025-1. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. [see comment] J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, Ward SJ, Childers SR. Identification of cannabinoid receptors in cultures of rat cerebellar granule cells. Brain Res. 1993;603:102–110. doi: 10.1016/0006-8993(93)91304-b. [DOI] [PubMed] [Google Scholar]
- Parolaro D, Massi P, Rubino T, Monti E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot Essent Fatty Acids. 2002;66:319–332. doi: 10.1054/plef.2001.0355. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Sawzdargo M, Nguyen T, Lee DK, Lynch KR, Cheng R, Heng HH, et al. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res Mol Brain Res. 1999;64:193–198. doi: 10.1016/s0169-328x(98)00277-0. [DOI] [PubMed] [Google Scholar]
- Sofia RD, Knobloch LC. Comparative effects of various naturally occurring cannabinoids on food, sucrose and water consumption by rats. Pharmacol Biochem Behav. 1976;4:591–599. doi: 10.1016/0091-3057(76)90202-1. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2005;144:1032–1036. doi: 10.1038/sj.bjp.0706134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk P, Verbakel S, Vankan Y, Hol S, Mancham S, Ploemacher R, et al. Anandamide, a natural ligand for the peripheral cannabinoid receptor is a novel synergistic growth factor for hematopoietic cells. Blood. 1997;90:1448–1457. [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, et al. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. [see comment] Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]