Abstract
Background and purpose:
Although the main therapeutic effect of angiotensin AT1 receptor antagonists is to decrease blood pressure, they also exert anti-inflammatory effects in the cardiovascular system. However, the underlying mechanisms remain unclear. We investigated the inhibitory effect of AT1 antagonists on the chemokine monocyte chemoattractant protein 1 (MCP-1) and its receptor C-C chemokine receptor 2 (CCR2) in rat monocytes and aortas.
Experimental approach:
Spontaneous hypertensive rats (SHRs) were treated with the AT1 antagonists losartan or telmisartan for 4 weeks, and Wistar-Kyoto rats (WKYs) were used as normotensive controls. Systolic arterial pressure was measured, and the number of macrophages in the aortic vessel wall was assessed by anti-ED-1 antibody immunolabelling.
Key results:
Compared with WKYs, SHRs showed significantly increased ED-1 positive macrophages in the aortic wall, which were decreased after high doses of losartan or telmisartan. Low doses of losartan did not improve blood pressure significantly as did the high doses, but markedly decreased macrophage infiltration in the vessel wall. AT1 antagonists, particularly at high doses, improved aortic remodeling in SHR. At the molecular level, AT1 antagonists attenuated the expression of MCP-1 and CCR2 in the aorta and peripheral blood monocytes and lowered the serum level of MCP-1. In addition, Western blotting showed that AT1 antagonists inhibited the phosphorylation of Akt in mouse monocytes.
Conclusions and implications:
AT1 antagonism inhibited vessel wall inflammation and inhibition of PI3K/Akt may be involved in the modulation of the MCP-1/CCR2 system by AT1 antagonists in SHRs.
Keywords: AT1 receptor antagonist, spontaneous hypersensitive rats (SHRs), MCP-1/CCR2, PI3K/Akt
Introduction
Hypertension is a risk factor for vascular diseases such as atherosclerosis, but the molecular mechanism by which hypertension leads to vascular inflammation is not well understood (Alexander, 2006; Pauletto and Rattazzi, 2006). Angiotensin II (Ang II) is a vasoactive peptide with a variety of effects, including alleviating vessel inflammation. Ang II induces inflammation through the production of reactive oxygen species, adhesion molecules and inflammatory cytokines such as monocyte chemoattractant protein-1 (MCP-1). As selective inhibitors of the Ang II receptor AT1, AT1 antagonists are used clinically for reducing arterial blood pressure. MCP-1 acts as a central mediator of the inflammatory response in hypertensive vascular disease (Usui et al., 2000; Sanz-Rosa et al., 2005; Willemsen et al., 2007). In response to Ang II, monocytes increase MCP-1 secretion, which plays an essential role in increased inflammation in the vessel (Ni et al., 2004). Previous studies reported that although activation of the renin-angiotensin system upregulates MCP-1, AT1 antagonism significantly decreases MCP-1 expression (Chen et al., 2003; Esteban et al., 2005; Ouyang et al., 2005). In addition, the C-C chemokine receptor 2 (CCR2), one receptor for MCP-1, is involved in Ang II-induced atherosclerosis (Bush et al., 2000; Bruemmer et al., 2003; Morita et al., 2003; Ishibashi et al., 2004a). Ang II-induced macrophage infiltration into the arterial wall has been shown to be markedly decreased in CCR2-deficient mice (Bush et al., 2000)
We hypothesized that the MCP-1/CCR2 system mediated the anti-inflammatory effect of AT1 antagonists in hypertensive animals. Our results showed that macrophage infiltration into vessel wall was greatly decreased with AT1A treatment. As well, the ratios of left-ventricular mass of the heart to body weight (LVM/BW) and aortic intima-medial thickness (IMT) to diameter (IMT/D), and aortic perivascular fibrosis ratio (PVFR), were all attenuated. The intracellular signalling pathway of phosphoinositide-3 kinase (PI3K)-Akt seems to be involved in this anti-inflammatory effect.
Materials and methods
Animal experiments
The animal study protocol was approved by the Jiaotong University, Medical Science School animal care and use committee. Ten-week-old male spontaneously hypertensive rats (SHRs) (n=24) were fed a standard chow diet. The rats were divided into four groups with the following treatments: vehicle-treated controls, 5 or 30 mg kg−1 day−1 losartan (purchased from Merck (Shanghai, China) or 30 mg kg−1 day−1 telmisartan (purchased from Boehringer Ingelheim Inc. (Shanghai, China). Age-matched Wistar-Kyoto rats (WKYs, n=6) were used as normotensive controls. Systolic arterial pressure was measured by tail-cuff plethysmography at 2 and 4 weeks after treatment. Peripheral arterial blood (∼5–6 ml) was collected immediately from the aorta before rats were killed. The aorta and left ventricle were isolated. All specimens were fixed in 10% buffered formalin or snap-frozen in liquid nitrogen for histological and immunohistochemical analysis.
Monocyte chemotaxis assay
MCP-1 chemotaxis was assessed with use of a double-chamber system (Costar Transwells, Corning, NY, USA). Peripheral blood monocytes (PBMCs) were isolated by density centrifugation with the use of a lymphocyte separation medium (Nycoprep 1.077A, purchased from Axis-Shied). For chemotaxis assays, 600 μl of RPMI 1640 medium (RPMI) containing 5% bovine serum albumin were placed in the lower chamber with or without MCP-1 (25 ng ml−1). PBMCs in 100 μl of RPMI (106 cells per well) were added to the upper chamber. After incubation for 4 h, the cells on the upper part of the membrane were scraped and fixed, and the migrated cells were stained with Giemsa and counted under a microscope.
Reverse transcription-polymerase chain reaction
The total RNA was isolated from PBMCs using RNAiso. RNA was reverse transcribed, and semiquantitative PCR was performed according to routine procedures. Glyceraldehyde-3-phosphate dehydrogenase expression was used as an internal control. The following primers were used: rat MCP-1, 5′-ATGCAGGTCTCTGTCACG (forward) and 5′-CTAGTTCTCTGTCATACT (reverse); CCR2, 5′-AGATGATCAGCATACTTGTG (forward) and 5′-AATGATAGGATTAACGCAGC (reverse).
Measurements of monocyte chemoattractant protein 1
After human blood monocyte (THP-1) cells were treated with various stimuli for different durations, MCP-1 released from the cells was measured. In brief, the supernatant was collected, filtered with 0.45 μm-pore filters and stored at −70 °C until use. Serum from 1 ml blood was centrifuged and MCP-1 concentration was measured by using BioSource ELISA kits (R&D, Shanghai, China).
Histology and immunohistochemistry
Rat descending aortas were embedded in paraffin and sectioned. The specimens were stained with haematoxylin and eosin staining or Masson's staining to show the internal lamina. In brief, sections (5 μm thick) were deparaffinized, rehydrated and blocked. Rat anti-mouse ED-1 at 1:100 dilution was used as the primary antibody and mouse IgG1 as a negative control. The goat anti-mouse SABC kit (Bostor Co. WuHan, China) was used for immunostaining. Sections were then counterstained with haematoxylin. Monocytes/macrophages accumulating in the aorta were counted. All images were captured with use of an Aixon 6.0 microscope equipped with a video camera and analysed by using Adobe Photoshop 6.0.
Western blotting
Monocytes (5 × 106) were plated into six-well plates and cultured for 20 h. LY294002 (5 μM) or CV-11974 (10 μM) was added to the cells for 30 min before Ang II treatment. Ang II (100 nM) was added to the cell cultures for 24 h. Cells were lysed with 150 mM radioimmunoprecipitation buffer. The whole-cell lysates underwent 10% SDS-PAGE. Western blot analysis was performed with the use of anti-Akt antibody or anti-phosphorylated Akt. An antibody against glyceraldehyde-3-phosphate dehydrogenase was used as a loading control and goat-anti-mouse horseradish peroxidase as a secondary antibody. The signal was revealed by chemiluminescence detection kit (Super Signal West Dura Extended Duration Substrate; Pierce Co., Rockford, USA).
Statistical analysis
Data were analysed by analysis of variance and Student's t-test with use of SPSS 13.0. The results are shown as mean±s.d. from three or five independent experiments. P<0.05 was considered statistically significant.
Materials
CV-11974 (an AT1 antagonist, an active metabolite of candesartan) was provided by Takeda Pharmaceutical Co. (Osaka, Japan). LY294004 (an inhibitor of PI3K) from Promega (Madison, WI, USA). Akt and phosphorylated Akt antibody and the glyceraldehyde-3-phosphate dehydrogenase antibody were purchased from Cell Signaling Co. (Boston, USA). Recombinant Rat MCP-1 (as used in the chemotaxis assays) was from Peprotech Inc. (Shanghai, China).
Results
AT1 antagonism inhibits monocyte infiltration into the aorta of spontaneous hypertensive rats
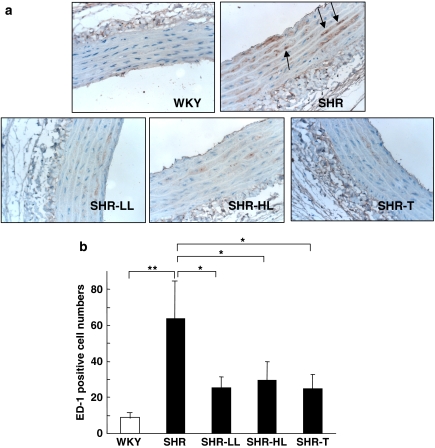
To investigate whether AT1 antagonists have an effect on monocyte/macrophage infiltration into the aorta in the presence of hypertension, we treated WKY controls and SHRs with or without losartan (5 or 30 mg kg−1) or telmisartan (30 mg kg−1) for 4 weeks. The systolic blood pressures of the five groups of rats are shown in Table 1. Immunostaining with anti-ED-1, a marker for monocyte/macrophage was enhanced in aortas from SHRs as compared with WKYs (Figure 1a). Treatment with either low or high doses of losartan or a high dose of telmisartan decreased the infiltration of monocytes/macrophages, as shown by decreased anti-ED-1 staining. Quantification of the infiltrated monocytes/macrophages (Figure 1b) demonstrates that AT1 antagonism inhibited the infiltration of monocytes/macrophages into the vascular wall in SHRs.
Table 1.
Systolic blood pressures in rats before and after treatment with AT1 antagonists (mm Hg, mean±s.d.)
| WKY | SHR | SHR-LL | SHR-HL | SHR-T | |
|---|---|---|---|---|---|
| Before treatment | 131.3±14.8 | 187.2±11.7a | 176.7±15.3a | 172.9±15.8a | 169.8±15.4a |
| After treatment 2 weeks | 132.2±12.7 | 186.8±11.6 | 175.2±15.3 | 162.7±14.9 | 166.4±17.6 |
| After treatment 4 weeks | 129.3±3.9 | 189.2±11.7 | 174.2±17.1 | 118.1±27.2b | 113.5±18.9b |
SHR, spontaneously hypertensive rats; SHR-HL, SHR treated with losartan 30 mg kg−1; SHR-LL, SHR treated with losartan 5 mg kg−1; SHR-T, SHR treated with telmisartan 30 mg kg−1; WKY, Wistar-Kyoto rats.
Compared with WKY groups, P<0.01.
Compared with before-treatment, P<0.05.
Figure 1.
AT1 antagonists inhibited the infiltration of monocytes/macrophages into the aorta of SHRs. (a) WKY and SHRs received 5 or 30 mg kg−1 losartan (SHR-LL and SHR-HL, respectively) or 30 mg kg−1 telmisartan (SHR-T) for 4 weeks. The isolated aortic specimens from the five groups (n=6 in each group) were immunostained with anti-ED-1, a marker for monocytes/macrophages. Images are representative of cross-sections (5 μm thick) (magnification × 40). Black arrows indicate the positive ED-1 staining. (b) ED-1-positive cells were counted and quantified; bar graphs are means±s.d. from 3–4 aortic specimens of each animal (**P<0.01 and *P>0.05). SHR, spontaneous hypertensive rats; WKY, Wistar-Kyoto rats.
AT1 antagonism inhibits MCP-1/CCR2-mediated chemotaxis
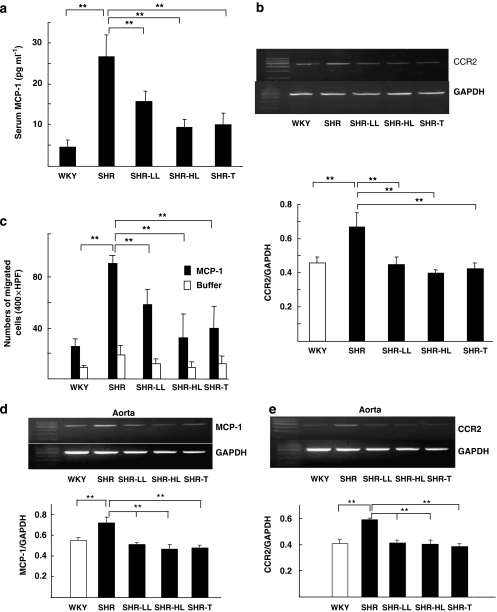
Given the key role of MCP-1 and its cognate receptor CCR2 in monocyte chemoattraction, we measured the serum level of MCP-1 and tissue content of CCR2 in both PBMCs and aortas after losartan and telmisartan treatment. The level of MCP-1 in the peripheral blood of SHRs was much higher than that of control WKYs (Figure 2a) and this level was significantly reduced in SHRs receiving losartan or telmisartan. The mRNA for CCR2 was increased in SHRs and was attenuated by losartan or telmisartan treatment (Figure 2b). In the MCP-1 chemotaxis assay, monocytes isolated from losartan- or telmisartan-treated SHRs showed attenuated MCP-1 chemotaxis as compared with control SHRs (Figure 2c).
Figure 2.
AT1 antagonists decreased MCP-1/CCR2-mediated monocyte chemotaxis. (a) Serum was collected from WKYs and SHRs receiving vehicle control or 5 mg kg−1 losartan, 30 mg kg−1 losartan or 30 mg kg−1 telmisartan for 4 weeks. The serum level of MCP-1 was determined by ELISA with the use of anti-MCP-1 antibody. (b) Level of CCR2 mRNA in PBMC detected by Reverse transcription-PCR. The upper panel indicates the amount of CCR2 mRNA, with the internal control GAPDH mRNA, and the lower panel shows the density of DNA bands. (c) Monocytes from the five groups of rats (n=6 in each group) were plated onto the upper chamber of the transwell, and MCP-1 (25 ng ml−1) was added to the lower chamber. At 4 h after incubation, the membrane was fixed and stained with Giemsa, and the migrated cells were counted. (**P<0.01) (d and e) Aortic extracts from the five groups of rats were processed by Reverse transcription-PCR to determine the mRNAs for MCP-1 and CCR2. Bar graphs in the lower panel show density of DNA bands. CCR2, C-C chemokine receptor 2; ELISA, enzyme-linked immunosorbant assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MCP-1, monocyte chemoattractant protein 1; PBMC, peripheral blood monocytes; SHR, spontaneous hypertensive rats; WKY, Wistar-Kyoto rats.
To compare data obtained from PBMCs (Figures 2b–c) with the levels of MCP-1 and CCR2 in the aorta, we measured the mRNA for MCP-1 and CCR2 in aortas from the five animal groups. The mRNA for MCP-1 and CCR2 in untreated SHRs was higher than that in WKYs (Figures 2d and e), but losartan or telmisartan treatment decreased both the mRNAs levels comparable to those in control untreated WKYs.
AT1 antagonism improves aortic remodeling in spontaneous hypertensive rats
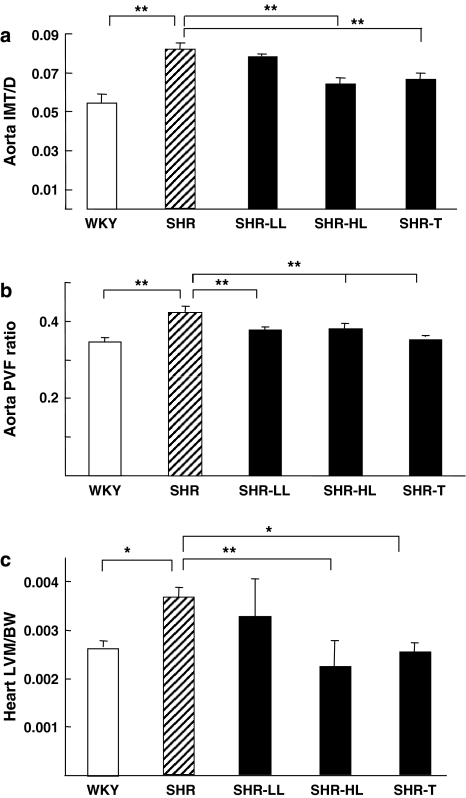
To study whether AT1 antagonists exerts protective effects on blood vessels, we compared aortic remodelling among the five rat groups. Treatment with 30 mg kg−1 losartan or telmisartan markedly decreased the IMT/D of the aorta as compared with that in untreated SHRs (Figure 3a). As well, all of the AT1 antagonists decreased the PVFR, compared with that in untreated rats (Figure 3b). The therapeutic effect of the AT1 antagonists was also exhibited in the heart. Thus, the heart LVM/BW ratio was significantly improved in SHRs receiving the higher dose of losartan or telmisartan (Figure 3c).
Figure 3.
AT1 antagonists reduced the remodelling of hypertension-targeted organs in SHRs. (a) Ratios of the aortic intima-medial thickness to the aortic diameter (IMT/D) after treatment with AT1 antagonists for 4 weeks. (b) Aortic perivascular fibrosis ratios (PVFRs). (c) Mass of the left ventricle normalized to body weight (LVM/BW). WKY aortas were measured at day 1 and SHR aortas after 4 weeks treatment. (*P<0.05 or **P<0.01). SHR, spontaneous hypertensive rats; WKY, Wistar-Kyoto rats.
PI3K/Akt is involved in inhibition of monocyte chemoattractant protein 1 by AT1 antagonists
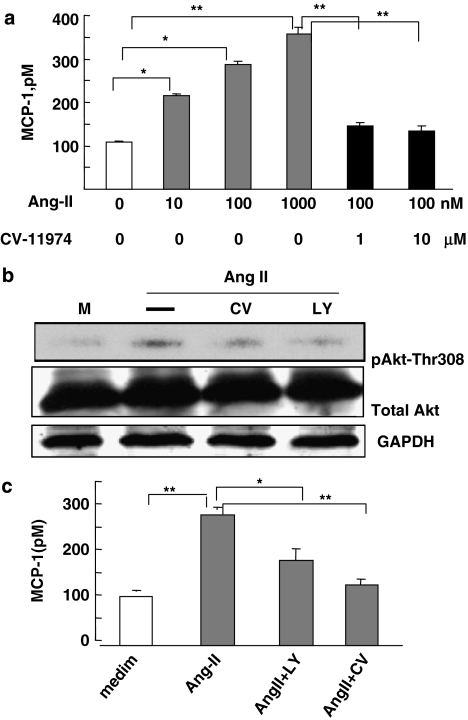
To confirm inhibition of MCP-1 secretion by AT1 antagonists at the molecular level, we pre-treated THP-1 monocytic cells with Ang II, or Ang II and CV-11974, another AT1 antagonist. Ang II dose-dependently induced the secretion of MCP-1 from THP-1 cells (Figure 4a). However, the addition of CV-11974 abolished this effect of Ang II. To dissect the signalling pathways involved in the inhibition of MCP-1 by AT1 antagonism, THP-1 cells were treated with Ang II with or without LY294002, a specific inhibitor of PI3K, or CV-11974. As already observed with CV-11974 inhibition, inhibition of PI3K attenuated the activation of Akt, as indicated by the decreased phosphorylation of Thr-308. Furthermore, inhibition of PI3K attenuated MCP-1 secretion (Figure 4c). Thus, we concluded that the PI3K–Akt pathway was involved in the inhibition of the MCP-1/CCR2 by AT1 antagonists.
Figure 4.
Inhibition of MCP-1 expression by AT1 antagonists was mediated through the PI3K/Akt pathway. (a) THP-1 monocyte cells were treated with 10, 100 or 1000 nM Ang II alone or 100 nM Ang II with 1 or 10 μM CV-11974. After 24 h, MCP-1 level in the supernatant was assayed by ELISA. The data are means±s.d. from five experiments (*P<0.05; **P<0.01). (b) THP-1 cells were pre-treated with 5 μM LY294002 (LY) or 10 μM CV-11974 (CV) for 30 min, then treated with 100 nM Ang II for 24 h. The cell lysates were analysed by 10% SDS-polyacrylamide gel electrophoresis. Western blot analysis involved anti-phospho-Akt (phosphorylation of Thr-308), anit-Akt or anti-GAPDH. The data shown are representative of three independent experiments. (c) THP-1 cells were pre treated with 5 μM LY294002 or 10 μM CV for 30 min, then with 100 nM Ang II for 24 h. The concentration of MCP-1 in the supernatant was assayed. Statistical analysis was as for (a). ELISA, enzyme-linked immunosorbant assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MCP-1, monocyte chemoattractant protein 1; PI3K, phosphoinositide-3 kinase.
Discussion
Hypertension is considered a major determinant of endothelial dysfunction, including but not limited to, promoting inflammatory responses and recruitment of PBMCs (Alexander, 2006). AT1 antagonists have anti-inflammatory effects on the vascular wall, in addition to lowering blood pressure. In this study, we showed that treatment with the AT1 antagonists, losartan and telmisartan, decreased the monocyte/macrophage infiltration into the vascular wall of SHRs, which was associated with improved protection of target organs, including vessels and heart. Further, AT1 antagonism decreased the secretion of MCP-1 from PBMCs and the level of CCR2 in PBMCs. Previous studies of animal models of hypertension have also shown AT1 antagonists alleviating the detrimental effects of Ang II on endothelial cells and reducing the level of inflammatory response in vessels (Cheng et al., 2005; Sanz-Rosa et al., 2005).
Interestingly, losartan at the lower dose of 5 mg kg−1, although not improving systolic pressure, still showed significant beneficial effects on the cardiovascular system, as shown by decreased MCP-1 and CCR2 expression, monocyte/macrophage infiltration and protection of the vessels and heart. Thus, low-dose AT1 antagonists may provide an anti-inflammatory effect clinically and prevent cardiovascular complications of hypertension. Two subtypes of Ang II receptors (AT1 and AT2) exist. Grafe et al. (1997) reported that human endothelial cells mainly express AT1R but not AT2R. It is through the AT1 receptors, that endothelial cells interact with leukocytes. Thus, AT1 antagonists could decrease the inflammatory response by blocking AT1 receptors.
Ang II augments MCP-1 expression in cell types such as endothelial cells, monocytes/macrophages and smooth muscle cells. MCP-1 plays a central role in the inflammatory response in vascular diseases related to hypertension, as shown by increased MCP-1 expression in the arterial walls of hypertensive animals (Capers et al., 1997), and inhibition of either MCP-1 or CCR2 prevents vascular inflammation in hypertensive rats (Bush et al., 2000; Koyanagi et al., 2000; Ishibashi et al., 2004b). Furthermore, Ang II infusion results in decreased accumulation of macrophages and vascular hypertrophy in CCR2-deficient mice (Bush et al., 2000). In this study, we showed MCP-1 was upregulated in SHRs, most likely because of the elevated Ang II, and this upregulation was attenuated by AT1 antagonists. One detrimental effect of Ang II-mediated vascular remodelling is the recruitment of inflammatory cells to the vascular wall. Our findings indicate that the decreased MCP-1 following AT1 antagonists was associated with decreased recruitment of inflammatory cells to the arterial wall.
The PI3K/Akt pathway is widely involved in various pathophysiological processes of vascular diseases such as cell proliferation and migration of endothelial cells, smooth muscle cells and monocytes/macrophages. Our data demonstrate that blocking of Ang II with AT1A diminished the activation of the PI3K/Akt pathway. Furthermore, inhibition of PI3K/Akt attenuated MCP-1 secretion. Thus, Ang II signalling through AT1 receptors could activate PI3K/Akt, which was critical for the enhanced expression of MCP-1/AT1R.
Conclusions from this study have increased our understanding of the functions and associated mechanism of AT1 antagonists in providing an anti-inflammatory effect by suppressing monocyte/macrophage recruitment. From the translational research point of view, treatment with AT1 antagonists, even at doses that did not lower blood pressure, improved the remodelling of organs such as the aorta and heart, which are important targets for the pathological consequences of hypertension.
Acknowledgments
We thank Miss Melissa L Petreaca's constructive discussion and preview. This project was support in part by MED-X fund (2005–2006) from Jiaotong University and Research Grant 02JC14035 from the Shanghai Science Committee, Shanghai, China.
Abbreviations
- AT1
AT1 receptor
- CCR2
C-C chemokine receptor 2
- IMT
aortic intima-medial thickness
- IMT/D
ratio of aortic intima-medial thickness to diameter
- LVM/BW
ratio of left ventricular mass of the heart to body weight
- MCP-1
monocyte chemoattractant protein 1
- PBMC
peripheral blood monocytes
- PVFR
aortic perivascular fibrosis ratio
- SHR
spontaneously hypertensive rats
- WKY
Wistar-Kyoto rats
Conflict of interest
The authors state no conflict of interest.
References
- Alexander RW. Leukocyte and endothelial angiotensin II type 1 receptors and microvascular thrombotic and inflammatory responses to hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:240–241. doi: 10.1161/01.ATV.0000199680.42737.ca. [DOI] [PubMed] [Google Scholar]
- Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush E, Maeda N, Kuiziel WA, Dawson TC, Wilcox JN, Deleon H, et al. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000;36:360–363. doi: 10.1161/01.hyp.36.3.360. [DOI] [PubMed] [Google Scholar]
- Capers Qt, Alexander RW, Lou P, De Leon H, Wilcox JN, Ishizaka N, et al. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997;30:1397–1402. doi: 10.1161/01.hyp.30.6.1397. [DOI] [PubMed] [Google Scholar]
- Chen R, Iwai M, Wu L, Suzuki J, Min LJ, Shiuchi T, et al. Important role of nitric oxide in the effect of angiotensin-converting enzyme inhibitor imidapril on vascular injury. Hypertension. 2003;42:542–547. doi: 10.1161/01.HYP.0000092440.52239.39. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Vapaatalo H, Mervaala E. Angiotensin II and vascular inflammation. Med Sci Monit. 2005;11:RA194–RA205. [PubMed] [Google Scholar]
- Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, Lorenzo O, Demaegdt H, et al. Angiotensin IV activates the nuclear transcription factor-kappaB and related proinflammatory genes in vascular smooth muscle cells. Circ Res. 2005;96:965–973. doi: 10.1161/01.RES.0000166326.91395.74. [DOI] [PubMed] [Google Scholar]
- Grafe M, Aujch-Schwelk W, Zakrzewicz A, Regitz-Zagrosek V, Bartsch P, Graf K, et al. Angiotensin II-induced leukocyte adhesion on human coronary endothelial cells is mediated by E-selectin. Circ Res. 1997;81:804–811. doi: 10.1161/01.res.81.5.804. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Egashira K, Zhao Q, Hiasa K, Ohtani K, Hara Y, et al. Bone marrow-derived monocyte chemoattractant protein-1 receptor CCR2 is critical in angiotensin II-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004a;24:e174–e178. doi: 10.1161/01.ATV.0000143384.69170.2d. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, et al. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004b;94:1203–1210. doi: 10.1161/01.RES.0000126924.23467.A3. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Egashira K, Kitamoto S, Ni W, Shimokawa H, Takeya M, et al. Role of monocyte chemoattractant protein-1 in cardiovascular remodeling induced by chronic blockade of nitric oxide synthesis. Circulation. 2000;102:2243–2248. doi: 10.1161/01.cir.102.18.2243. [DOI] [PubMed] [Google Scholar]
- Morita T, Imai T, Yamaguchi T, Sugiyama T, Katayama S, Yoshino G. Induction of heme oxygenase-1 in monocytes suppresses angiotensin II-elicited chemotactic activity through inhibition of CCR2: role of bilirubin and carbon monoxide generated by the enzyme. Antioxid Redox Signal. 2003;5:439–447. doi: 10.1089/152308603768295186. [DOI] [PubMed] [Google Scholar]
- Ni W, Kitamoto S, Ishibashi M, Usui M, Inoue S, Hiasa K, et al. Monocyte chemoattractant protein-1 is an essential inflammatory mediator in angiotensin II-induced progression of established atherosclerosis in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004;24:534–539. doi: 10.1161/01.ATV.0000118275.60121.2b. [DOI] [PubMed] [Google Scholar]
- Ouyang X, Le TH, Roncal C, Gersch C, Herrera-Acosta J, Rodriguez-Iturbe B, et al. Th1 inflammatory response with altered expression of profibrotic and vasoactive mediators in AT1A and AT1B double-knockout mice. Am J Physiol Renal Physiol. 2005;289:F902–F910. doi: 10.1152/ajprenal.00141.2005. [DOI] [PubMed] [Google Scholar]
- Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant. 2006;21:850–853. doi: 10.1093/ndt/gfl019. [DOI] [PubMed] [Google Scholar]
- Sanz-Rosa D, Oubina MP, Cediel E, de Las Heras N, Vegazo O, Jimenez J, et al. Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: role of NF-kappaB/IkappaB system. Am J Physiol Heart Circ Physiol. 2005;288:H111–H115. doi: 10.1152/ajpheart.01061.2003. [DOI] [PubMed] [Google Scholar]
- Usui M, Egashira K, Tomita H, Koyanagi M, Katoh M, Shimokawa H, et al. Important role of local angiotensin II activity mediated via type 1 receptor in the pathogenesis of cardiovascular inflammatory changes induced by chronic blockade of nitric oxide synthesis in rats. Circulation. 2000;101:305–310. doi: 10.1161/01.cir.101.3.305. [DOI] [PubMed] [Google Scholar]
- Willemsen JM, Westerink JW, Dallinga-Thie GM, Van Zonneveld AJ, Gaillard CA, Rabelink TJ, et al. Angiotensin II type 1 receptor blockade improves hyperglycemia-induced endothelial dysfunction and reduces proinflammatory cytokine release from leukocytes. J Cardiovasc Pharmacol. 2007;49:6–12. doi: 10.1097/FJC.0b013e31802b31a7. [DOI] [PubMed] [Google Scholar]