Abstract
An alteration in the character and function of platelets is manifested in patients with inflammatory diseases, and these alterations have been dissociated from the well-characterized involvement of platelets in thrombosis and haemostasis. Recent evidence reveals platelet activation is sometimes critical in the development of inflammation. The mechanisms by which platelets participate in inflammation are diverse, and offer numerous opportunities for future drug intervention. There is now acceptance that platelets act as innate inflammatory cells in immune responses, with roles as sentinel cells undergoing surveillance, responding to microbial invasion, orchestrating leukocyte recruitment, and migrating through tissue, causing damage and influencing repair processes in chronic disease. Some of these processes are targeted by drugs that are being developed to target platelet participation in atherosclerosis. The actions of platelets therefore influence the pathogenesis of diverse inflammatory diseases in various body compartments, encompassing parasitic and bacterial infection, allergic inflammation (especially asthma and rhinitis), and non-atopic inflammatory conditions, for example, chronic obstructive pulmonary disease (COPD), rheumatoid arthritis (RA), inflammatory bowel disease (IBD) and atherosclerosis. This review will first discuss the evidence for platelet activation in these various inflammatory diseases, and secondly discuss the mechanisms by which this pathogenesis occurs and the various anti-platelet agents which have been developed to combat platelet activation in atherosclerosis and their potential future use for the treatment of other inflammatory diseases.
Keywords: platelets, inflammation, P2Y1, P2Y12, P-selectin, GPIIb/IIIa, CD40, S1P, PPAR
Platelet activation in asthma and rhinitis
A participation of platelets in the pathogenesis of asthma and rhinitis has been documented for a number of years. Primarily, platelet activation occurs during antigen-induced airway reactions in asthmatic patients. An altered functionality is manifested as heightened platelet activation in vivo, while platelets from the same allergic patients are found to be refractory to a variety of stimuli ex vivo, possibly resulting from platelet ‘exhaustion', due to the inability of platelets to replenish many released mediators that require de novo synthesis because platelets lack a nucleus (Harker et al., 1980; Pareti et al., 1980). Platelet ‘exhaustion' has been reported as an inability of noradrenaline and adenosine di-phosphate (ADP) to induce full aggregation of platelets, with no second-phase aggregation, an occurrence that has been correlated with increased serum immunoglobulin E (IgE) in asthmatic patients (Maccia et al., 1977; Palma-Carlos et al., 1991). However, full aggregation of platelets in vitro returns in the same patients when studies are repeated outside of the allergy (pollen) season (Maccia et al., 1977).
This alteration in platelet function has been associated with bronchial hyperresponsiveness that accompanies nocturnal asthma (Gresele et al., 1993). The phenomenon of platelet activation in response to allergen-induced anaphylaxis has been shown to be beyond the control of agents that stimulate cyclic adenosine monophosphate and metabolites of the arachidonate pathway. Platelet responses to allergen are thus different to platelet responses to normal aggregatory stimuli. Indeed, while non-steroidal anti-inflammatory drugs (NSAIDs) block platelet aggregation, platelet–leukocyte interactions are not blocked by NSAIDs and represent a mechanism of platelet activation during inflammation that is distinct from platelet aggregation (Storey et al., 2002; Li et al., 2003).
Atopy is also accompanied by a prolonged bleeding time, increased platelet mass and volume, and decreased platelet survival. This accelerated platelet consumption correlates to a shortened time taken to regenerate the platelet population. These phenomena can be corrected by treatment of asthmatic patients with glucocorticoids, or platelets treated with di-sodium chromoglycate in vitro before re-infusion, although these anti-inflammatory drugs have no known direct affects on platelet activation (Taytard et al., 1985; Tunon-De-Lara et al., 1992).
Large numbers of pulmonary megakaryocytes (precursors to platelets) and platelets have been obtained at autopsy from patients who have died from status asthmaticus. In addition, platelets are localized to various tissue compartments in the lung parenchyma of biopsies taken from asthmatic patients (Metzger et al., 1987; Jeffery et al., 1989) and this event is accompanied with bone marrow karyopoiesis and thrombopoiesis (Slater et al., 1985). This is perhaps the result of localized platelet recruitment and activation within lungs, since circulating venous platelet numbers have been shown to fall during both early- and late-phase responses to allergen (Kowal et al., 2006). Platelet–platelet, and platelet–leukocyte aggregates have also been detected in patients with spontaneous asthma attacks (Gresele et al., 1993). This occurs in a biphasic manner following allergen challenge and results in an increase in the expression of CD11b, an activation marker on the surface of leukocytes (Pitchford et al., 2003).
Raised levels of platelet-derived mediators such as the chemokines – β-thromboglobulin (β-TG) and platelet-factor 4 (PF-4) – are observed in plasma and broncho-alveolar lavage fluid of atopic individuals compared to normal individuals during allergen exposure (Slater et al., 1985; Gresele et al., 1993), while an increase in serum CD40 ligand (CD40L) of platelet origin has also been reported recently (Kowal et al., 2006a). Other platelet-derived mediators have also been observed in atopic patients after allergen provocation, including regulated upon activation normally T-cell expressed and secreted (RANTES, CCL5), platelet selectin (P-selectin), 5-hydroxytryptamine (5-HT), adenosine, histamine, platelet-derived growth factor (PDGF), platelet-activating factor (PAF), the de novo production of arachidonic acid metabolites including prostaglandin E2 and thromboxane (TXA2), platelet-specific lipoxygenase products including hydroyeicosatetraenoic acid, lysosomal enzymes such as matrix metalloproteinases (MMPs) and mediators sequestered from the circulation (for example, IgE) (reviewed in Pitchford and Page, 2002).
Production of antigen-specific IgE in response to allergen provocation is integral to atopic diseases. Interestingly, IgE binds to between 20 and 30% of platelets from normal individuals, this binding affinity rises up to the binding of 50% of platelets from patients with allergies (Maccia et al., 1977; Joseph et al., 1986). Platelets from atopic individuals are characterized by a much greater IgE content stored in α-granules compared to non-atopics, which correlates to serum IgE levels from atopic patients. Stimulation of platelets from atopic patients resulted in the release of 65% of stored IgE levels by PAF stimulation but not by platelet mediators involved in aggregation, for example thrombin and ADP (Klouche et al., 1997).
Platelet activation in chronic obstructive pulmonary disease
The involvement of platelets in chronic obstructive pulmonary disease (COPD) is less well researched than the involvement of platelets in asthma. However, the occurrence of platelet hyperreactivity has been demonstrated in ex vivo studies where platelets had an increased sensitivity to various agonists, and elevated levels of plasma β-TG and soluble P-selectin of platelet origin have been reported (Cordova et al., 1985; Ferroni et al., 2000). These reports reflect the occurrence of in vivo platelet activation as measured by increased synthesis of TxA2 in patients with COPD, and the administration of a TxA2 antagonist was beneficial in improving respiratory distress in patients with chronic pulmonary emphysema (Davi et al., 1997).
Platelet activation in rheumatoid arthritis
Clinical studies have demonstrated that activation of circulating platelets occurs in patients with rheumatoid arthritis (RA) (Endresen, 1989; Joseph et al., 2001), and platelets have been observed in the synovial fliud of patients with RA (Farr et al., 1984; Endresen, 1989; Endresen and Forre, 1992). Interestingly, heterotypic platelet–monocyte and platelet–neutrophil complexes occur in the circulating blood of patients with RA (Endresen and Forre, 1992; Bunescu et al., 2004), and in common with other inflammatory conditions, these interactions may contribute to leukocyte activation and recruitment to the synovium.
Platelet activation in inflammatory bowel disease
Patients suffering from exacerbations of Crohn's disease and ulcerative colitis have an increase in circulating platelet numbers (Morowitz et al., 1968). This is often associated with a reduced platelet lifespan and reduction in mean platelet volume (Webberley et al., 1993; Jaremo and Sandberg-Gertzen, 1996). Furthermore, platelets from inflammatory bowel disease (IBD) patients are more sensitive to platelet agonists in vitro (van Wersch et al., 1990), while the platelet-specific chemokines PF-4 and β-TG are detected in plasma, revealing activation in vivo (Collins et al., 1994; Vrij et al., 2000). A role for platelets in mediating leukocyte recruitment to the inflamed colon is likely since platelet P-selectin and RANTES are also detected (Fagerstam et al., 2000), and this is localized to the intestinal microcirculation (Collins et al., 1997). Recent evidence suggests that increased circulating levels of soluble CD40L are of platelet origin in IBD patients (Danese et al., 2003a). Furthermore, platelets mediate leukocyte recruitment via CD40–CD40L interactions in patients with IBD and in a murine model of colonic inflammation induced by dextran sodium sulphate (Danese et al., 2003b; Vowinkel et al., 2007). Interestingly platelet activation may also be involved in chronic inflammatory events occurring in IBD as CD40–CD40L interactions have been shown to be necessary for angiogenesis in a murine model of IBD (Danese et al., 2007).
Platelet activation in atherosclerosis
Inflammatory processes are a recognized feature of atherosclerotic lesions, eventually causing plaque rupture. The link between immune system activation and cardiovascular disease has been demonstrated through the involvement of inflammatory cytokines (Ross, 1999). In particular, activated endothelium attracts the adherence and accumulation of monocytes and CD4 and CD8T cells (Hansson et al., 1989; Hansson and Libby, 1996). In addition to this, evidence is accumulating to suggest that chemokines play a central role in the development of atherosclerotic plaques, with stromal cell-derived factor-1 (SDF-1, CXCL-12), monocyte chemoattractant protein-1 (MCP-1, CCL-2), RANTES, interleukin-8 (IL-8) and eotaxin observed in atherosclerotic plaques (Wilcox et al., 1994; Abi-Younes et al., 2000; Haley et al., 2000). This leads to macrophage infiltration into fatty streaks where the production of cytokines such as tumour necrosis factor-α (TNFα), IL-1, transforming growth factor-β (TGFβ), proteolytic enzymes and growth factors secreted by immune cells precede plaque destabilization and rupture.
Platelet adhesion and thrombus formation is a ubiquitous feature in the initiation and generation of atherosclerotic lesions. However, interactions between platelets and inflammatory cells take place during atherosclerosis and this stimulation of an inflammatory response within the atherosclerotic plaque may trigger acute coronary events via reactive oxygen species (ROS) production and MMP secretion (Poubelle and Borgeat, 2002). Substantial clinical evidence demonstrates activation of circulating platelets in diseases with a substantial inflammatory component acting on the vasculature, for example, acute coronary syndromes such as myocardial infarction and unstable angina (Sarma et al., 2002) and atherosclerosis (Massberg et al., 2002). These studies suggest a participation of platelets in the inflammatory responses as well as the recognized events leading to thrombus formation. Platelet binding to leukocytes occurs during acute coronary events, and these heterotypic aggregates are formed as a result of activation by inflammatory mediators (Arber et al., 1991; Ott et al., 1996) and are largely bound via P-selectin/P-selectin glycoprotein ligand-1 (PSGL-1) interactions (Sarma et al., 2002). It is worth noting that an increased expression of CD40L occurs on the surface of platelets in acute coronary syndromes (Garlichs et al., 2001), although the significance of this is not yet known, it reveals another mechanism whereby platelets may further stimulate the inflammatory response during atherosclerosis.
Inflammatory mechanisms affected by platelets
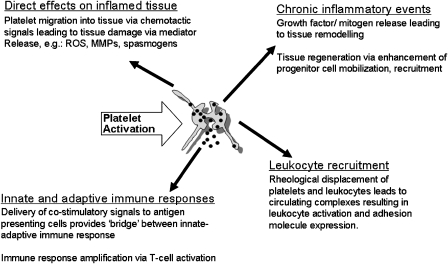
Various mechanisms common to many diseases have been documented whereby platelets modulate the inflammatory response. Such mechanisms include intravascular ‘priming' of leukocytes for efficient recruitment to tissue, chronic inflammatory events leading to tissue remodelling and regeneration, release of platelet-derived mediators that cause tissue damage directly, and the involvement of platelets linking the innate and adaptive immune responses (Figure 1).
Figure 1.
Platelet participation in inflammation.
Leukocyte recruitment and activation: influence of platelets
Circulating platelet–leukocyte complexes are a feature of a wide cross-section of inflammatory diseases, and it is believed that this phenomenon ‘primes' resting circulating leukocytes for efficient recruitment to inflamed tissue. For example, studies with un-separated leukocyte populations reveal a significant increase in platelet–leukocyte complexes in allergic mice and in human asthmatics (Pitchford et al., 2003, 2005). Similar processes occur in patients with COPD (Ferroni et al., 2000), atherosclerosis (Arber et al., 1991; Ott et al., 1996; Neumann et al., 1997; Sarma et al., 2002; Huo et al., 2003) and RA (Joseph et al., 2001; Bunescu et al., 2004). In this regard, experimental models of disease have provided evidence for a requirement of platelets in pulmonary eosinophil and lymphocyte recruitment in rabbits, guinea-pigs and mice in models of allergic inflammation (Lellouch-Tubiana et al., 1988; Coyle et al., 1990; Pitchford et al., 2003, 2005); and neutrophil and monocyte recruitment in atherosclerosis (Arber et al., 1991; Neumann et al., 1997; Hayward et al., 1999) and RA (Schmitt-Sody et al., 2005). This phenomenon requires intact platelets expressing mediators on the cell surface, and in common with the occurrence of leukocyte recruitment in inflammatory diseases, platelet P-selectin is of particular importance (Diacovo et al., 1996a, 1996b; Schober et al., 2002; Huo et al., 2003; Pitchford et al., 2005). With regard to asthma, this mechanism has been confirmed by various in vitro studies, revealing eosinophil attachment to inflamed endothelium is greatly enhanced in the presence of platelets taken from asthmatic patients, and P-selectin expressed by platelets is responsible for platelet–eosinophil interactions in particular (Jawien et al., 2002; Ulfman et al., 2003). Circulating leukocytes attached to platelets display significant increases in CD11b and very late antigen-4 integrin (VLA-4) expression, compared to leukocytes not attached to platelets, and circulating platelet–leukocyte complexes in non-inflamed animals (Pitchford et al., 2005). Thus, platelets have the ability to activate leukocytes at the level of contact-dependent signalling and prime them for endothelial attachment. We and others have shown that the occurrence of platelet–leukocyte complexes is abolished by the administration of antibodies to P-selectin and its counter ligand PSGL-1, demonstrating the importance of platelet P-selectin on this mechanism (Mayadas et al., 1993; Katayama et al., 2000; Pitchford et al., 2005).
Selectin-mediated rolling is thus an essential step towards firm cell–cell adhesion directed by β2-integrins. This can result in the absence of exogenous stimuli (Yeo et al., 1994) and is supported by CD11a/CD18 (LFA-1) and CD11b/CD18 (Mac 1) expression induced by P-selectin–PSGL-1 interactions, as PSGL-1 functions as a signalling molecule (Evangelista et al., 1996; Blanks et al., 1998; Konstantopoulos et al., 1998). Engagement of PSGL-1 by P-selectin results in tyrosine phosphorylation of a 110 kDa protein (Evangelista et al., 1999) and activation of mitogen-activated protein kinase (Hidari et al., 1997) in leukocytes complexed to platelets (Evangelista et al., 1999). However, P110 tyrosine phosphorylation also requires integrin–counterligand interactions on the surface of platelets, resulting eventually in the complete adhesion (Evangelista et al., 1999). Recent evidence reveals the importance of Src-family tyrosine kinases (SFKs) in stabilizing CD11b/CD18 interactions with platelets (Evangelista et al., 2007). A principal β2-integrin present on activated platelets is intercellular adhesion molecule-2 (ICAM-2) (Diacovo et al., 1994). Platelet-derived ICAM-2 mediates lymphocyte–platelet adhesion via CD11a/CD18; and ICAM-2 may also contribute to neutrophil rolling and firm arrest, mediated by CD11b/CD18 under flow conditions (Kuijper et al., 1998). Glycoprotein-Ibα (GPIbα) has also been identified as a ligand for CD11b/CD18 (Simon et al., 2000), and leukocyte engagement of platelet GPIbα via CD11b/CD18 has been shown to be critical for leukocyte accumulation in a mouse femoral artery injury model (Wang et al., 2005).
Several other immunoglobulin-type receptors have also been described on platelets, including platelet endothelial cell adhesion molecule-1 (PECAM-1), endothelial cell selective adhesion molecule, junctional adhesion molecule-(JAM)-1 and -3, which are localized around tight junctions of endothelium and epithelium and modulate barrier function around the cleft of adjacent cells (Ozaki et al., 1999). The physiological function of JAMs on platelets remains unclear; however, it is plausible that these receptors play a part in platelet adhesion to the sub-endothelium (Nasdala et al., 2002). JAM-1 has been described as a counter-receptor for CD11a/CD18 (Ostermann et al., 2002). Moreover, JAM-3, another novel counter receptor for CD11b/CD18 facilitates platelet–leukocyte interactions, and together with GPIbα, appears to be the predominant counter-receptor for CD11b/CD18 (Santoso et al., 2002). Analysis of different blood cell populations indicates that JAM-3 is exclusively expressed on platelets (Santoso et al., 2002). Recent evidence suggests inhibition of JAM-3 and PECAM-1 completely inhibited neutrophil trans-endothelial migration in vitro and soluble JAM-3 administration significantly reduced neutrophil emigration in a murine model of peritonitis (Chavakis et al., 2004). Therefore, JAMs may modulate the final process of platelet–leukocyte transmigration via the most apical regions of the inflamed endothelium.
Interestingly, and perhaps because of the diverse array of ligands by which stable platelet–leukocyte interactions occur, other cellular events unrelated to the tethering of leukocytes to endothelium have been reported. These include inflammatory gene activation. For example, P-selectin bound to antigen-primed CD4+ T cells differentially modulates the production of pro-inflammatory cytokines (Damle et al., 1992), and thus T-cell activation may be facilitated via adhesion with activated platelets. Furthermore, CD11b/CD18 can modulate NF-κB activity via IL-1 receptor signalling pathway (Shi et al., 2001). Biochemical events may also occur, since SFK activation via CD11b/CD18 activation results in respiratory burst and oxygen-free radical release, contributing to tissue damage (Lowell et al., 1996). Lastly, evidence supports a direct role for platelet P-selectin in phagocytosis by neutrophils, which is a recognized CD11b/CD18-dependent function (Cooper et al., 1994).
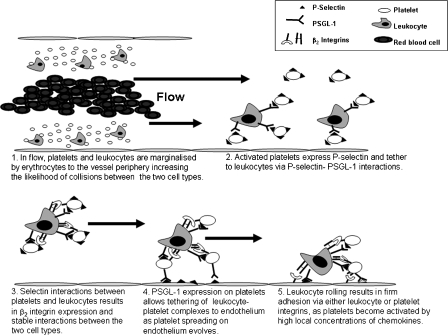
Platelet–leukocyte complexes form as a result of the rheological displacement of blood cells. This ‘traps' leukocytes into an environment rich in platelets towards the vessel periphery, greatly enhancing the possibility of collisions between platelets and leukocytes (Figure 2). However, unless platelets become activated by inflammatory stimuli, the result of these collisions on circulating platelet–leukocyte complex formation appears to be unresponsive in terms of adhesion to the vascular endothelium, as recognized by the presence of circulating platelet–leukocyte complexes found in control subjects (Pitchford et al., 2003, 2005). It would appear that platelets require additional inflammatory stimuli above that which is required for stable interactions between platelets and leukocytes before firm adhesion to the endothelium takes place.
Figure 2.
Enhancement of leukocyte trafficking by platelet tethering.
It is feasible that platelet activation and formation of platelet–leukocyte complexes is directed by a number of chemokines, since SDF-1, monocyte-derived chemokine (MDC, CCL22), thymus and activation-regulated cytokine (TARC, CCL17) and fractalkine can activate platelets in the presence of ADP (Abi-Younes et al., 2000, 2001; Kowalska et al., 2000; Schafer et al., 2004) via their receptors CXCR4, CCR1, CCR3, CCR4 and CX3CR1 (Clemetson et al., 2000; Schafer et al., 2004). Platelet activation by chemokines in the presence of low levels of ADP (insufficient alone to cause aggregation) is a very rapid process, resulting in near maximal activation within 5 s of stimulation. The rapidity of this response is highlighted by chemokine stimulation of platelet adhesion under flow, and exposed P-selectin to the platelet surface (Gear et al., 2001), which is temporally similar to leukocyte adhesion under flow (Alon and Feigelson, 2002). Thus, arterial blood flow concentrates platelets close to the endothelium, drawing platelets into the vicinity of higher local concentrations of chemokines released from endothelial cells. This signalling may result in the upregulation of selectins and integrins on the surface of platelets (Gear et al., 2001), enabling platelets to bind and activate circulating leukocytes. A relationship between platelet P-selectin expression and platelet–leukocyte endothelial arrest after chemokine activation has since been revealed in several studies (Schober et al., 2002; Huo et al., 2003; Von Hundelshausen et al., 2005).
Platelets and tissue remodelling events
One consequence of persistent, chronic inflammation is alteration to tissue structure and function. In atherosclerosis, this may result in neo-intima formation (Ross, 1999), while in asthma, chronic inflammation may contribute to changes in airway architecture observed in this disease, referred to as airway remodelling (Vignola et al., 2000). Some of these processes may be independent of the separate requirement of platelets for leukocyte recruitment, since platelets may release a number of mitogens and enzymes that may contribute to tissue remodelling directly. As an example, airway remodelling occurs in experimental models where leukocyte recruitment has been inhibited by glucocorticosteroid administration, but not in animals depleted of platelets (Pitchford et al., 2004a). Thus, platelets may directly affect chronic inflammatory events that lead to smooth muscle proliferation, angiogenesis, myofibroblast proliferation and fibrosis (Tutluoglu et al., 2005; Zernecke et al., 2005). Mechanisms that drive tissue remodelling are not fully understood. However, the recruitment and proliferation of circulating stem and progenitor cell populations, for example mesenchymal stem cells, endothelial progenitor cells and fibrocytes, have been reported (Schmidt et al., 2003; Zernecke et al., 2005; Jin et al., 2006; Massberg et al., 2006). While it is equally feasible that resident structural cells partake in tissue remodelling, there is an increasing body of evidence from studies of diseases with remodelling phenomena which suggest these processes may be greatly enhanced by the participation of progenitor cells, as they become recruited and undergo in situ proliferation and differentiation according to the micro-environment (Schmidt et al., 2003; Zernecke et al., 2005; Jin et al., 2006; Massberg et al., 2006). Interestingly, platelet-derived SDF-1α has been shown to be necessary for ‘hermangioblast' mobilization from the bone marrow (Jin et al., 2006), while platelet P-selectin has been shown to be required for their recruitment to inflamed endothelium in models of atherosclerosis (Massberg et al., 2006). Furthermore, smooth muscle progenitor cells have been shown to require chemokine presentation by platelets for efficient recruitment (Zernecke et al., 2005), and platelets are also required for re-epithelialization of damaged corneal tissue (Li et al., 2006), with similar mechanisms occurring in airway wall remodelling in asthma. Thus platelets may directly participate in the tissue regenerative responses that occur as a result of progenitor cell mobilization from the bone marrow.
Platelets may also directly contribute to a favourable microenvironment for wound repair since platelets contain cellular mitogens such as PDGF, epidermal growth factor, insulin-like growth factor, TGFβ and vascular endothelial growth factor (VEGF) among other growth factors (Rendu and Brohard-Bohn, 2002). Interestingly, the major product of arachidonic acid metabolism in platelets, TxA2, is known to induce the proliferation of smooth muscle cells and also endothelial cell migration and angiogenesis (Dorn, 1997; Daniel et al., 1999). PDGF affects human, rat and rabbit smooth muscle mitogenesis (Hirst et al., 1992). PDGF also acts as a potent chemoattractant for fibroblasts and has been implicated in pulmonary fibrosis (Bonner et al., 1998). TGFβ increases smooth muscle cell mitogenesis in culture, and it has also been suggested to increase airway obstruction by participating in sub-epithelial fibrosis via its chemotactic properties for fibroblasts and neutrophils (Okona-Mensah et al., 1998). VEGF is necessary for angiogenesis during vascular remodelling of ischaemic tissues and also contributes to increases in airflow resistance in obstructive lung disease (Lee et al., 2004). Angiogenesis is also a feature of airway remodelling in asthmatic individuals. The formation of new vessels within the lung parenchyma is inversely correlated to airway calibre and airways hyperresponsiveness (Hoshino et al., 2001).
Platelets may themselves directly alter the composition of the extracellular matrix. Within lysosomes, platelets contain a number of enzymes, termed MMPs, These enzymes are believed to disrupt the composition and integrity of cell membranes by degrading GPs, glycolipids and glycosaminoglycans (Ciferri et al., 2000; Falcinelli et al., 2005). The outcome of this is thought to induce the diapedesis of leukocytes, as well as release membrane-bound growth factors for wound repair (Corry et al., 2002). The implications of these actions are profound in the progression of disease as they may facilitate inflammatory cell diapedesis and stimulate tissue remodelling.
Platelet involvement in antigen recognition
Platelets may also interact with immuno-modulatory cells, or platelets may become directly activated by immunoglobulins such as antigen-specific IgE in allergic inflammation. The actions discussed below strongly indicate that platelets serve an important role in the development of adaptive immunity.
Platelets secrete, or express, a number of factors that have been shown to activate T-lymphocytes, for example the chemokines: RANTES, monocyte chemoattractant protein-3 (MCP-3, CCL7) and macrophage inflammatory protein-1α (MIP-1α, CCL3) may be released by platelets when in contact with T-lymphocytes via CD40–CD40L interactions (Sallusto et al., 1998). CD40–CD40L interactions can induce many cell-mediated inflammatory and immune responses. The release of such mediators can amplify the immune response to antigen by inducing further activation of T-lymphocytes (Danese et al., 2004). CD40L has been identified on activated platelets (Henn et al., 1998), and is functionally active, mediating IgM–IgG isotype switching, a crucial event in humoral immunity (Elzey et al., 2003). CD40L can also lead to the activation of endothelial cells to have a pro-inflammatory phenotype (Danese et al., 2004). Indeed, stimulation of endothelial cells by platelets expressing CD40L significantly contributes to inflammatory cell recruitment in atherosclerosis (Buchner et al., 2003). Interestingly, the production of allergen-specific IgE and airway hyper-responsiveness are suppressed in allergen-sensitized mice deficient in either CD40 or CD40L (Mehlhop et al., 2000). Such interactions may also be important in linking innate responses to that of an adaptive immune response involving platelets, since platelets activated by thrombin induce the activation and maturation of primary bone marrow dendritic cells (Czapiga et al., 2004). This process has been shown to be dependent on platelets delivering co-stimulatory signals via CD40L–CD40 expressed by antigen-presenting cells (APCs; Czapiga et al., 2004). Stimulation via this pathway leads to IL-12 production by APCs, and the surface expression of CD80 and CD83, and as such platelets may provide a bridge between tissue trauma and acquired immunity (Czapiga et al., 2004). It has also been demonstrated that platelets are able to undergo chemotaxis to formyl-Met-Leu-Phe (Czapiga et al., 2005), and are found proximal to dendritic cells in various tissue compartments (Pitchford et al., 2006). This contact may interfere with dendritic cell differentiation and cytokine production (Kissel et al., 2006).
Production of antigen-specific IgE in response to allergen provocation is a fundamental hallmark of atopic diseases (Burrows et al., 1989; Hamelmann et al., 1999). The crosslinking of antigen to IgE on the surface of mast cells is believed to provide the stimulus for mast cell degranulation in early-phase allergic reactions, an event that precipitates a cascade of inflammatory events in response to allergen (Martin et al., 1989, 1993; Oshiba et al., 1996). Patients allergic to Dermatophagoides pteronyssinus (Der p1) and exposed to synthetic peptides derived from the allergen Der p1 were shown to have activated platelets. This was a process mediated by IgE, that did not stimulate platelets from healthy subjects or non-Der p1 allergic patients, illustrating the specific activation of platelets to allergic stimuli (Cardot et al., 1992).
Platelets contain both the high- (10−9 M) and low-affinity (10−7 M) receptors for IgE (FcɛRI and FcɛRII/CD23, respectively) on the surface membrane (Joseph et al., 1986, 1997; Cines et al., 1986; Hasegawa et al., 1999). However, it is apparent that only a few platelets express both FcɛRI and FcɛRII simultaneously (Joseph et al., 1997), and these may represent a subset of platelets that react in a dichotic manner to inflammatory stimuli compared to ‘normal' platelets. The involvement of platelets in allergic inflammation may well represent inappropriate actions of platelets commonly displayed in IgE-mediated immunity against helminth and protozoan parasitic infections (Joseph et al., 1983, 1985; Momi et al., 2000). Platelet activation via FcɛRI has been shown to induce the release of 5-HT, ROS and RANTES, demonstrating that platelets may play an important role in the progression of allergic inflammation via IgE-dependent mechanisms (Joseph et al., 1986; Klouche et al., 1997). It has since been shown that platelets accumulate in the lungs and de-granulate following antigen challenge in sensitized mice, preceding histamine release from mast cells, and platelets may therefore participate towards anaphylaxis directly in response to IgE (Yoshida et al., 2002). Platelets from asthmatic patients and allergic mice have been observed to undergo chemotaxis in response to allergen exposure, via platelet-bound, antigen-specific IgE, and this in vitro phenomenon is reciprocated in vivo as platelets migrate through lung tissue in response to allergen exposure towards the airway wall (as the focus of allergen exposure) (Zhang et al., 1993; Pitchford et al., 2004b).
The process of platelet activation by IgE has been demonstrated to be inhibited by drugs used for the treatment of atopic asthma and allergies, such as nedocromil sodium, disodium cromoglycate and cetirizine (Thorel et al., 1988; Tsicopoulos et al., 1988; De Vos et al., 1989; Joseph et al., 1989, 1993; Tunon-De-Lara et al., 1992). IgE stimulation of platelets represents a non-thrombotic pathway by which platelets can be specifically activated by allergen, and thus directly contribute to the inflammatory responses observed in allergy.
Anti-platelet drugs that modulate inflammation
Some anti-platelet drugs are in use clinically with actions that are known to affect the inflammatory pathways in which platelets are involved, for example purinergic receptor antagonists. An example is clopidogrel, which is used in the treatment of thrombosis, and has beneficial effects on atherosclerosis. A new generation of P2Y1 and P2Y12 antagonists has since been developed, and it will be interesting to observe how their anti-inflammatory properties translate to diseases other than atherosclerosis. P-selectin antagonists, on the other hand, have been developed for their anti-inflammatory properties, and the translation from atherosclerosis to other inflammatory diseases has been more forthcoming, while studies of drugs that anatagonise the actions of pleiotropic mediators released by platelets have also shown efficacy in animal models of inflammation. In the future, drug development may focus on the recent increase in understanding of platelet-dependent mechanisms that control certain inflammatory pathways, and also exploit differences in platelet activation in thrombosis compared to inflammation.
Purinergic receptor antagonists
Three purinergic receptors are expressed on the surface of platelets. The P2X1 cation channel is activated by adenosine tri-phosphate (ATP), while two G protein-coupled receptors – P2Y1 and P2Y12 – are both activated by ADP (Kunapuli, 1998). All three receptors have a role in platelet activation and aggregation. However, differences in the activation kinetics of these last two receptors opens distinct possibilities for the use of antagonists to these receptors as anti-inflammatory compounds.
Activation of P2Y1 coupled to Gαq leads to Ca2+ release, resulting in platelet shape change and a transient aggregation to ADP. While activation of platelets to ADP via P2Y1 is of low potency, it is a requisite step towards further activation of platelets by ADP and collagen. However, P2Y1 does not significantly contribute to the platelet aggregation by other agonists. Selective P2Y1 antagonists have been developed and include MRS2179, MRS2500 and MRS2279, which mimic ATP. Activation of P2Y12 coupled to Gi2 results in full-platelet aggregation and irreversible clot formation in vivo. Activation of platelets via P2Y12 amplifies aggregation initiated by P2Y1; however, it is also necessary for complete aggregation induced by other platelet agonists, for example collagen, thrombin, TXA2, adrenaline and 5-HT. P2Y12 is the target of established inhibitors clopidogrel, ticlopidine and prasugrel; and newer antagonists such as AR-C69931X, AR-C66096MX, AZD6140 and C1330-7. Despite differences in the individual contribution of P2Y1 and P2Y12 activation on platelet aggregation, co-activation is necessary for full ADP-induced aggregation since antagonism of either receptor results in a decrease in the aggregatory response (Hechler et al., 1998; Jin and Kunapuli, 1998; Cattaneo, 2005).
Evidence now suggests that purinergic receptors are important for platelet-mediated inflammation and offer a new opportunity for suppression. Both P2Y1 and P2Y12 activation leads to the expression of platelet P-selectin and the formation of platelet–leukocyte complexes (Leon et al., 2003, 2004). The activation of platelets via chemokines and low levels of primary agonists such as ADP have been shown to be dependent on the purinergic P2Y1 receptor rather than the P2Y12 receptor (Suttitanamongkol and Gear, 2001), which is believed to have a greater role in sustained thrombus formation (Leon et al., 2003; Nylander et al., 2003; Mazzucato et al., 2004). Furthermore, P2Y1 is involved in platelet–monocyte complex formation when platelets are stimulated by lysophosphatidic acid (Haseruck et al., 2004). Although the phenomenon of platelet–leukocyte complex formation and adhesion has not been tested after platelet activation via chemokines, ADP signalling through P2Y1 may contribute to the initial stages of platelet activation in an inflammatory setting. This may resemble a ‘bottleneck' in the directing of leukocyte migration into inflamed tissue via the orchestration of chemokines. Thus, selective inhibition of the P2Y1 in inflammatory diseases may be beneficial because antagonism by MRS2179- and P2Y1-deficient mice leads to only a moderate prolongation of bleeding time (Leon et al., 1999; Fabre et al., 1999; Baurand et al., 2001). This could be advantageous as an anti-inflammatory drug as effects on the normal function of platelets in haemostasis may not be compromised.
Reported inhibition of both the P2Y1 and P2Y12 receptors leads to a decrease in inflammatory parameters in vivo. The administration of MRS2179 leads to a suppression of pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation (Pitchford and Page, 2006), while P2Y1 and apolipoprotein E (ApoE) double ‘knockout' mice have a significant reduction in atherosclerotic plaque size (Gachet, 2006). Recently, a role for platelets has been reported for leukocyte recruitment in a murine model of chronic contact hypersensitivity, where clopidogrel administration reduced cell infiltration into skin tissue and the production of chemokines: MIP-1α, RANTES and TARC (Tamagawa-Mineoka et al., 2007). Specific P2Y1 antagonism reduces platelet P-selectin expression and the occurrence of platelet–leukocyte complexes (Storey et al., 2000; Leon et al., 2003), which may represent a mechanism by which P2Y1 antagonism has efficacy in reducing the extent of inflammation in vivo. Furthermore, the administration of clopidogrel also reduces platelet P-selectin expression, decreased platelet–PMN adhesion and platelet-dependent ROS production in neutrophils (Evangelista et al., 2005), findings reciprocated with thienopyridine and AR-C69931MX (Storey et al., 2002a, 2002b; Leon et al., 2003), but not aspirin (Storey et al., 2002a).
Clinically, the effectiveness of clopidogrel has been compared to aspirin in the ‘Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events' trial (CAPRIE study) in patients with atherosclerotic disease (CAPRIE Steering Committee, 1996). An 8.7% relative risk reduction was demonstrated with the use of clopidogrel compared to aspirin in the occurrence of vascular death, ishaemic stroke or myocardial infarction. This beneficial effect was multiplied in high-risk patients (Bhatt et al., 2001) and in patients suffering from diabetes (Bhatt et al., 2002). Thus, the anti-inflammatory effects may account for the increased efficacy with the use of the P2Y12 antagonist compared to aspirin. Indeed, clopidogrel withdrawal results in the re-emergence of inflammation in patients with diabetes and coronary artery disease (Angiolillo et al., 2006). Clopidogrel significantly reduces platelet-associated inflammatory markers in renal transplant patients with no clinical signs of atherosclerosis (Graff et al., 2005) and patients with stable coronary artery disease, for example P-selectin and CD40L release, platelet–leukocyte complexes and MMP9 release (Klinkhardt et al., 2002; Azar et al., 2006). This increased inflammatory activity of platelets in renal transplant patients may account for the high cardiovascular mortality rate as a result of the development of atherosclerotic lesions in such patients.
While the majority of reports illustrates the anti-inflammatory nature of P2Y receptor antagonism, some investigations suggest otherwise. In particular, clopidogrel administration has been shown to increase the expression of RANTES and MIP-1β from peripheral blood mononuclear cells, and platelet P-selectin and CD63 (a marker of platelet lysosome release) expression remained unchanged in patients with coronary artery disease (Waehre et al., 2006). Since the group of patients studied had stabilized coronary heart disease, the baseline inflammation and platelet activation may have been lower than in other studies, thus making it difficult to accurately access the effect of clopidogrel, although a lack of efficacy on RANTES levels has been reported elsewhere (Bahrmann et al., 2002). Nevertheless, evidence overwhelmingly points to an efficacy of P2 antagonists on inhibition of platelet–leukocyte complex formation and platelet P-selectin expression, and it is necessary to investigate the effects of these drugs on inflammatory diseases other than atherosclerosis.
P-selectin inhibition
The surface expression of P-selectin on activated platelets, the requirement of this adhesion molecule in the formation of platelet–leukocyte aggregates, the ensuing leukocyte activation and subsequent diapedesis have made P-selectin a potential anti-platelet target. Investigations have used a diverse array of compounds to inhibit P-selectin, from blocking antibodies, soluble protein ligands, oligosaccharides and small molecule antagonists.
Efforts have been made to engineer blocking antibodies for P-selectin or PSGL-1. In particular, the antibody RB40.34 has proven in vivo efficacy in models of ischaemia (Lehmberg et al., 2006). Furthermore, pulmonary eosinophil and lymphocyte recruitment were inhibited with the administration of RB40.34 in a murine model of allergic inflammation (Pitchford et al., 2005). The concept has been advanced towards the clinic with the production of a humanized antibody (mEP.SC7), which has been shown, to block binding of a leukaemia cell line to P- and endothelial-selectin (E-selectin), and has favourable pharmacokinetic properties when administered to Rhesus monkeys (He et al., 1998). Other attempts to block P-selectin have been made by targeting the counter ligand PSGL-1, either via the administration of antibodies or recombinant proteins. For example, intimal hyperplasia of the carotid artery was prevented with an anti-PSGL-1 immunoglobulin in a balloon injury model in pigs (Wang et al., 2001) and attenuated infarct size during ischaemia–reperfusion injury in dogs (Wang et al., 2002). These effects may be attributable to the ability of rPSGL-Ig to reduce leukocyte rolling and adhesion to acute inflamed endothelium (Eppihimer and Schaub, 2001; Theoret et al., 2001). Separate from cardiovascular disease, an rPSGL-1 antibody ameliorates cell accumulation, TNFα levels and joint severity in a murine model of RA (Sumariwalla, 2004). P-selectin-mediated cell adhesion has also been specifically inhibited by phage display-derived peptide antagonists with high potency (Molenaar et al., 2002), which are reported to be most effective in tetrameric form.
Other P-selectin inhibitors include fucoidans extracted from brown seaweed. Fucoidans effectively inhibit leukocyte recruitment to inflamed peritoneum in rats (Preobrazhenskaya et al., 1997; Cumashi et al., 2007). In vitro evaluation showed inhibition of P-selectin-mediated neutrophil adhesion to platelets under flow conditions.
Synthetic low-molecular weight P-selectin antagonists have also been produced that mimic the carbohydrate moieties on the P-selectin counter ligands, being largely based on Sialyl LewisX. These have potent in vivo and in vitro activity. For example, oligosaccharides have been shown to inhibit eosinophil and neutrophil adhesion to immobilized platelets (Kim et al., 1998), and the monosaccharide dimer: bimosiamose (TBC1269) decreases reperfusion injury after myocardial infarction in rats (Onai et al., 2003). Although the activity of bimosiamose may also be attributable to antagonism of E-selectin, it has shown promising results during clinical development, having improved skin lesions in psoriasis patients and airway reactivity in mild asthmatics (Beeh et al., 2006; Friedrich et al., 2006). Interestingly, a synthetic pentasaccharide devoid of anti-coagulant properties and derived from fondaparinux (Frank et al., 2006) has recently been reported to reduce inflammation in a murine model of kidney ischaemia. Previous studies reveal this anti-inflammatory activity may be attributed to the ability of fondaparinux to inhibit P-selectin-dependent adhesion of U937 cells in vitro and a reduction in the recruitment of neutrophils to the peritoneum of thioglycolate-treated mice that is also dependent on platelet P-selectin (Frank et al., 2005). It must be stated, however, that other oligosaccharides, for example the pentasaccharide CY1503, that have been developed for reperfusion injury have not demonstrated clinical efficacy (Kaila and Thomas, 2002), but this has been attributed to poor bioavailability.
Lastly, small molecule inhibitors have been developed in the guise of quinoline salicylic acids. Having been evaluated for their ability to antagonise P-selectin, quinilone salicylic acid antagonists act by competing with the sialyl Lewisx moieties on P-selectin ligands (Kaila et al., 2007). Antagonism has been shown to be efficacious in a rat antigen-induced arthritis model of RA (Kaila et al., 2007). These perhaps offer an exciting subset of compounds that antagonise P-selectin with favourable pharmacodynamic profiles that may allow them to progress with efficacy through clinical trials.
Antagonists of pleiotropic mediators released by platelets
Experimental data suggest that established anti-platelet agents (ridogrel) indicated in the treatment of other inflammatory diseases inhibit pathological processes with similarities to processes involved in the pathogenesis of asthma (Anderson et al., 2001). Inhibitors of TXA2 are effective in inhibiting pulmonary leukocyte recruitment in murine models of allergic inflammation (Shi et al., 1998) and may act via the inhibition of TXA2 synthase by platelets and antagonism of TXA2 on effector cells. Indeed, these have been shown to be effective when used either alone or in combination for suppressing antigen-induced bronchoconstriction in guinea pigs (Yoshimi et al., 2001). Other drugs have been developed with dual leukotriene D4 and TXA2 receptor antagonism with efficacy in experimental models of allergic inflammation (Yamada et al., 2003; Ishimura et al., 2006). However, the efficacy of TXA2 synthase inhibitors and receptor antagonists does not spread to all inflammatory diseases where activated platelets are a component. TXA2 release is a feature of Crohn's disease but ridogrel lacks efficacy (Carty et al., 2001), despite showing efficacy in experimental colitis models (Vilaseca et al., 1990).
Inhibitors of 5-HT, for example ketanserin (De Bie et al., 1998), are effective in inhibiting indices of allergic inflammation, acting on 5-HT2 receptors. Indeed, studies reveal 5-HT originating from platelets is capable of altering the pathogenesis of asthma, since drug (tianeptine) induced 5-HT uptake by platelets has been shown to reduce the clinical severity of asthmatic patients (Lechin et al., 1998).
GPIIb/IIIa integrin blockers: a cautionary note
The GP IIb/IIIa integrin is found on the platelet membrane and is the final common pathway in platelet aggregation. Intravenous antagonists of the GPIIb/IIIa integrin have significant clinical benefits in patients with acute coronary syndromes undergoing percutaneous coronary intervention (Bhatt and Topol, 2000; Topol et al., 2001). However, sub-therapeutic doses of GPIIb/IIIa antagonists especially orally active compounds have been shown to have deleterious outcomes on patients (Chew et al., 2001; Quinn et al., 2002). While this outcome may be the result of partial agonism at sub-therapeutic concentrations (Cox et al., 2000), leading to ‘platelet escape' and thrombus formation, a pro-inflammatory profile of GPIIb/IIIa antagonists is apparent. In vitro studies reveal that both monoclonal antibodies and non-peptide inhibitors increase platelet P-selectin expression and platelet–leukocyte complexes (Caron et al., 2002; Klinkhardt et al., 2002), as well as the release of CD40L and tissue factor (Zhao et al., 2003) and may explain the negative clinical effects of GPIIb/IIIa antagonists. These studies also demonstrate that pathways leading to platelet aggregation are distinct to pathways leading to platelet activation of P-selectin expression and CD40L release. Thus, future drug design may be successful in inhibiting pro-inflammatory platelet activation but not platelet aggregation in inflammatory diseases where an inhibition of clotting could be deleterious.
Future drug targets
Mediators and receptors that can be selectively targeted to inhibit platelet activation rather than platelet aggregation may be clinically relevant in the treatment of asthma, COPD, RA, IBD and atherosclerosis. Indeed, exploiting mediators involved in platelet activation that are disease specific would obviously have advantages. Antagonists for specific chemokine receptors may be advantageous, for example the targeting of platelet CCR3 and CCR4 might be of benefit in treating asthmatics (Gonzalo et al., 1999; Sekiya et al., 2000), while the targeting of RANTES may be beneficial in the treatment of atherosclerosis since platelet-derived RANTES deposited on the surface of endothelial cells is necessary for monocyte accumulation (Von Hundelshausen et al., 2005). Indeed, RANTES receptor antagonists inhibit the infiltration of macrophages, and importantly, reduce neointima formation in ApoE-deficient mice (Schober et al., 2002; Huo et al., 2003).
CD40L is a principal mediator in inflammation, and the majority (>90%) of CD40L produced in the body is derived from platelets (Henn et al., 1998). CD40L is therefore a very attractive drug target for inhibiting platelet activation as it can induce an inflammatory cascade centred on the activation of endothelium and T cells (Buchner et al., 2003; Danese et al., 2004). Few reports exist of studies describing CD40 antagonism, although a monoclonal antibody (5D12) is currently being tested for the treatment of Crohn's disease (Kasran et al., 2005).
Another attractive target is sphingosine-1-phosphate (S1P), a lipid mediator stored in platelets, which are the major source of S1P in plasma (Yatomi et al., 2000). The mechanisms by which S1P modulate the pathogenesis of inflammation are ill defined but S1P acts via five specific receptors, S1P1−5 (Chun et al., 2002). S1P can activate monocytes, endothelial cells, mast cells, eosinophils, smooth muscle cells and promote tissue recruitment of leukocytes (Roviezzo et al., 2004). Studies reveal an involvement for S1P in asthma, RA, IBD and atherosclerosis (Ammit et al., 2001; Kitano et al., 2006; Daniel et al., 2007; Nofer et al., 2007). Attempts have been made to produce antagonists to the S1P receptors, for example FTY720, and it has been demonstrated to ameliorate disease pathogenesis in models of colitis and atherosclerosis (Daniel et al., 2007; Nofer et al., 2007).
Peroxisome proliferator-activated receptors (PPARs) are another novel target for inhibiting platelet activation, acting as transcription factors for lipid and glucose metabolism. While being nuclear receptors, all three subtypes are expressed in platelets – α, β and γ – despite platelets being anucleate. Selective agonists for all three receptors (fenofibrate: PPARα; GW0742 and L165041: PPARβ; and rosiglitazone: PPARγ) are capable of inhibiting platelet aggregation (Ali et al., 2005). Recently, PPARγ agonists acting on platelets have been shown to have anti-inflammatory properties, inhibiting platelet release of CD40L and TXA2 production (Akbiyik et al., 2004; Ray et al., 2006). The efficacy of PPAR agonists in inflammatory diseases needs to be thoroughly investigated.
A dichotomy of platelet function in thrombosis compared to inflammation is highlighted in the fact that only a subset of platelets displays receptors for IgE. This dichotomy in platelet function needs to be thoroughly researched and a better understanding of the molecular pathways by which inflammatory mediators activate platelets as opposed to aggregatory pathways may hold promise for future therapeutics. Similarly, the molecular mechanisms governing platelet granule and lysosome release are currently being investigated and may have potential. For example, the exocytotic pathway in platelets is unique as platelet-shape change leads to an organized rearrangement of secretory granules. Specific membrane proteins control the fusion of granules to the platelet membrane, and these include vesicle membrane proteins: vSNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) from the synaptobrevin/VAMP (vesicle-associated membrane protein) bind to complexes in the target membrane tSNAREs composed of syntaxins- and synaptosomal-associated proteins (for example, SNAP-23). Dense core and α-granule secretion is mediated by the tSNARES: SNAP-23 and syntaxin 2 (Chen et al., 2000; Feng et al., 2002). However, differences are apparent in the requirement of different VAMPs for dense and α-granule secretion (Polgar et al., 2002). Exploitation of these differences and differences that may be apparent compared to other secretory cells could, therefore, lead to selective inhibitors of platelet granule release of pro-inflammatory mediators and adhesion molecules.
Concluding remarks
Despite recent advances revealing the phenomenon of platelet participation in inflammation, detailed investigations of platelet-dependent mechanisms of disease pathogenesis are in their infancy. It is therefore not surprising that as yet no specific therapy has been developed for the treatment of inflammatory diseases based on inhibiting platelet function. Clearly, the mechanisms that differentiate the necessary role of platelets in haemostasis to that of platelet activation in an inflammatory setting need to be distinguished to allow the emergence of targets for novel safe therapies. Nevertheless, the efficacy of platelet inhibition in the suppression of inflammation in disease models; suggests that potential drug targets directed at inhibiting platelet function may provide alternative and powerful treatments for inflammatory diseases in the future.
Abbreviations
- 5-HT
5-hydroxytryptamine
- ADP
adenosine di-phosphate
- ATP
adenosine tri-phosphate
- APC
antigen-presenting cell
- ApoE
apolipoprotein E
- β-TG
β-thromboglobulin
- COPD
chronic obstructive pulmonary disease
- Der p1
Dermatophagoides pteronyssinus
- E-selectin
endothelial selectin
- GP
glycoprotein
- Ig
immunoglobulin
- IBD
inflammatory bowel disease
- CD11b
integrin αM
- CD18
integrin β2
- ICAM-2
intercellular adhesion molecule-2
- IL
interleukin
- JAM
junctional adhesion molecule
- LFA-1, CD11a/CD18, αLβ2 integrin
leukocyte functional antigen-1
- Mac 1, CD11b/CD18, αMβ2 integrin
macrophage-1 integrin
- MMP
matrix metalloproteinase
- MCP-1 (CCL-2)
monocyte chemoattractant protein-1
- MCP-3 (CCL7)
monocyte chemoattractant protein-3
- MDC (CCL22)
monocyte-derived chemokine
- MIP-1α, CCL3
macrophage inflammatory protein-1 alpha
- NSAIDs
non-steroidal anti-inflammatory drugs
- PPAR
peroxisome proliferator-activated receptor
- PECAM-1
platelet endothelial cell adhesion molecule-1
- PDGF
platelet-derived growth factor
- PF-4
platelet-factor 4
- P-selectin (CD62P)
platelet selectin
- PSGL-1 (CD154)
P-selectin glycoprotein ligand-1
- PAF
platelet-activating factor
- TARC (CCL17)
thymus and activation-regulated cytokine
- TXA2
thromboxane
- RANTES (CCL5)
regulated upon activation normally T-cell expressed and secreted
- ROS
reactive oxygen species
- RA
rheumatoid arthritis
- SNAP-23
synaptosomal-associated proteins
- S1P
sphingosine-1-phosphate
- SFKs
Src-family tyrosine kinases
- SDF-1 (CXCL12)
stromal cell-derived factor-1
- TGFβ
transforming growth factor-β
- TNFα
tumour necrosis factor-α
- VAMP
vesicle-associated membrane protein
- VEGF
vascular endothelial growth factor
- VLA-4
α4β1 integrin, very late antigen-4 integrin
Conflict of interestThe author states no conflict of interest.
References
- Abi-Younes S, Sauty A, Mach F, Sukhova GK, Libb Y P, Luster AD. The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circ Res. 2000;86:131–139. doi: 10.1161/01.res.86.2.131. [DOI] [PubMed] [Google Scholar]
- Abi-Younes S, Si-Tahar M, Luster AD. The CC chemokines MDC and TARC induce platelet activation via CCR4. Thromb Res. 2001;101:279–289. doi: 10.1016/s0049-3848(00)00402-3. [DOI] [PubMed] [Google Scholar]
- Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPAR gamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104:1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- Ali FY, Davidson SJ, Moraes LA, Traves SL, Paul-Clark M, Bishop-Bailey D, et al. Role of nuclear receptor signaling in platelets: antithrombotic effects of PPARβ. FASEB J. 2005;20:326–328. doi: 10.1096/fj.05-4395fje. [DOI] [PubMed] [Google Scholar]
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, et al. Sphingosine 1-phosphate modulates human airway smooth muscle functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- Anderson HV, Mcnatt J, Clubb FJ, Herman M, Maffrand J-P, Declerk F, et al. Platelet inhibition reduces cyclic flow variations and neointimal proliferation in normal and hypercholesterolemic–atherochlerotic canine coronary arteries. Circulation. 2001;104:2331–2337. doi: 10.1161/hc4401.098434. [DOI] [PubMed] [Google Scholar]
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, et al. Clopidogrel withdrawal is associated with pro-inflammatory and pro-thrombotic effects in patients with diabetes and coronary artery disease. Diabetes. 2006;55:780–784. doi: 10.2337/diabetes.55.03.06.db05-1394. [DOI] [PubMed] [Google Scholar]
- Arber N, Berliner S, Pras E, Arber L, Fishelson Z, Kahn Y, et al. Heterotypic leukocyte aggregation in the peripheral blood of patients with leukaemia, inflammation and stress. Nouv Rev Fr Hematol. 1991;33:251–255. [PubMed] [Google Scholar]
- Azar RR, Kassab R, Zoghbi A, Aboujaoude S, El-Osta H, Ghorra P, et al. Effects of clopidogrel on soluble CD40 ligand and on high-sensitivity C-reactive protein in patients with stable coronary artery disease. Am Heart J. 2006;151:521–524. doi: 10.1016/j.ahj.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Bahrmann P, Sigusch HH, Surber R, Figulla HR. Oral anti-platelet therapies have no effect on circulating levels of RANTES in patients with coronary artery disease. Pharmazie. 2002;57:863–864. [PubMed] [Google Scholar]
- Baurand A, Raboisson P, Freund M, Leon C, Cazenave JP, Bourguignon JJ, et al. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Beier J, Meyer M, Buhl R, Zahlten R, Wolff G. Bimosiamose, an inhaled small-molecule pan-selectin antagonist, attenuates late asthmatic reactions following allergen challenge in mild asthmatics: a randomized, double blind, placebo-controlled clinical cross-over trial. Pulm Pharmacol Ther. 2006;19:233–241. doi: 10.1016/j.pupt.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Kacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation. 2001;103:363–368. doi: 10.1161/01.cir.103.3.363. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol. 2002;90:625–628. doi: 10.1016/s0002-9149(02)02567-5. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Topol EJ. Current role of platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. JAMA. 2000;284:1549–1558. doi: 10.1001/jama.284.12.1549. [DOI] [PubMed] [Google Scholar]
- Blanks JE, Moll T, Eytner R, Vestweber D. Stimulation of P-selectin glycoprotein ligand-1 on mouse neutrophils activates beta 2-integrin mediated cell attachment to ICAM-1. Eur J Immunol. 1998;28:433–443. doi: 10.1002/(SICI)1521-4141(199802)28:02<433::AID-IMMU433>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Lindroos PM, Rice AB, Moomaw CR, Morgan DL. Induction of PDGF receptor-alpha in rat myofibroblasts during pulmonary fibrogenesis in vivo. Am J Physiol. 1998;274:L72–L80. doi: 10.1152/ajplung.1998.274.1.L72. [DOI] [PubMed] [Google Scholar]
- Buchner K, Henn V, Grafe M, De Boer OJ, Becker AE, Kroczek RA. CD40 ligand is selectively expressed on CD4+ T cells and platelets: implications for CD40–CD40L signalling in atherosclerosis. J Pathol. 2003;201:288–295. doi: 10.1002/path.1425. [DOI] [PubMed] [Google Scholar]
- Bunescu A, Seideman P, Lenkei R, Levin K, Egberg N. Enhanced Fcγ receptor I, αMβ2 integrin receptor expression by monocytes and neutrophils in rheumatoid arthritis: interaction with platelets. J Rheumatol. 2004;31:2347–2355. [PubMed] [Google Scholar]
- Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- CAPRIE Steering Committee A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- Cardot E, Pestel J, Callebaut I, Lassalle P, Tsicopoulos A, Gras-Masse H, et al. Specific activation of platelets from patients allergic to Dermatophagoides pteronyssinus by synthetic peptides derived from the allergen Der p I. Int Arch Allergy Immunol. 1992;98:127–134. doi: 10.1159/000236175. [DOI] [PubMed] [Google Scholar]
- Caron A, Theoret J-F, Mousa SA, Merhi Y. Anti-platelet effects of GPIIb/IIIa and P-selectin antagonism, platelet activation, and binding to neutrophils. J Cardiovasc Pharmacol. 2002;40:296–306. doi: 10.1097/00005344-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Carty E, Rampton DS, Schneider H, Rutgeerts P, Wright JP. Lack of efficacy of ridogrel, a thromboxane synthase inhibitor, in a placebo controlled, double blind, multi-centre clinical trial in active Crohn's disease. Aliment Pharmacol Ther. 2001;15:1323–1329. doi: 10.1046/j.1365-2036.2001.01056.x. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. The P2 receptors and congenital platelet function defects. Semin Thromb Hemost. 2005;31:168–173. doi: 10.1055/s-2005-869522. [DOI] [PubMed] [Google Scholar]
- Chavakis T, Keiper T, Matz-Westphal R, Hersemeyer K, Sachs UJ, Nawroth PP, et al. The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J Biol Chem. 2004;279:55602–55608. doi: 10.1074/jbc.M404676200. [DOI] [PubMed] [Google Scholar]
- Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95:921–929. [PubMed] [Google Scholar]
- Chew DP, Bhatt DL, Sapp S, Topol EJ. Increased mortality with oral platelet glycoprotein IIb/IIIa antagonists: a meta analysis of phase III multicenter randomized trials. Circulation. 2001;103:201–206. doi: 10.1161/01.cir.103.2.201. [DOI] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International union of pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Ciferri S, Emiliani C, Guglielmini G, Orlacchio A, Nenci GG, Gresele P. Platelets release their lysosomal content in vivo in humans upon activation. Thromb Haemost. 2000;83:157–164. [PubMed] [Google Scholar]
- Cines DB, Van Der KH, Levinson AI. In vitro binding of an IgE protein to human platelets. J Immunol. 1986;136:3433–3440. [PubMed] [Google Scholar]
- Clemetson KJ, Clemetson JM, Proudfoot AE, Power CA, Baggiolini M, Wells TN. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood. 2000;96:4046–4054. [PubMed] [Google Scholar]
- Collins CE, Cahill MR, Newland AC, Rampton DS. Platelets circulate in an activated state in inflammatory bowel disease. Gastroeneterology. 1994;106:840–845. doi: 10.1016/0016-5085(94)90741-2. [DOI] [PubMed] [Google Scholar]
- Collins CE, Rampton DS, Rogers J, Williams NS. Platelet aggregation and neutrophil and neutrophil sequestration in the mesenteric circulation in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:1213–1217. [PubMed] [Google Scholar]
- Cooper D, Butcher CM, Berndt MC, Vadas MA. P-selectin interacts with a beta 2-integrin to enhance phagocytosis. J Immunol. 1994;153:3199–3209. [PubMed] [Google Scholar]
- Cordova C, Musca A, Violi F, Alessandri C, Perrone A, Balsano F. Platelet hyperfunction in patients with chronic airways obstruction. Eur J Respir Dis. 1985;66:9–12. [PubMed] [Google Scholar]
- Corry DB, Rishi K, Kanellis J, Kiss A, Song LZ, Xu J, et al. Decrease allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Smith R, Quinn M, Theroux P, Crean P, Fitzgerald DJ. Evidence of platelet activation during treatment with a GPIIb/IIIa antagonist in patients presenting with acute coronary syndromes. J Am Coll Cardiol. 2000;36:1514–1519. doi: 10.1016/s0735-1097(00)00919-0. [DOI] [PubMed] [Google Scholar]
- Coyle AJ, Page CP, Atkinson L, Flanagan R, Metzger WJ. The requirement for platelets in allergen-induced late asthmatic airway obstruction. Eosinophil infiltration and heightened airway responsiveness in allergic rabbits. Am Rev Respir Dis. 1990;142:587–593. doi: 10.1164/ajrccm/142.3.587. [DOI] [PubMed] [Google Scholar]
- Cumashi A, Ushakova NA, Preobrazhenskaya ME, D'incecco A, Piccoli A, Totani L, et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiol. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- Czapiga M, Gao JL, Kirk A, Lekstrom-Himes J. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp Hematol. 2005;33:73–84. doi: 10.1016/j.exphem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Czapiga M, Kirk AD, Lekstrom-Himes J. Platelets deliver costimulatory signals to antigen-presenting cells: a potential bridge between injury and immune activation. Exp Hematol. 2004;32:135–139. doi: 10.1016/j.exphem.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Damle NK, Klussman K, Dietsch MT, Mohagheghpour N, Aruffo A. GMP-140 (P-selectin/CD62) binds to chronically stimulated but not resting CD4+ T lymphocytes and regulates their production of proinflammatory cytokines. Eur J Immunol. 1992;22:1789–1793. doi: 10.1002/eji.1830220718. [DOI] [PubMed] [Google Scholar]
- Danese S, De La Motte C, Rivera Reyes BM, Sans M, Levine AD, Fiocchi C. T cells trigger CD40-dependent platelet activation and granular RANTES release: a novel pathway for immune response amplification. J Immunol. 2004;172:2011–2015. doi: 10.4049/jimmunol.172.4.2011. [DOI] [PubMed] [Google Scholar]
- Danese S, De La Motte C, Sturm A, Vogel JD, West GA, Strong SA, et al. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology. 2003b;124:1249–1264. doi: 10.1016/s0016-5085(03)00289-0. [DOI] [PubMed] [Google Scholar]
- Danese S, Katz J, Saibeni S, Papa A, Gasbarrini A, Vecchi M, et al. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003a;52:1435–1441. doi: 10.1136/gut.52.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese S, Scaldaferri F, Vetrano S, Stefanelli T, Graziani C, Repici A, et al. Critical role of the CD40–CD40 ligand pathway in governing mucosal inflammation driven angiogenesis in inflammatory bowel disease Gut 2007(doi 1136/gut.2006.111989; E-pub ahead of print) [DOI] [PMC free article] [PubMed]
- Daniel C, Sartory N, Zahn N, Geisslinger G, Radeke HH, Stein JM. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178:2458–2468. doi: 10.4049/jimmunol.178.4.2458. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999;59:4574–4577. [PubMed] [Google Scholar]
- Davi G, Basili S, Vieri M, Cipollone F, Santarone S, Alessandri C, et al. Enhanced thromboxane biosynthesis in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:1794–1799. doi: 10.1164/ajrccm.156.6.9706026. [DOI] [PubMed] [Google Scholar]
- De Bie JJ, Henricks PA, Cruikshank WW, Hofman G, Jonker EH, Nijkamp FP, et al. Modulation of airway hyperresponsiveness and eosinophilia by selective histamine and 5-HT receptor antagonists in a mouse model of allergic asthma. Br J Pharmacol. 1998;124:857–864. doi: 10.1038/sj.bjp.0701901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos C, Joseph M, Leprevost C, Vorng H, Tomassini M, Capron M, et al. Inhibition of human eosinophil chemotaxis and of the IgE-dependent stimulation of human blood platelets by cetirizine. Int Arch Allergy Appl Immunol. 1989;88:212–215. doi: 10.1159/000234789. [DOI] [PubMed] [Google Scholar]
- Diacovo TG, De Fougerolles AR, Bainton DS, Springer TA. A functional integrin ligand on the surface of platelets: intercellular adhesion molecules-2. J Clin Invest. 1994;94:1243–1251. doi: 10.1172/JCI117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacovo TG, Puri KD, Warnock RA, Springer TA, Von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996a;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996b;88:146–157. [PubMed] [Google Scholar]
- Dorn GW. Role of thromboxane A2 in mitogenesis of vascular smooth muscle cells. Agents Actions Suppl. 1997;48:42–62. doi: 10.1007/978-3-0348-7352-9_3. [DOI] [PubMed] [Google Scholar]
- Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, et al. Platelet mediated modulation of adapted immunity. A communication link between innate and adaptive immune compartments. Immunity. 2003;19:9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- Endresen GK. Evidence for activation of platelets in the synovial fluid from patients with rheumatoid arthritis. Rheumatol Int. 1989;9:19–24. doi: 10.1007/BF00270285. [DOI] [PubMed] [Google Scholar]
- Endresen GK, Forre O. Human platelets in synovial fluid. A focus on the effects of growth factor on the inflammatory responses in rheumatoid arthritis. Clin Exp Rheumatol. 1992;10:181–187. [PubMed] [Google Scholar]
- Eppihimer MJ, Schaub RG. Soluble P-selectin antagonist mediates rolling velocity and adhesion of leukocytes in acutely inflamed venules. Microcirculation. 2001;8:15–24. [PubMed] [Google Scholar]
- Evangelista V, Manarini S, Dell'elba G, Martelli N, Napoleone E, Di Santo A, et al. Clopidogrel inhibits platelet–leukocyte adhesion and platelet-dependent leukocyte activation. Thromb Haemost. 2005;93:568–577. [PubMed] [Google Scholar]
- Evangelista V, Manarini S, Rotondo S, Martelli N, Polischuk R, Mcgregor JL, et al. Platelet/polymorphonuclear leukocyte interaction in dynamic conditions: evidence of adhesion cascade and cross talk between P-selectin and the beta 2 integrin CD11b/CD18. Blood. 1996;88:4183–4194. [PubMed] [Google Scholar]
- Evangelista V, Manarini S, Sideri R, Rotondo S, Martelli N, Piccoli A, et al. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 1999;93:876–885. [PubMed] [Google Scholar]
- Evangelista V, Pamuklar Z, Piccoli A, Manarini S, Dell'elba G, Pecce R, et al. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood. 2007;109:2461–2469. doi: 10.1182/blood-2006-06-029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1 deficient mice. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- Fagerstam JP, Whiss PA, Strom M, Andersson RG. Expression of platelet P-selectin and detection of soluble P-selectin, NPY and RANTES in patients with inflammatory bowel disease. Inflamm Res. 2000;49:466–472. doi: 10.1007/s000110050618. [DOI] [PubMed] [Google Scholar]
- Falcinelli E, Guglielmini G, Torti M, Gresele P. Platelets release matrix metalloproteinase-2 (MMP-2) in vivo in humans at a localised site of platelet activation. J Thromb Haemost. 2005;3:2526–2535. doi: 10.1111/j.1538-7836.2005.01614.x. [DOI] [PubMed] [Google Scholar]
- Farr M, Wainwright A, Salmon M, Hollywell CA, Bacon PA. Platelets in the synovial fluid of patients with rheumatoid arthritis. Rheumatol Int. 1984;4:13–17. doi: 10.1007/BF00683878. [DOI] [PubMed] [Google Scholar]
- Feng D, Crane K, Rozenvayn N, Dvorak AM, Flaumenhalt R. Subcellular distribution of 3 functional platelet SNARE proteins: human cellubrevin, SNAP-23, and syntaxin 2. Blood. 2002;99:4006–4014. doi: 10.1182/blood.v99.11.4006. [DOI] [PubMed] [Google Scholar]
- Ferroni P, Basili S, Martini F, Vieri M, Labbadia G, Cordova C, et al. Soluble P-selectin as a marker of platelet hyperactivity in patients with chronic obstructive pulmonary disease. J Investig Med. 2000;48:21–27. [PubMed] [Google Scholar]
- Frank RD, Holscher T, Schabbauer G, Tencati M, Pawlinski R, Weitz JI, et al. A non-anticoagulant synthetic pentasaccharide reduces inflammation in a murine model of kidney ischaemia–reperfusion injury. Thromb Haemost. 2006;96:802–806. [PubMed] [Google Scholar]
- Frank RD, Schabbauer G, Holscher T, Sato Y, Tencati M, Pawlinski R, et al. The synthetic pentasaccharide fondaparinux reduces coaggulation, inflammation and neutrophil accumulation in kidney ischaemia–reperfusion injury. J Thromb Haemost. 2005;3:531–540. doi: 10.1111/j.1538-7836.2005.01188.x. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Bock D, Philipp S, Ludwig N, Sabat R, Wolk K, et al. Pan-selectin antagonism improves psoriasis manifestation in mice and man. Arch Dermatol Res. 2006;297:345–351. doi: 10.1007/s00403-005-0626-0. [DOI] [PubMed] [Google Scholar]
- Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- Garlichs CD, Eskafi S, Raaz D. Patients with acute coronary syndromes express enhanced levels of CD40 ligand/CD154 on platelets. Heart. 2001;86:649–655. doi: 10.1136/heart.86.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear ARL, Suttitanamongkol S, Viisoreanu D, Polanowska-Grabowska RK, Raha S, Camerini D. Adenosine diphosphate strongly potentiates the ability of the chemokines MDC, TARC, and SDF-1 to stimulate platelet function. Blood. 2001;97:937–945. doi: 10.1182/blood.v97.4.937. [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- Graff J, Harder S, Wahl O, Scheuermann E-H, Gossmann J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: effects on platelet–leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin Pharmacol Ther. 2005;78:468–476. doi: 10.1016/j.clpt.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Gresele P, Dottorini M, Selli ML, Iannacci L, Canino S, Todisco T, et al. Altered platelet function associated with the bronchial hyperresponsiveness accompanying nocturnal asthma. J Allergy Clin Immunol. 1993;91:894–902. doi: 10.1016/0091-6749(93)90347-i. [DOI] [PubMed] [Google Scholar]
- Haley KJ, Lilly CM, Yang JH, Feng Y, Kennedy SP, Turi TG, et al. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation. 2000;102:2185–2189. doi: 10.1161/01.cir.102.18.2185. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Tadeda K, Oshiba A, Gelfand EW. Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness – a murine model. Allergy. 1999;54:297–305. doi: 10.1034/j.1398-9995.1999.00085.x. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Jonasson I, Seifert PS, Stemme S. Immune mechanisms in atherosclerosis. Atherosclerosis. 1989;9:567–578. doi: 10.1161/01.atv.9.5.567. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Libby P.The role if the lymphocyte Atherosclerosis and Coronary Artery Disease 19961Lippincott-Raven: Philadelphia; 557–568.In: Fuster V, Ross R, Topol EJ (eds). [Google Scholar]
- Hasegawa S, Pawankar R, Suzuki K, Nakahata T, Furukawa S, Okumura K, et al. Functional expression of the high affinity receptor for IgE (FcepsilonRI) in human platelets and its' intracellular expression in human megakaryocytes. Blood. 1999;93:2543–2551. [PubMed] [Google Scholar]
- Haseruck N, Erl W, Pandey D, Tigyi G, Ohlmann P, Ravanat C, et al. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet–monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood. 2004;103:2585–2592. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- Harker LA, Malpass TW, Branson HE, Hessel EA, Slichter SJ. Mechanism of abnormal bleeding in patients undergoing cardiopulmonary bypass: acquired transient platelet dysfunction associated with selective alpha-granule release. Blood. 1980;56:824–834. [PubMed] [Google Scholar]
- Hayward R, Campbell B, Shin YK, Scalia R, Lefer AM. Recombinant soluble P-selectin glycoprotein ligand-1 protects against myocardial ischemic reperfusion injury in cats. Cardiovasc Res. 1999;41:65–76. doi: 10.1016/s0008-6363(98)00266-1. [DOI] [PubMed] [Google Scholar]
- He XY, Xu Z, Melrose J, Mullowney A, Vasquez M, Queen C, et al. Humanization and pharmacokinetics of a monoclonal antibody with a specificity for both E- and P-selectin. J Immunol. 1998;160:1029–1035. [PubMed] [Google Scholar]
- Hechler B, Eckley A, Ohlmann P, Cazenave JP, Gachet C. The P2Y1 receptor, necessary but not sufficient to support full ADP-induced platelet aggregation, is not the target of the drug clopidogrel. Br J Haematol. 1998;103:858–866. doi: 10.1046/j.1365-2141.1998.01056.x. [DOI] [PubMed] [Google Scholar]
- Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, et al. CD40L on activated platelets platelets triggers an inflammatory reaction on endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- Hidari KI, Weyrich AS, Zimmerman GA, McEver RP. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem. 1997;272:28750–28756. doi: 10.1074/jbc.272.45.28750. [DOI] [PubMed] [Google Scholar]
- Hirst SJ, Barnes PJ, Twort CH. Quantifying proliferation of cultured human and rabbit airway smooth muscle cells in response to serum and platelet-derived growth factor. Am J Respir Cell Mol Biol. 1992;7:574–581. doi: 10.1165/ajrcmb/7.6.574. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107:1034–1038. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- Huo Y, Schober A, Forlow B, Smith DF, Hyman MC, Jung S, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nature Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- Ishimura M, Kataoka S, Suda M, Maeda T, Hiyama Y. Effects of KP-496, a novel dual antagonist for leukotriene D4 and thromboxane A2 receptors, on contractions induced by various agonists in the guinea pig trachea. Allergol Int. 2006;55:403–410. doi: 10.2332/allergolint.55.403. [DOI] [PubMed] [Google Scholar]
- Jawien J, Chlopicki S, Gryglewski RW. Interactions between human platelets and eosinophils are mediated by selectin-P. Pol J Pharmacol. 2002;54:157–160. [PubMed] [Google Scholar]
- Jaremo P, Sandberg-Gertzen H. Platelet density and size in inflammatory bowel disease. Thromb Haemost. 1996;75:560–561. [PubMed] [Google Scholar]
- Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB. Bronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivity. Am Rev Respir Dis. 1989;140:1745–1753. doi: 10.1164/ajrccm/140.6.1745. [DOI] [PubMed] [Google Scholar]
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, et al. Cytokine mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4 hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M, Auriault C, Capron M, Ameisen JC, Pancre V, Torpier G, et al. IgE-dependent platelet cytotoxicity against helminths. Adv Exp Med Biol. 1985;184:23–33. doi: 10.1007/978-1-4684-8326-0_2. [DOI] [PubMed] [Google Scholar]
- Joseph M, Auriault C, Capron A, Vorng H, Viens P. A new function for platelets: IgE-dependent killing of schistosomes. Nature. 1983;303:810–812. doi: 10.1038/303810a0. [DOI] [PubMed] [Google Scholar]
- Joseph M, Capron A, Ameisen JC, Capron M, Vorng H, Pancre V, et al. The receptor for IgE on blood platelets. Eur J Immunol. 1986;16:306–312. doi: 10.1002/eji.1830160318. [DOI] [PubMed] [Google Scholar]
- Joseph M, Gounni AS, Kusnierz JP, Vorng H, Sarfati M, Kinet JP, et al. Expression and functions of the high-affinity IgE receptor on human platelets and megakaryocyte precursors. Eur J Immunol. 1997;27:2212–2218. doi: 10.1002/eji.1830270914. [DOI] [PubMed] [Google Scholar]
- Joseph M, Thorel T, Tsicopoulos A, Tonnel AB, Capron A. Nedocromil sodium inhibition of IgE-mediated activation of human mononuclear phagocytes and platelets from asthmatics. Drugs. 1989;37 Suppl 1:32–36. doi: 10.2165/00003495-198900371-00008. [DOI] [PubMed] [Google Scholar]
- Joseph M, Tsicopoulos A, Tonnel AB, Capron A. Modulation by nedocromil sodium of immunologic and nonimmunologic activation of monocytes, macrophages, and platelets. J Allergy Clin Immunol. 1993;92:165–170. doi: 10.1016/0091-6749(93)90100-t. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Harrison P, Mackie IJ, Isenberg DA, Machin SJ. Increased circulating platelet–leukocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erthematosus and rheumatoid arthritis. Br J Haematol. 2001;115:451–459. doi: 10.1046/j.1365-2141.2001.03101.x. [DOI] [PubMed] [Google Scholar]
- Kaila N, Janz K, Debernardo S, Bedard PW, Camphausen RT, Tam S, et al. Synthesis and biological evaluation of quinoline salicylic acids as P-selectin antagonists. J Med Chem. 2007;50:21–39. doi: 10.1021/jm0602256. [DOI] [PubMed] [Google Scholar]
- Kaila N, Thomas BE. Design and synthesis of sialyl lewisx mimics as E-selectin and P-selectin inhibitors. Med Res Rev. 2002;22:566–601. doi: 10.1002/med.10018. [DOI] [PubMed] [Google Scholar]
- Kasran A, Boon L, Wortel CH, Van Hogezand RA, Schreiber S, Goldin E, et al. Safety and tolerability of antagonist anti-human CD40 Mab ch5D12 in patients with moderate to severe Crohn's disease. Aliment Pharmacol Thera. 2005;22:111–122. doi: 10.1111/j.1365-2036.2005.02526.x. [DOI] [PubMed] [Google Scholar]
- Katayama T, Ikeda Y, Handa M, Tamatani T, Sakamoto S, Ito M, et al. Immunoneutralization of glycoprotein Ibalpha attenuates endotoxin-induced interactions of platelets and leukocytes with rat venular endothelium in vivo. Circ Res. 2000;86:1031–1037. doi: 10.1161/01.res.86.10.1031. [DOI] [PubMed] [Google Scholar]
- Kim MK, Brandley BK, Anderson MB, Bockner BS. Antagonism of selectin-dependent adhesion of human eosinophils and neutrophils by glycomimetics and oligosaccharide compounds. Am J Respir Cell Mol Biol. 1998;19:836–841. doi: 10.1165/ajrcmb.19.5.3032. [DOI] [PubMed] [Google Scholar]
- Kissel K, Berber S, Nockher A, Santoso S, Bein G, Hackstein H. Human platelets target dendritic cell differentiation and production of proinflammatory cytokines. Transfusion. 2006;46:818–827. doi: 10.1111/j.1537-2995.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- Kitano M, Hla T, Sekiguchi M, Kawahito Y, Yoshimura R, Miyazawa K, et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 siganlling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 2006;54:742–753. doi: 10.1002/art.21668. [DOI] [PubMed] [Google Scholar]
- Klinkhardt U, Graff J, Harder S. Clopidogrel, but not abciximab, reduces platelet leukocyte conjugates and P-selectin expression in a human ex vivoin vitro model. Clin Pharmacol Ther. 2002;71:176–185. doi: 10.1067/mcp.2002.122018. [DOI] [PubMed] [Google Scholar]
- Klouche M, Klinger MH, Kuhnel W, Wilhelm D. Endocytosis, storage, and release of IgE by human platelets: differences in patients with type I allergy and nonatopic subjects. J Allergy Clin Immunol. 1997;100:235–241. doi: 10.1016/s0091-6749(97)70230-6. [DOI] [PubMed] [Google Scholar]
- Konstantopoulos K, Neelamegham S, Burns AR, Hentzen E, Kansas GS, Snapp KR, et al. Venous levels of shear support neutrophil-platelet adhesion and neutrophil aggregation in blood via P-selectin and beta2-integrin. Circulation. 1998;98:873–882. doi: 10.1161/01.cir.98.9.873. [DOI] [PubMed] [Google Scholar]
- Kowal K, Pampuch A, Kowal-Bielecka O, Dubuske LM, Bodzenta-Lukaszyk A. Platelet activation in allergic asthma patients during allergen challenge with Dermatophagoides pteronyssinus. Clin Exp Allergy. 2006;36:426–432. doi: 10.1111/j.1365-2222.2006.02446.x. [DOI] [PubMed] [Google Scholar]
- Kowal K, Pampuch A, Kowal-Bielecka O, Iacoviello L, Bodzenta-Lukaszyk A. Soluble CD40 ligand in asthma patients during allergen challenge. J Thromb Haemost. 2006a;4:2718–2720. doi: 10.1111/j.1538-7836.2006.02206.x. [DOI] [PubMed] [Google Scholar]
- Kowalska MA, Ratajczak MZ, Majka M, Jin J, Kunapuli S, Brass L, et al. Stromal cell-derived factor-1 and macrophage-derived chemokine: 2 chemokines that activate platelets. Blood. 2000;96:50–57. [PubMed] [Google Scholar]
- Kuijper PH, Gallardo Tores HI, Lammers JW, Sixma JJ, Koenderman L, Zwaginga JJ. Platelet associated fibrinogen and ICAM-2 induced firm adhesion of neutrophils under flow conditions. Thromb Haemost. 1998;80:443–448. [PubMed] [Google Scholar]
- Kunapuli SP. Multiple P2 receptor subtypes on platelets: a new interpretation of their function. Trends Pharmacol Sci. 1998;19:391–394. doi: 10.1016/s0165-6147(98)01248-6. [DOI] [PubMed] [Google Scholar]
- Lechin F, Van Der DB, Orozco B, Jara H, Rada I, Lechin ME, et al. The serotonin uptake-enhancing drug tianeptine suppresses asthmatic symptoms in children: a double-blind, crossover, placebo-controlled study. J Clin Pharmacol. 1998;38:918–925. doi: 10.1002/j.1552-4604.1998.tb04387.x. [DOI] [PubMed] [Google Scholar]
- Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH-2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmberg J, Beck J, Baethmann A, Uhl E. Effect of P-selectin inhibition on leukocyte endothelium interaction and survival after global cerebral ischaemia. J Neurol. 2006;253:357–363. doi: 10.1007/s00415-005-0996-4. [DOI] [PubMed] [Google Scholar]
- Lellouch-Tubiana A, Lefort J, Simon MT, Pfister A, Vargaftig BB. Eosinophil recruitment into guinea pig lungs after PAF-acether and allergen administration. Modulation by prostacyclin, platelet depletion, and selective antagonists. Am Rev Respir Dis. 1988;137:948–954. doi: 10.1164/ajrccm/137.4.948. [DOI] [PubMed] [Google Scholar]
- Leon C, Alex M, Klocke A, Morgenstern E, Moosbauer C, Eckly A, et al. Platelet ADP receptors contribute to the initiation of intravascular coagulation. Blood. 2004;103:594–600. doi: 10.1182/blood-2003-05-1385. [DOI] [PubMed] [Google Scholar]
- Leon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y(1) receptor null mice. J Clin Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon C, Ravanat C, Freund M, Cazenave JP, Gachet C. Differential involvement of the P2Y1 and P2Y12 receptors in platelet procoagulant activity. Arterioscler Thromb Vasc Biol. 2003;23:1941–1947. doi: 10.1161/01.ATV.0000092127.16125.E6. [DOI] [PubMed] [Google Scholar]
- Li N, Hu H, Hjemdahl P. Aspirin treatment does not attenuate platelet or leukocyte activation as monitored by whole blood flow cytometry. Thromb Res. 2003;111:165–170. doi: 10.1016/j.thromres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Li Z, Rumbaut RE, Burns AR, Smith CW. Platelet response to corneal abrasion is necessary for acute inflammation and efficient re-epithelialization. Invest Ophthal Vis Sci. 2006;47:4794–4802. doi: 10.1167/iovs.06-0381. [DOI] [PubMed] [Google Scholar]
- Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61 hck and p58c-fgr results in defective adhesion dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccia CA, Gallagher JS, Ataman G, Glueck HI, Brooks SM, Bernstein IL. Platelet thrombopathy in asthmatic patients with elevated immunoglobulin E. J Allergy Clin Immunol. 1977;59:101–108. doi: 10.1016/0091-6749(77)90210-x. [DOI] [PubMed] [Google Scholar]
- Martin TR, Galli SJ, Katona IM, Drazen JM. Role of mast cells in anaphylaxis. Evidence for the importance of mast cells in the cardiopulmonary alterations and death induced by anti-IgE in mice. J Clin Invest. 1989;83:1375–1383. doi: 10.1172/JCI114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TR, Takeishi T, Katz HR, Austen KF, Drazen JM, Galli SJ. Mast cell activation enhances airway responsiveness to methacholine in the mouse. J Clin Invest. 1993;91:1176–1182. doi: 10.1172/JCI116277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, et al. Platelets secrete stromal cell-derived factor 1α and recruit bone marrow derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;205:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- Mazzucato M, Cozzi MR, Pradella P, Ruggeri ZM, De Marco L. Distinct roles of ADP receptors in von Willebrand factor-mediated platelet signalling and activation under high flow. Blood. 2004;104:322–327. doi: 10.1182/blood-2004-03-1145. [DOI] [PubMed] [Google Scholar]
- Mehlhop PD, Van De Rijn M, Brewer JP, Kisselgof AB, Geha RS, Oettgen HC, et al. CD40L, but not CD40, is required for allergen-induced bronchial hyperresponsiveness in mice. Am J Respir Cell Mol Biol. 2000;23:646–651. doi: 10.1165/ajrcmb.23.5.3954. [DOI] [PubMed] [Google Scholar]
- Metzger WJ, Sjoerdsma K, Richerson HB, Moseley P, Zavala D, Monick M. Platelets in bronchoalveolar lavage from asthmatic patients and allergic rabbits with allergen-induced late phase responses. Agents Actions Suppl. 1987;21:151–159. doi: 10.1007/978-3-0348-7451-9_13. [DOI] [PubMed] [Google Scholar]
- Molenaar TJ, Appeldoorn CC, De Haas SA, Michon IN, Bonnefoy A, Hoylaerts MF, et al. Specific inhibition of P-selectin-mediated cell adhesion by phage display-derived peptide antagonists. Blood. 2002;10:3570–3577. doi: 10.1182/blood-2002-02-0641. [DOI] [PubMed] [Google Scholar]
- Momi S, Perito S, Mezzasoma AM, Bistoni F, Gresele P. Involvement of platelets in experimental mouse trypanosomiasis: evidence of mouse platelet cytotoxicity against Trypanosoma equiperdum. Exp Parasitol. 2000;95:136–143. doi: 10.1006/expr.2000.4517. [DOI] [PubMed] [Google Scholar]
- Morowitz DA, Allen LW, Kirsner JB. Thrombocytosis in chronic inflammatory bowel disease. Ann Intern Med. 1968;68:1013–1021. doi: 10.7326/0003-4819-68-5-1013. [DOI] [PubMed] [Google Scholar]
- Nasdala I, Wolburg-Buchholz K, Wolburg H. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J Biol Chem. 2002;277:16294–16303. doi: 10.1074/jbc.M111999200. [DOI] [PubMed] [Google Scholar]
- Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, et al. Induction of cytokine expression in leukocytes by binding of thrombin stimulated platelets. Circulation. 1997;95:2387–2394. doi: 10.1161/01.cir.95.10.2387. [DOI] [PubMed] [Google Scholar]
- Nofer JR, Bot M, Brodde M, Taylor PJ, Salm P, Brinkmann V, et al. FTY720, a synthetic sphingosine 1 phosphate analogue, inhibits development of atherosclerosis in low-density lipoprotein receptor deficient mice. Circulation. 2007;115:501–508. doi: 10.1161/CIRCULATIONAHA.106.641407. [DOI] [PubMed] [Google Scholar]
- Nylander S, Mattsson C, Ramstrom S, Lindahl TL. The relative importance of the ADP receptors, P2Y12 and P2Y1, in thrombin-induced platelet activation. Thromb Res. 2003;111:65–73. doi: 10.1016/j.thromres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Okona-Mensah KB, Shittu E, Page C, Costello J, Kilfeather SA. Inhibition of serum and transforming growth factor beta (TGF-beta1)-induced DNA synthesis in confluent airway smooth muscle by heparin. Br J Pharmacol. 1998;125:599–606. doi: 10.1038/sj.bjp.0702046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai Y, Suzuki J, Nishiwaki Y, Gotoh R, Berens K, Dixon R, et al. Blockade of cell adhesion by small molecule selectin antagonist attenuates myocardial ischaemia/reperfusion injury. Eur J Pharmacol. 2003;481:217–225. doi: 10.1016/j.ejphar.2003.09.040. [DOI] [PubMed] [Google Scholar]
- Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, et al. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest. 1996;97:1398–1408. doi: 10.1172/JCI118560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann G, Weber KS, Zernecke A, Schroeder A, Weber C. JAM-1 is a ligand of the beta-2 integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- Ott I, Neumann FJ, Gawaz M, Schmitt M, Schomig A. Increased neutrophil-platelet adhesion in patients with unstable angina. Circulation. 1996;94:1239–1246. doi: 10.1161/01.cir.94.6.1239. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, et al. Cutting edge: combined treatment of TNF-α and IFNγ causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163:553–557. [PubMed] [Google Scholar]
- Palma-Carlos AG, Palma-Carlos ML, Santos MC, De Sousa JR. Platelet aggregation in allergic reactions. Int Arch Allergy Appl Immunol. 1991;94:251–253. doi: 10.1159/000235374. [DOI] [PubMed] [Google Scholar]
- Pareti FI, Capitanio A, Mannucci L, Ponticelli C, Mannucci PM. Acquired dysfunction due to the circulation of ‘exhausted' platelets. Am J Med. 1980;69:235–240. doi: 10.1016/0002-9343(80)90383-6. [DOI] [PubMed] [Google Scholar]
- Pitchford SC, Page CP.Platelets and allergic diseases Platelets in Thrombotic and Non-Thrombotic Disorders 2002Cambridge University Press: Cambridge, UK; 852–868.In: Gresele, P, Page C, Fuster V, Vermylen J (eds). [Google Scholar]
- Pitchford S, Momi S, Casali L, Gresele P.Platelets migrate in response to allergen in vivo and in vitro: role of IgE Pathophysiol Haemost Thromb 2004b33Suppl 2OC013 p24 [Google Scholar]
- Pitchford SC, Bajwa M, Moffatt JD, Page CP. Platelet co-localisation to airway dendritic cells after allergen sensitization in mice. Proc Am Thorac Soc. 2006;3:A341. [Google Scholar]
- Pitchford SC, Momi S, Giannini S, Casali L, Spina D, Page CP, et al. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005;105:2074–2081. doi: 10.1182/blood-2004-06-2282. [DOI] [PubMed] [Google Scholar]
- Pitchford SC, Page CP. MRS2179, a P2Y1 antagonist suppresses leukocyte recruitment in a murine model of allergic inflammation. Proc Am Thorac Soc. 2006;3:A340. [Google Scholar]
- Pitchford SC, Riffo-Vasquez Y, Sousa A, Momi S, Gresele P, Spina D, et al. Platelets are necessary for airway wall remodelling in a murine model of chronic allergic inflammation. Blood. 2004a;103:639–647. doi: 10.1182/blood-2003-05-1707. [DOI] [PubMed] [Google Scholar]
- Pitchford SC, Yano H, Lever R, Riffo-Vasquez Y, Ciferri S, Giannini S, et al. Platelets are essential for leukocyte recruitment in allergic inflammation. J Allergy Clin Immunol. 2003;112:109–118. doi: 10.1067/mai.2003.1514. [DOI] [PubMed] [Google Scholar]
- Polgar J, Chung S-H, Reed GL. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100:1081–1083. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- Poubelle PE, Borgeat P.Platelet–cell interactions in inflammation Platelets in Thrombotic and Non-Thrombotic Disorders 2002Cambridge University Press: Cambridge, UK; 869–884.In: Gresele P, Page C, Fuster V, Vermylen J (eds). [Google Scholar]
- Preobrazhenskaya ME, Berman AE, Mikhalov VI, Ushakova NA, Mazurov AV, Semenov AV, et al. Fucoidan inhibits leukocyte recruitment in a model of peritoneal inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochem Mol Biol Int. 1997;43:443–451. doi: 10.1080/15216549700204231. [DOI] [PubMed] [Google Scholar]
- Quinn MJ, Plow EF, Topol EJ. Platelet glycoprotein IIb/IIIa inhibitors. Recognition of a two-edged sword? Circulation. 2002;106:379–385. doi: 10.1161/01.cir.0000019581.22812.b2. [DOI] [PubMed] [Google Scholar]
- Ray DM, Spinelli SL, O'brien JJ, Blumberg N, Phipps RP. Platelets as a novel target for PPARgamma ligands: implications for inflammation, diabetes, and cardiovascular disease. Biodrugs. 2006;20:2331–2341. doi: 10.2165/00063030-200620040-00004. [DOI] [PubMed] [Google Scholar]
- Rendu R, Brohard-Bohn B.Platelets organelles Platelets in Thrombotic and Non-Thrombotic Disorders 2002Cambridge University Press: Cambridge, UK; 104–112.In: Gresele P, Page C, Fuster V, Vermylen J (eds). [Google Scholar]
- Roviezzo F, Del Galdo F, Abbate G, Bucci M, D'agostino B, Antunes E, et al. Human eosinophil chemotaxis and selective in vivo recruitment by sphingosine 1-phosphate. Proc Natl Acad Sci USA. 2004;101:11170–11175. doi: 10.1073/pnas.0401439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atheroschlerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- Santoso S, Sachs UJ, Kroll H. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma J, Laan CA, Alam S, Jha A, Fox KAA, Dransfield I. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002;105:2166–2171. doi: 10.1161/01.cir.0000015700.27754.6f. [DOI] [PubMed] [Google Scholar]
- Schafer A, Schulz C, Eigenthaler M, Fraccarollo D, Kobsar A, Gawaz M, et al. Novel role of the membrane-bound chemokine fractalkine in platelet activation and adhesion. Blood. 2004;103:407–412. doi: 10.1182/blood-2002-10-3260. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in astthma. J Immunol. 2003;170:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Schmitt-Sody M, Klose A, Gottschalk O, Metz P, Gebhard H, Zysk S, et al. Platelet–endothelial cell interactions in murine antigen-induced arthritis. Rheumatol. 2005;44:885–889. doi: 10.1093/rheumatology/keh638. [DOI] [PubMed] [Google Scholar]
- Schober A, Manka D, Von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, et al. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- Sekiya T, Miyamasu M, Imanishi M, Yamada H, Nakajima T, Yamaguchi M, et al. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol. 2000;165:2205–2213. doi: 10.4049/jimmunol.165.4.2205. [DOI] [PubMed] [Google Scholar]
- Shi C, Zhang X, Chen Z, Robinson MK, Simon DL. Leukocyte integrin Mac-1 recruits toll/interleukin-1 receptor superfamily signaling intermediates to modulate NF-kappaB activity. Circ Res. 2001;89:859–865. doi: 10.1161/hh2201.099166. [DOI] [PubMed] [Google Scholar]
- Shi H, Yokoyama A, Kohno N, Hirasawa Y, Kondo K, Sakai K, et al. Effect of thromboxane A2 inhibitors on allergic pulmonary inflammation in mice. Eur Respir J. 1998;11:624–629. [PubMed] [Google Scholar]
- Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LD, et al. Platelet glycoprotein Ibα is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater D, Martin J, Trowbridge A. The platelet in asthma. Lancet. 1985;1:110. doi: 10.1016/s0140-6736(85)92002-1. [DOI] [PubMed] [Google Scholar]
- Storey RF, Judge HM, Wilcox RG, Heptinstall S. Inhibition of ADP-induced P-selectin expression and platelet–leukocyte conjugate formation by clopidogrel and the P2Y12 receptor antagonist AR-C69931MX but not aspirin. Thromb Haemost. 2002a;88:488–494. [PubMed] [Google Scholar]
- Storey RF, Sanderson HM, White AE, May JA, Cameron KE, Heptinstall S. The central role of the P(2 T) receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol. 2000;110:925–934. doi: 10.1046/j.1365-2141.2000.02208.x. [DOI] [PubMed] [Google Scholar]
- Storey RF, Wilcox RG, Heptinstall S. Comparison of the pharmacodynamic effects of the platelet ADP receptor antagonists clopidogrel and AR-C69931MX in patients with ischaemic heart disease. Platelets. 2002;13:407–413. doi: 10.1080/0953710021000024402. [DOI] [PubMed] [Google Scholar]
- Sumariwalla PF. P-selectin glycoprotein ligand 1 therapy ameliorates established collagen-induced arthritis in DBA/1 mice partly through the suppression of tumour necrosis factor. Clin Exp Immunol. 2004;136:67–75. doi: 10.1111/j.1365-2249.2004.02421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttitanamongkol S, Gear ARL. ADP receptor antagonists inhibit platelet aggregation induced by the chemokines SDF-1, MDC and TARC. FEBS Lett. 2001;490:84–87. doi: 10.1016/s0014-5793(00)02413-3. [DOI] [PubMed] [Google Scholar]
- Tamagawa-Mineoka R, Katch N, Ueda E, Takenaka H, Kita M, Kishimoto S. The role of platelets in leukocyte recruitment in chronic contact hypersensitivity induced by repeated elicitation. Am J Pathol. 2007;170:1–11. doi: 10.2353/ajpath.2007.060881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taytard A, Guenard H, Vuillemin L, Bouvet JL, Vergeret J, Ducassou D, et al. Platelet kinetics in stable asthmatic patients. Effect of corticosteroid therapy. Am Rev Respir Dis. 1985;131:A285. doi: 10.1164/arrd.1986.134.5.983. [DOI] [PubMed] [Google Scholar]
- Theoret JF, Bienvenu JG, Kumar A, Merhi Y. P-selectin antagonism with recombinant p-selectin glycoprotein ligand-1 (rPSGL-Ig) inhibits circulating activated platelet binding to neutrophils induced by damaged arterial surfaces. J Pharmacol Exp Ther. 2001;298:658–664. [PubMed] [Google Scholar]
- Thorel T, Joseph M, Tsicopoulos A, Tonnel AB, Capron A. Inhibition by nedocromil sodium of IgE-mediated activation of human mononuclear phagocytes and platelets in allergy. Int Arch Allergy Appl Immunol. 1988;85:232–237. doi: 10.1159/000234508. [DOI] [PubMed] [Google Scholar]
- Topol EJ, Moliterno DJ, Hermann HC, Powers ER, Grines CL, Cohen DJ, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischaemic events with percutaneous coronary revascularization. N Engl J Med. 2001;344:1888–1894. doi: 10.1056/NEJM200106213442502. [DOI] [PubMed] [Google Scholar]
- Tsicopoulos A, Tonnel AB, Wallaert B, Joseph M, Ameisen JC, Ramon P, et al. Decrease of IgE-dependent platelet activation in hymenoptera hypersensitivity after specific rush desensitization. Clin Exp Immunol. 1988;71:433–438. [PMC free article] [PubMed] [Google Scholar]
- Tunon-De-Lara JM, Rio P, Marthan R, Vuillemin L, Ducassou D, Taytard A. The effect of sodium cromoglycate on platelets: an in vivo and in vitro approach. J Allergy Clin Immunol. 1992;89:994–1000. doi: 10.1016/0091-6749(92)90222-n. [DOI] [PubMed] [Google Scholar]
- Tutluoglu B, Gurel CB, Ozdas SB, Musellim B, Erturan S, Anakkaya AN, et al. Platelet function and fibrinolytic activity in patients with bronchial asthma. Clin Appl Thromb Haemost. 2005;11:77–81. doi: 10.1177/107602960501100109. [DOI] [PubMed] [Google Scholar]
- Ulfman LH, Joosten DPH, Van Aalst CW, Lammers J-WJ, Van De Graaf EA, Koenderman L, et al. Platelets promote eosinophil adhesion of patients with asthma to endothelium under flow conditions. Am J Respir Cell Mol Biol. 2003;28:512–519. doi: 10.1165/rcmb.4806. [DOI] [PubMed] [Google Scholar]
- Van Wersch JWJ, Houben P, Rijken J. Platelet count, platelet function, coagulation activity and fibrinolysis in the acute phase of inflammatory bowel disease. J Clin Chem Clin Biochem. 1990;28:513–517. doi: 10.1515/cclm.1990.28.8.513. [DOI] [PubMed] [Google Scholar]
- Vignola AM, Kips J, Bousquet J. Tissue remodelling as a feature of asthma. J Allergy Clin Immunol. 2000;105:1041–1053. doi: 10.1067/mai.2000.107195. [DOI] [PubMed] [Google Scholar]
- Vilaseca J, Salas A, Guarner F. Participation of thromboxane and other eicosanoid synthesis in the course of experimental inflammatory colitis. Gastroenterology. 1990;98:269–277. doi: 10.1016/0016-5085(90)90814-h. [DOI] [PubMed] [Google Scholar]
- Von Hundelshausen P, Koenen RR, Sack M, Mause SF, Adriaens W, Proudfoot AE, et al. Heterophilic interactions of platelet factor 4 and RANTES promote monocyte arrest on endothelium. Blood. 2005;105:924–930. doi: 10.1182/blood-2004-06-2475. [DOI] [PubMed] [Google Scholar]
- Vowinkel T, Anthoni C, Wood KC, Stokes KY, Russell J, Gray L, et al. CD40–CD40 ligand mediates the recruitment of leukocytes and platelets in the inflamed murine colon. Gastroenterology. 2007;132:955–965. doi: 10.1053/j.gastro.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Vrij AA, Rijken J, Van Wersh JW, Stockbrugger RW. Platelet factor 4 and beta-thromboglobulin in inflammatory bowel disease and giant cell arteritis. Eur J Clin Invest. 2000;30:188–194. doi: 10.1046/j.1365-2362.2000.00616.x. [DOI] [PubMed] [Google Scholar]
- Waehre T, Damas JK, Pederson TM, Gullestad L, Yndestad A, Andreassen AK, et al. Clopidogrel increases expression of chemokines in peripheral blood mononuclear cells in patients with coronary artery disease: results of a double-blind placebo-controlled study. J Thromb Haemost. 2006;4:2140–2147. doi: 10.1111/j.1538-7836.2006.02131.x. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhou X, Zhou Z, Tarakji K, Qin JX, Sitges M, et al. Recombinant soluble P-selectin glycoprotein ligand-Ig (rPSGL-Ig) attenuates infarct size and myeloperoxidase activity in a canine model of ischaemia–reperfusion. Thromb Haemost. 2002;88:149–154. [PubMed] [Google Scholar]
- Wang K, Zhou Z, Zhou X, Tarakji K, Topol EJ, Lincoff AM. Prevention of intimal hyperplasia with recombinant soluble P-selectin glycoprotein ligand immunoglobulin in the porcine coronary artery balloon injury model. J Am Coll Cardiol. 2001;38:577–582. doi: 10.1016/s0735-1097(01)01347-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, et al. Leukocyte engagement of platelet glycoprotein 1bα via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- Webberley MJ, Hart MT, Melikian V. Thromboembolism in inflammatory bowel disease: role of platelets. Gut. 1993;34:247–251. doi: 10.1136/gut.34.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox JN, Nelken NA, Coughlin SR, Gordon D, Schall TJ. Local expression of inflammatory cytokines in human atherosclerotic plaques. J Alheroscler Thromb. 1994;1 Suppl 1:S10–S13. doi: 10.5551/jat1994.1.supplemment1_s10. [DOI] [PubMed] [Google Scholar]
- Yamada T, Takahashi Y, Ishizaki M, Musoh K, Ohashi T, Tanaka H, et al. Effects of RS-601, a novel leukotriene D(4)/thromboxane A(2) dual receptor antagonist, on asthmatic responses in guinea pigs. Pharmacology. 2003;69:51–58. doi: 10.1159/000071267. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, Sano T, et al. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]
- Yeo EL, Sheppard JA, Feuerstein IA. Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model) Blood. 1994;83:2498–2507. [PubMed] [Google Scholar]
- Yoshida A, Ohba M, Wu X, Sasano T, Nakamura M, Endo Y. Accumulation of platelets in the lung and liver and their degranulation following antigen-challenge in sensitized mice. Br J Pharmacol. 2002;137:146–152. doi: 10.1038/sj.bjp.0704852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimi Y, Fujimura M, Myou S, Tachibana H, Hirose T. Effect of thromboxane A(2) (TXA(2)) synthase inhibitor and TXA(2) receptor antagonist alone and in combination on antigen induced bronchoconstriction in guinea pigs. Prostaglandins. 2001;65:1–9. doi: 10.1016/s0090-6980(01)00099-5. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Schober A, Bot I, Von Hundelshaussen P, Liehn EA, Mopps B, et al. SDF-1α/CXCR4 axis is instrumental in neo-intimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- Zhao L, Bath PMW, May J, Losche W, Heptinstall S. P-selectin, tissue factor and CD40 ligand expression on platelet–leukocyte conjugates in the presence of a GPIIb/IIIa antagonist. Platelets. 2003;14:473–480. doi: 10.1080/09537100310001638562. [DOI] [PubMed] [Google Scholar]
- Zhang X, Selli ML, Baglioni S, Hauri A, Chiari R, Dottorini M, et al. Platelets from asthmatic patients migrate in vitro in response to allergen stimulation. Thromb Haemost. 1993;69:1356. [Google Scholar]