Abstract
Background and purpose:
Illegal ‘ecstasy' tablets frequently contain 3,4-methylenedioxymethamphetamine (MDMA)-like compounds of unknown pharmacological activity. Since monoamine transporters are one of the primary targets of MDMA action in the brain, a number of MDMA analogues have been tested for their ability to inhibit [3H]noradrenaline uptake into rat PC12 cells expressing the noradrenaline transporter (NET) and [3H]5-HT uptake into HEK293 cells stably transfected with the 5-HT transporter (SERT).
Experimental approach:
Concentration–response curves for the following compounds at both NET and SERT were determined under saturating substrate conditions: 4-hydroxy-3-methoxyamphetamine (HMA), 4-hydroxy-3-methoxymethamphetamine (HMMA), 3,4-methylenedioxy-N-hydroxyamphetamine (MDOH), 2,5-dimethoxy-4-bromophenylethylamine (2CB), 3,4-dimethoxymethamphetamine (DMMA), 3,4-methylenedioxyphenyl-2-butanamine (BDB), 3,4-methylenedioxyphenyl-N-methyl-2-butanamine (MBDB) and 2,3-methylenedioxymethamphetamine (2,3-MDMA).
Key results:
2,3-MDMA was significantly less potent than MDMA at SERT, but equipotent with MDMA at NET. 2CB and BDB were both significantly less potent than MDMA at NET, but equipotent with MDMA at SERT. MBDB, DMMA, MDOH and the MDMA metabolites HMA and HMMA, were all significantly less potent than MDMA at both NET and SERT.
Conclusions and implications:
This study provides an important insight into the structural requirements of MDMA analogue affinity at both NET and SERT. It is anticipated that these results will facilitate understanding of the likely pharmacological actions of structural analogues of MDMA.
Keywords: noradrenaline, 5-hydroxytryptamine, transport, MDMA, PC12 cells, HEK cells
Introduction
3,4-Methylenedioxymethamphetamine (MDMA; ‘ecstasy') is a highly popular but illegal (schedule 1/class A) recreational psychoactive drug that is known for its empathogenic, euphoric and stimulant effects. So great is the popularity of this drug that it is now regarded as the second most commonly abused controlled substance in Europe (outside of cannabis), and it is estimated that approximately 25 million people worldwide consume amphetamine-type stimulants such as MDMA every year (Morton, 2005; UNODC, 2006).
In spite of the increasing prevalence of MDMA abuse (Schifano et al., 2006), there is considerable debate about the mode of action of the drug (Lyvers, 2006). One of the main limitations in assessing the physiological effects of illegal MDMA drug abuse in humans is the unknown purity of the ingested substance. MDMA tablets are known to be regularly spliced with a number of alternative compounds, ranging in lethality from caffeine to ketamine (Becker et al., 2003; Refstad, 2003; Cheng et al., 2006; Tanner-Smith, 2006; Teng et al., 2006). Furthermore, both the number and combination of other amphetamine-like compounds present in ‘ecstasy' tablets is continually increasing (Teng et al., 2006). According to a recent estimate, only 39% of all tablets marketed as ‘ecstasy' consist of pure MDMA, 46% contain substances other than MDMA and 15% are mixtures of MDMA and other substances (Tanner-Smith, 2006). It is therefore imperative that information regarding the biological activity of these MDMA-like compounds be made available as soon as possible.
With these considerations in mind, we have adopted an approach of rational synthesis and constructed a range of structural analogues of MDMA (Figure 1). It is anticipated that, through the analysis of these compounds, we will increase our understanding of their pharmacological activity before they become commonplace on the illegal drug market. Two of these compounds, 2,5-dimethoxy-4-bromophenylethylamine (2CB) and 3,4-methylenedioxyphenyl-N-methyl-2-butanamine (MBDB), have already been identified as contaminants in MDMA tablets, whereas 2,3-methylenedioxymethamphetamine (2,3-MDMA) has not been detected in either tablets or samples taken from drug users (Giroud et al., 1998; de Boer et al., 1999; Simonsen and Kaa, 2001; Vaiva et al., 2001a; Tanner-Smith, 2006). We have also synthesized the primary human MDMA metabolites, 4-hydroxy-3-methoxymethamphetamine (HMMA) and 4-hydroxy-3-methoxyamphetamine (HMA), for investigation in this study (de la Torre et al., 2004; Monks et al., 2004; Escobedo et al., 2005; Jones et al., 2005; Milhazes et al., 2006).
Figure 1.
Structures of 3,4-methylenedioxymethamphetamine (MDMA) and its analogues.
Neuronal monoamine transport is believed to be the primary biological target through which MDMA mediates both its pharmacological and toxicological effects. MDMA is a known substrate and an inhibitor of the 5-HT transporter (SERT), noradrenaline transporter (NET) and dopamine transporter (DAT; Verrico et al., 2005). Once inside the cell, MDMA causes the release of endogenous neurotransmitter back into the extracellular space, again via the monoamine transporters, resulting in a substantial rise in the level of extracellular neurotransmitter (Green et al., 2003; Pifl et al., 2005). Although amphetamine-induced neurotransmitter release is thought to be key in the manifestation of the neurotoxic effects of MDMA (Rothman et al., 2001; Green et al., 2003), the exact molecular mechanism by which this release occurs has yet to be determined (Siedel et al., 2005; Sulzer et al., 2005). In light of the central role of monoamine transporters in mediating the pharmacological actions of MDMA, it was decided to investigate the effects of the MDMA analogues on NET and SERT transport in vitro, using two separate mammalian cell lines. Although the significance of DAT in mediating both the physiological and potentially toxicological effects of MDMA should not be underestimated (Colado et al., 2004; Breier et al., 2006; Rothman and Baumann, 2006), MDMA has typically demonstrated a greater inhibitory potency at NET and SERT compared to DAT in vitro (Rothman and Baumann, 2003; Pifl et al., 2005; Verrico et al., 2005). Consequently, it was decided to perform this investigation using NET and SERT. The rat phaeochromocytoma (PC12) cell line was chosen as the experimental model for the noradrenergic system (Greene and Tischler, 1976), whereas the T-REx SERT HEK 293 cell line was selected as the experimental model for the 5-hydroxytryptaminergic system (Tate et al., 2003).
Methods
Synthesis of MDMA analogues as their (±)-HCl salts
3,4-Methylenedioxymethamphetamine and the following analogues were synthesized by the general procedure A, which employs the methodology described by Braun et al. (1980) and Nichols et al. (1986): 2,3-MDMA, DMMA, BDB, MBDB, MDOH.
General procedure A
The amine (as ammonium acetate, methylamine (2.0 M in MeOH), ethylamine, propylamine or hydroxylamine; 26.4 mmol) was added to a solution of the required ketone (3,4-methylenedioxyphenylacetone, 2,3-methylenedioxyphenylacetone, 3,4-dimethoxyphenylacetone, 3,4-methylenedioxyphenylbutan-2-one or 4-methoxyphenylacetone; 22 mmol) in methanol (10 ml) at room temperature. After stirring for 30 min, sodium cyanoborohydride (1.66 g, 26.4 mmol) was added and the suspension was stirred for 3 days at room temperature under an atmosphere of nitrogen. During this time, the pH of the reaction mixture was maintained between 4 and 6 by careful addition of hydrochloric acid mixed with methanol (1:1 mixture). The solution was then adjusted to pH 2 with concentrated hydrochloric acid, stirred for 1 h, diluted with water (80 ml) and then extracted with dichloromethane (2 × 40 ml) and the combined organic phases discarded. The aqueous phase was separated and washed with further dichloromethane (3 × 80 ml) and again the organic phases were discarded. The aqueous layer was basified with 30% NaOH and then extracted with dichloromethane (3 × 80 ml), the organic fractions were combined, dried over magnesium sulphate and concentrated in vacuo. The oil was dissolved in isopropanol (5 ml) and precipitation of the product occurred after adding a few drops of concentrated hydrochloric acid followed by diethyl ether addition. Filtration and drying afforded the required compounds as white/grey solids.
2,3-MDMA
(45% yield) 1H NMR (300 MHz, DMSO-d6): δ 1.08 (d, 3H, J=5.7 Hz), 2.51 (s, 3H), 2.61–2.69 (m, 1H J1), 3.03 (d, J=11.1), 5.96 (s, 2H), 6.74 (m, 3H), 9.08 (bs, 2H); Vmax (KBr) 3568–3340, 3260–2829, 1361, 1257, 1045, 948, 810 cm−1; Anal. calcd. for C11H16ClNO2: C, 57.52; H, 7.02; N, 6.10: found C, 57.89; H, 6.97; N, 6.20.
DMMA
(58% yield) 1H NMR (300 MHz, DMSO-d6): δ 1.06 (d, 3H, J=6.6 Hz), 2.47–2.59 (m, 5H), 3.08 (dd, 1H, J=9.1, 3.9 Hz), 3.69 (s, 3H), 3.72 (s, 3H), 6.72 (dd, 1H, J=6.4, 1.8 Hz), 6.84–6.88 (m, 2H), 9.08 (bs, 1H); Vmax (KBr) 3625–3223, 2962, 2834, 2455, 1591, 1577, 1529, 1458, 1272, 1238, 1161, 1142, 1035, 804 cm−1; Anal. calcd. for C12H20ClNO2: C, 58.64; H, 8.20; N, 5.72: found C, 58.21; H, 8.11; N, 5.56.
BDB
(46% yield) 1H NMR (300 MHz, DMSO-d6): δ 0.88 (t, 3H, J=7.5 Hz), 1.42–1.53 (m, 2H), 2.41 (m, 1H), 2.62–2.78 (m, 1H), 2.79–2.86 (m, 1H), 5.96 (s, 2H), 6.62–6.84 (m, 3H), 8.05 (bs, 2H); Vmax (KBr) 3629–3262, 2960, 2789, 1589, 1243, 1159, 1150, 1140, 795 cm−1; Anal. calcd. for C11H15ClNO2: C, 57.77; H, 6.61; N, 6.12: found C, 58.08; H, 6.78; N, 6.09.
MBDB
(58% yield) 1H NMR (300 MHz, DMSO-d6): δ 0.86 (t, 3H, J=7.5 Hz), 1.50–1.60 (m, 2H), ∼2.50 (m, 1H), 2.62–2.79 (m, 1H), 2.91–2.96 (m, 1H), 3.98 (s, 2H), 6.01 (s, 2H), 6.71–6.98 (m, 3H), 9.37 (bs, 1H); Vmax (KBr) 3629–3262, 2960, 2789, 1589, 1243, 1159, 1150, 1140, 795 cm−1; Anal. calcd. for C12H17ClNO2: C, 59.38; H, 7.05; N, 5.78: found C, 59.12; H, 6.98; N, 5.83.
MDOH
(76% yield) 1H NMR (300 MHz, DMSO-d6): δ 1.07 (d, 3H, J=6.6 Hz), 2.45–2.47 (m, 1H), 3.05–3.15 (m, 1H), 3.40–3.52 (m, 1H), 5.96 (s, 2H), 6.65–6.84 (m, 3H), 10.86 (bs, 1H), 11.45 (bs, 1H); Vmax (KBr) 3603–3267, 3080, 2900, 1541, 1503, 1488, 1442, 1247, 1033, 927, 805 cm−1; Anal. calcd. for C10H14NO3Cl: C, 51.84; H, 6.09; N, 6.05: found C, 51.67; H, 5.96; N, 5.88.
The MDMA metabolites, HMA and HMMA, were prepared according to the method of Forsling et al. (2002) described in procedure B.
Procedure B
To a solution of methylamine hydrochloride (2.60 g, 38.5 mmol) in methanol (25 ml), 4-hydroxy-3-methoxyphenylacetone (2.00 g, 11.1 mmol) was added and the solution was stirred at room temperature for 3.5 h. After the addition of sodium cyanoborohydride (0.86 g, 13.7 mmol), the white suspension was stirred for 2 days at room temperature under an atmosphere of nitrogen. The pH was maintained between 4 and 6 by careful addition of hydrochloric acid mixed with methanol. The solution was then adjusted to pH 2 with concentrated HCl, stirred for 1 h and then extracted with ethyl acetate (3 × 20 ml) and the organic phase discarded. The aqueous phase was adjusted to pH 10 with the addition of NaOH and saturated with sodium chloride. The solution was again extracted with ethyl acetate (3 × 20 ml), the organic fractions were combined, dried over sodium sulphate and concentrated in vacuo. The oil was dissolved in ethanol (5 ml) and precipitation of the product occurred after adding a few drops of concentrated HCl followed by diethyl ether addition. Filtration and drying afforded HMMA.HCl as a white powder (0.62 g, 24%): 1H NMR (300 MHz, DMSO-d6): δ 1.10 (d, 3H, J=6.6 Hz), 2.51 (m, 4H), 3.00 (dd, 1H J1=4.5 Hz, J2=13.3Hz), 3.35 (m, 1H), 3.77 (s, 3H), 6.62 (dd, 1H, J1=7.9 Hz, J2=1.9 Hz), 6.73 (d, 1H, J=7.9 Hz), 6.81 (d, 1H, J=1.9 Hz), 8.7 (bs, 2H), 8.89 (bs, 1H).
Similarly, use of ammonium acetate (2.24 g, 29.1 mmol), 4-hydroxy-3-methoxyphenylacetone (1.50 g, 8.3 mmol) and sodium cyanoborohydride (0.86 g, 13.7 mmol) afforded HMA.HCl as a white powder (0.65 g, 36%): 1H NMR (300 MHz, DMSO-d6): δ 1.11 (d, 3H, J=6.6 Hz), 2.50 (m, 41H), 2.85 (dd, 1H J1=5.7 Hz, J2=13.5 Hz), 3.43 (m, 1H), 3.77 (s, 3H), 6.61 (dd, 1H, J1=7.9 Hz, J2=1.9 Hz), 6.73 (d, 1H, J=7.9 Hz), 6.79 (d, 1H, J=1.9 Hz), 7.85 (bs, 3H), 8.87 (bs, 1H).
2,5-Dimethoxy-4-bromophenylethylamine was prepared in three steps from 2,5-dimethoxybenzaldehyde according to the methods of Varma and Kabalka (1985) and Anderson et al. (1987). Nitroethane (4.51 g, 60.17 mmol) was added to a solution of 2,5-dimethoxybenzaldehyde (10.00 g, 60.17 mmol) in methanol (200 ml). After stirring for 10 min, the reaction mixture was cooled to 0 °C and sodium hydroxide (4 ml of 10.5 M) was added dropwise over a 30-min period. The reaction was allowed to stir at room temperature for 3H and was then added slowly to an HCl solution maintained at 60 °C. A yellow crystalline material formed, which was filtered and dried to afford (E)-1-(2,5-dimethoxyphenyl)-2-nitroethane (16.00 g, 89% yield) as yellow crystals (m. p. 121–123 °C).
Sodium borohydride (4.29 g, 113.5 mmol) was stirred in tetrahydrofuran (100 ml) and to this boron trifluoride etherate (18.2 ml, 143.4 mmol) was added and the resulting mixture was stirred at room temperature for 15 min. Then, (E)-1-(2,5-dimethoxyphenyl)-2-nitroethane (4.50 g, 21.4 mmol) in tetrahydrofuran (60 ml) was added dropwise over 30 min and the resulting mixture was refluxed for 4 h. After allowing the reaction to cool to room temperature, the reaction was quenched by careful addition of ice water (250 ml) and acidification with concentrated HCl and further heating at 80–85 °C for 2 h. The mixture was again allowed to cool to room temperature and the acidic layer was washed with diethyl ether (2 × 50 ml). The amine product was liberated through the addition of sodium hydroxide (30% solution) and was extracted into diethyl ether (3 × 30 ml). The combined organic extracts were dried over magnesium sulphate and concentrated to afford 2,5-dimethoxyamphetamine (1.20 g, 31% yield) as a pale brown oil.
2,5-Dimethoxyamphetamine (1.10 g, 6.06 mmol) was dissolved in acetic acid (1.5 ml) at room temperature and bromine (1.06 g, 6.6 mmol) dissolved in acetic acid (1.5 ml) was carefully added. After reaction for 5 min, solids were observed to have formed with simultaneous evolution of heat. The reaction mixture was allowed to return to room temperature, the solids were filtered off, washed with diethyl ether to afford 2CB as its HBr salt (2.00 g). This salt was dissolved in water/acetic acid and concentrated HCl was added leading to the immediate formation of the hydrochloride salt of 2CB, which was filtered, washed with diethyl ether and dried to afford 2CB.HCl (1.57 g, 87% yield) as beige crystals (m. p. 237–239 °C), 1H NMR (300 MHz, DMSO-d6): 2.79–2.98 (m, 4H), 3.71–3.81 (m, 6H), 7.0 (s, 1H), 7.16 (s, 1H), 8.15 (bs, 2H); Vmax (KBr) 3577–3330, 3263–2835, 1255, 1209, 1044, 948, 816 cm−1; Anal. calcd. for C10H15ClBrNO2: C, 40.49; H, 5.10; N, 4.73: found C, 40.57; H, 5.25; N, 4.80.
PC12 cell culture
Undifferentiated PC12 cells were a gift from Dr Veronica Campbell (Trinity College, Dublin). The cells were grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 5% fetal bovine serum, 10% heat-inactivated horse serum and 1000 units ml−1 of penicillin/streptomycin on T75 flasks at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The cells were cultured as a monolayer and routinely passaged three times weekly.
T-REx SERT HEK293 cell culture
The T-REx-SERT cells were a gift from Dr Jana Haase, UCD Conway Institute. The original cell line was created and supplied by Dr Chris Tate (University of Cambridge, UK). Full details of the transfection procedure may be found in Tate et al. (2003). Briefly, rat SERT cDNA was used as a template to generate a PCR fragment containing the complete coding sequence but lacking the stop codon. The sequences of the primers used were 5′-CCGCTCGAGCAGGATGGAGACCACACC-3′ and 5′-TTGGTACCACAGCATTCATGCGGAT-3′. The PCR product was digested with XhoI and KpnI and cloned the SalI–KpnI-digested vector pFLAG-CMV-5b (Sigma-Aldrich, Dorset, UK) resulting in plasmid pSERT-FLAG. The complete insert of the plasmid was checked by DNA sequencing. The plasmid was used to transfect an HEK293 cell line (T-REx-293) that expressed the tetracycline repressor protein, Tet, as described (Tate et al., 2003). Expression of SERT was confirmed by western blotting, inhibitor-binding assays on crude membrane preparations, [3H]5-HT uptake assays into whole cells and by confocal microscopy of immunostained cells. Cells were grown in DMEM containing 10% Tet system-approved fetal bovine serum, L-glutamine (2 mM), blasticidin (5 μg ml−1), zeocin (200 μg ml−1) and gentamicin (10 μg ml−1) in T75 flasks in a humidified atmosphere of 5% CO2 and 95% air. The cells were cultured as a monolayer and routinely passaged two–three times weekly. Cells were not induced with tetracycline prior to performing a transport assay, as the basal level of SERT expression was sufficient for these experiments.
[3H]NA transport assay in PC12 cells
Measurements of the rate of [3H]noradrenaline ([3H]NA) uptake into PC12 cells were performed in poly-L-lysine (0.1 mg ml−1)-coated 24-well plates. When 90% confluent, the cells were seeded at a density of 0.25 × 106 cells ml−1 into each well of the 24-well plate. On reaching 80% confluency (typically 1–2 days), the cells were used in [3H]NA uptake assays. The growth medium was removed by aspiration and the cells were gently washed with 500 μl of normal HEPES medium containing 25 mM HEPES, 150 mM NaCl, 2 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 1.3 mM CaCl2, 5.6 mM glucose, 100 μM pargyline and 1.4 mM ascorbate, pH 7.4. The transport assay was initiated by the addition of 500 μl HEPES buffer to each well, containing 10 μM [3H]NA (specific activity approximately 0.72 Ci mol−1) and the desired concentration of amphetamine analogue. After a test incubation period of 5 min (initial rate conditions) at 37 °C, [3H]NA uptake was immediately terminated by aspiration of the test solution, followed by a gentle wash of the cell layer with 500 μl ice-cold 1 mM NA in HEPES buffer. This procedure was repeated once. The cells were then solubilized overnight in 500 μl 0.25 M NaOH. An aliquot of 300 μl of solubilized cells was added to 4 ml of scintillation cocktail (Ecoscint A) and the level of radioactivity incorporated into the cells was determined by liquid scintillation spectroscopy. A 200-μl sample of the cell lysate was retained for protein determination by the Bradford method. The results are expressed as pmol [3H]NA taken up per mg protein per min.
A substrate concentration range of 10 nM–10 μM [3H]NA was used for kinetic analysis of transport. Nonspecific [3H]NA uptake was determined in the presence of the potent and selective noradrenaline transport inhibitor, nisoxetine (1 μM; Fuller et al., 1975; Lemberger et al., 1976). Specific transport was calculated by subtraction of the rate of nonspecific uptake from total uptake. Nisoxetine-sensitive [3H]NA uptake in PC12 cells displayed Michaelis–Menten saturation kinetics with a Vmax of 49.3±1.3 pmol per mg protein per min and a Km value of 961±90 nM. Nonspecific transport accounted for approximately 13% of total transport. On the basis of this kinetic assessment, a saturating substrate concentration of 10 μM [3H]NA was chosen at which the inhibitory potency of each of the MDMA analogues was to be assessed. The mean rate of specific [3H]NA transport at this concentration in the absence of any inhibitor was 43.7±5.7 pmol per mg protein per min.
As clearly demonstrated, PC12 cells stably express functional NET and are therefore regarded as a suitable model for catecholaminergic neurons in vitro (Greene and Tischler, 1976). PC12 cells do not possess the molecular machinery necessary for 5-HT uptake as no significant difference was observed between total and nonspecific [3H]5-HT (10 nM–10 μM) transport in PC12 cells (as defined by 100 nM citalopram; n=3, data not shown).
[3H]5-HT transport assay in T-REx SERT cells
[3H]5-HT transport assays were performed in poly-L-lysine (0.1 mg ml−1)-coated 24-well plates When 80% confluent, cells were seeded at a density of 0.25 × 106 cells ml−1 into each well of the 24-well plate and a transport assay was performed once the cells reached 80% confluency (typically 1–2 days). Just prior to the assay, the medium was removed and a 0.4-ml transport buffer (TB; 10 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM KCl, 1.2 mM ascorbate, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose; 37 °C) was added. The assay was started by the addition of [3H]5-HT (final specific activity per well 0.62 Ci mmol−1) and was terminated after 6 min (initial rate conditions) by the aspiration of test solution and immediate washing of the cells with ice-cold TB containing 10 μM paroxetine. This procedure was repeated once. Cells were lysed and both radioactivity and protein content determined as per the [3H]NA transport assay. Nonspecific uptake was defined using the potent and selective inhibitor of 5-HT transport, paroxetine (10 μM; Thomas et al., 1987). Specific transport was calculated by subtraction of the rate of nonspecific uptake from total uptake and the results are expressed as pmol [3H]5-HT taken up per mg protein per min. Nonspecific transport accounted for approximately 7% of total transport.
Basal SERT expression levels were used for all experiments and a substrate concentration range of 10 nM–10 μM [3H]5-HT was used for kinetic analysis of transport. Paroxetine-sensitive [3H]5-HT uptake in T-REx SERT HEK 293 cells displayed Michaelis–Menten saturation kinetics with a Vmax of 65.8±2.2 pmol per mg protein per min and a Km of 771±66 nM. On the basis of this kinetic assessment, a saturating substrate concentration of 10 μM [3H]5-HT was chosen at which the inhibitory potency of each of the MDMA analogues was to be assessed. The mean rate of specific [3H]5-HT transport at this concentration in the absence of any inhibitor was 64.5±4.1 pmol per mg protein per min.
Data analysis
All transport experiments were conducted under conditions of initial velocity. Data presented are mean values±s.e.mean obtained from n experiments as indicated in figure legends. Total uptake data were corrected for nonspecific uptake and analysed by fitting to a sigmoidal concentration–response curve (variable slope) using the Prism 4 software from GraphPad Software Inc, San Diego, CA, USA. The maximum and minimum values were constrained to 100 and 0% respectively during all analyses so as to allow the accurate determination of the IC50, Hill slopes and Ki values in each experiment (maximum=transport in the absence of any inhibitor and minimum=nonspecific transport). Data were analysed by one-way ANOVA followed by a Tukey's post hoc test for multiple comparisons. In each case, the difference between means was considered significant at P-values of <0.05. Hill slope data were analysed by the F-test and when Hill slopes were not significantly different from −1 (P>0.05), the IC50 value was converted into an affinity constant (Ki). All transport data were calculated as pmol per mg protein per min, but are graphed as percentage of control values for comparative purposes.
Materials
[3H]Noradrenaline (specific activity ∼37 MBq (36 Ci) mmol−1 and [3H]5-HT (specific activity ∼37 MBq (15.7 Ci) mmol−1) were purchased from Amersham Biosciences, Buckinghamshire, UK. DMEM, penicillin/streptomycin, fetal bovine serum, blasticidin and zeocin were purchased from Invitrogen Corporation, Carlsbad, CA, USA. Tet system-approved fetal bovine serum was supplied by BD Biosciences, Erembodegem, Belgium. Scintillation cocktail (Ecoscint A) was supplied by National Diagnostics USA, Atlanta, Georgia, USA. Life Sciences. All other chemicals and reagents were purchased from Sigma-Aldrich and were of the highest grade commercially available.
Results
MDMA is a potent inhibitor of [3H]NA uptake in PC12 cells
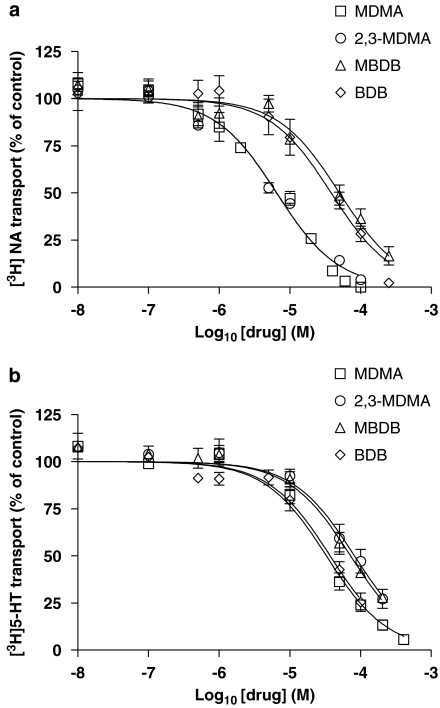
The IC50 values for inhibition of 10-μM [3H]NA and 10-μM [3H]5-HT uptake were determined from log concentration–response curves generated at both NET and SERT using a concentration range of 1 nM–0.4 mM MDMA (Figures 2a and b). The IC50 value for inhibition of [3H]NA transport by MDMA was 6.6±1.1 μM, whereas the value for MDMA-mediated inhibition of [3H]5-HT transport was 34.8±1.1 μM (Table 1). At a substrate concentration of 1 μM ([3H]NA) or 100 nM ([3H]5-HT), the potency of MDMA inhibition increased, resulting in a characteristic shift of both IC50 curves to the left. At NET, the MDMA IC50 value decreased approximately fourfold from its value at 10 μM [3H]NA to 1.7±0.9 μM at 1 μM [3H]NA. Similarly at SERT, the MDMA IC50 value decreased approximately 15-fold from its value at 10 μM [3H]5-HT to 2.2±1.2 μM at 100 nM [3H]5-HT (results not shown). Hill slope values for MDMA at both NET and SERT were not significantly different from −1 and the calculated affinity constants (Ki) were 0.6 (NET) and 2.5 μM (SERT). These data are consistent with MDMA acting as a competitive inhibitor of [3H]NA transport in PC12 cells and of [3H]5-HT transport in the T-REx SERT HEK 293 cell line.
Figure 2.
The effect of 3,4-methylenedioxymethamphetamine (MDMA) analogues on [3H]noradrenaline ([3H]NA) and [3H]5-HT transport. Log concentration–response (IC50) curve showing the inhibition of noradrenaline transporter (NET)-specific [3H]NA transport in rat phaeochromocytoma (PC12) cells (a) and 5-HT transporter (SERT)-specific [3H]5-HT transport in T-REx SERT HEK 293 cells (b) by MDMA, 2,3-methylenedioxymethamphetamine (2,3-MDMA), 3,4-methylenedioxyphenyl-N-methyl-2-butanamine (MBDB) and 3,4-methylenedioxyphenyl-2-butanamine (BDB). Cells were incubated with the appropriate [3H]neurotransmitter (10 μM) in the presence or absence (control) of a range of inhibitor concentrations (10 nM–0.5 mM) and the rate of uptake was determined as described in Methods. Data are presented as the mean±s.e.mean of at least four independent determinations performed in quadruplicate.
Table 1.
IC50 and Ki values for inhibition of [3H]neurotransmitter uptake by MDMA and its analogues at NET and SERT
| Drug |
NET |
SERT |
||||
|---|---|---|---|---|---|---|
| IC50 (μM) | Hill slope | Ki (μM) | IC50 (μM) | Hill slope | Ki (μM) | |
| MDMA | 6.6±1.1 | −1.06±0.11 | 0.6 | 34.8±1.1 | −1.18±0.12 | 2.5 |
| 2,3-MDMA | 6.2±1.2 | −0.86±0.10 | 0.6 | 82.0±1.1++,b | −1.08±0.15 | 5.9 |
| BDB | 39.5±1.1***,a | −1.18±0.15 | 3.6 | 37.0±1.2 | −1.02±0.14 | 2.7 |
| MBDB | 49.7±1.2***,a | −0.94±0.13 | 4.5 | 72.2±1.1+,b,c | −1.04±0.15 | 5.2 |
| HMA | 51.2±1.5***,a | −0.78±0.23 | 4.6 | 191.0±1.1+++,b | −1.08±0.22 | 13.7 |
| HMMA | 83.0±1.2***,a | −0.86±0.12 | 7.5 | 161.4±1.1+++,b | −1.14±0.17 | 11.5 |
| MDOH | 87.8±1.1***,a | −0.92±0.14 | 6.3 | 215.3±1.2+++,b | −1.14±0.23 | 15.4 |
| DMMA | 253.4±1.2***,a | −1.30±0.45 | 22.8 | 108.0±1.1+++,b | −1.05±0.09 | 7.7 |
| 2CB | 312.9±1.4***,a | −0.96±0.41 | 27.4 | 67.1±1.2 | −0.65±0.11d | |
Abbreviations: BDB=3,4-methylenedioxyphenyl-2-butanamine; 2CB=2,5-dimethoxy-4-bromophenylethylamine; DMMA=3,4-dimethoxymethamphetamine; HMA=4-hydroxy-3-methoxyamphetamine; HMMA=4-hydroxy-3-methoxymethamphetamine; MBDB=3,4-methylenedioxyphenyl-N-methyl-2-butanamine; MDMA; 3,4-methylenedioxymethamphetamine; 2,3-MDMA=2,3-methylenedioxymethamphetamine; MDOH=3,4-methylenedioxy-N-hydroxyamphetamine; NET=noradrenaline transporter; PC12=rat phaeochromocytoma; SERT=5-HT transporter.
Log concentration–response curves were generated for each of the MDMA analogues at NET in PC12 cells and SERT in T-REx HEK 293 cells. Cells were incubated with 10 μM of the appropriate [3H]neurotransmitter for 5 (NET) or 6 min (SERT) in the presence or absence of a range of inhibitor concentrations (1 nM–0.5 mM), as specified in Materials and methods. IC50 data are presented as the means±s.e.mean of at least four individual experiments, each performed in quadruplicate. Multiple comparisons by the Tukey's post hoc test were performed after one-way ANOVA.
With respect to MDMA at NET.
With respect to MDMA at SERT.
With respect to BDB at SERT (+P<0.05, ++P<0.01; ***, +++P<0.001). Hill slope data were analysed by the F-test. When Hill slopes were not significantly different from −1, the IC50 was converted to an affinity constant Ki (dP<0.05).
MDMA-methylenedioxy functionality is a requirement for substrate inhibition at both NET and SERT
2,3-Methylenedioxymethamphetamine, HMMA, HMA, DMMA and 2CB all carry a modification to the methylenedioxy bridge at positions 3 and 4 of the phenyl ring of MDMA (Figure 1). As can be seen from Table 1, a shift of the methylenedioxy bridge from positions 3 and 4 to positions 2 and 3 of the phenyl ring (2,3-MDMA) did not affect the potency of MDMA at NET (Figure 2a; Table 1). However, 2,3-MDMA was significantly less potent than MDMA at SERT, this time demonstrating a twofold decrease in inhibitory potency (Figure 2b; Table 1). In contrast, compounds in which the 3,4-methylenedioxy bridge was replaced by either a hydroxy or methoxy substitution at positions 3 and 4 of the phenyl ring (such as HMA, HMMA and DMMA) were all significantly less potent inhibitors of monoamine transport at both NET and SERT when compared to MDMA (Table 1). The N-demethylation of HMMA, resulting in HMA (Figure 1) did not alter its inhibitory potency. DMMA, in particular, showed itself to be a very weak inhibitor of [3H]NA uptake. 2,5-Dimethoxy-4-bromophenylethylamine was also significantly less potent than MDMA at both NET and SERT. None of these compounds, with the exception of 2CB at SERT, had Hill slopes significantly different from −1, which is indicative of competitive inhibition. The calculated Ki values for HMA, HMMA and DMMA are shown in Table 1.
Modifications to the amphetamine α-methyl group greatly reduce analogue affinity at NET
3,4-Methylenedioxyphenyl-N-methyl-2-butanamine (MBDB) is the α-ethyl homologue of MDMA and therefore consists of a side chain containing an extra methylene group (Figure 1). From both Figure 2a and Table 1, it is clear that this simple addition to the amphetamine side chain significantly reduced the inhibitory potency of MDMA at NET. This was also true for BDB, the N-demethylated derivative of MBDB (Figure 1). BDB, although capable of reducing [3H]NA uptake to levels equal to that of nonspecific transport (albeit at a concentration of 250 μM BDB; Figure 2a), was approximately six times less potent than MDMA at inhibiting [3H]NA transport. MBDB was incapable of inducing maximal inhibition of NET-specific [3H]NA transport even at a concentration as high as 400 μM (Figure 2a) and was almost eight times less potent than MDMA at NET (Table 1).
In contrast, BDB is almost identical in potency to MDMA at SERT and demonstrates a very similar inhibitory profile with comparable threshold, IC50 and maximal inhibition values (Table 1; Figure 2b). MBDB is significantly less potent than MDMA at SERT (Table 1). However, the decrease in MBDB inhibitory potency at SERT is only twofold when compared to MDMA, as opposed to the eightfold decrease in comparison to MDMA at NET (Table 1). These data suggest that MBDB may possess greater inhibitory potency at SERT than NET. Neither MBDB nor BDB had Hill slopes significantly different from −1 and the calculated Ki values for each of these compounds are shown in Table 1.
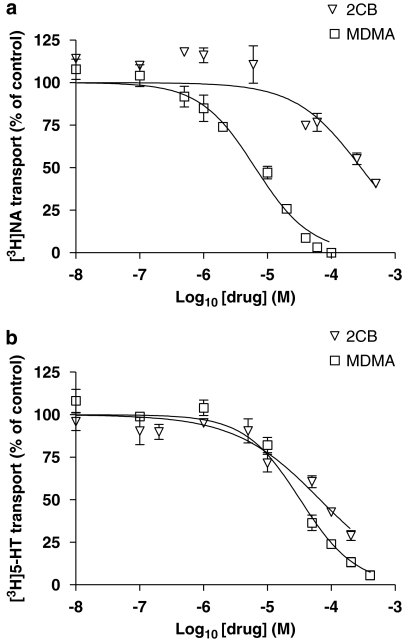
2,5-Dimethoxy-4-bromophenylethylamine (2CB) differs from MDMA in that the 3,4-methylenedioxy bridge has been replaced by a methoxy group at both positions 2 and 5 of the phenyl ring as well as a bromo substituent at position 4 (Figure 1). However, in spite of the para substitution of a metabolically resistant bromo group on the phenyl ring, the reduction of the amphetamine side chain from three to two carbon atoms, combined with the di-methoxy substitution of the phenyl ring (as seen with DMMA) appears to have abolished the inhibitory potency of MDMA at NET (Table 1; Figure 3a). In contrast, 2CB proved to be a much more effective inhibitor of SERT, but the Hill slope for 2CB at SERT was significantly different from −1, indicating that 2CB was not a competitive inhibitor of 5-HT at SERT (Figure 3b; Table 1).
Figure 3.
The effect of 2,5-dimethoxy-4-bromophenylethylamine (2CB) on [3H]noradrenaline ([3H]NA) and [3H]5-HT transport. Log concentration–response (IC50) curve showing the inhibition of noradrenaline transporter (NET)-specific [3H]NA transport in rat phaeochromocytoma (PC12) cells (a) and 5-HT transporter (SERT)-specific [3H]5-HT transport in T-REx SERT HEK 293 cells (b) by 3,4-methylenedioxymethamphetamine (MDMA) and 2CB. Cells were incubated with the appropriate [3H]neurotransmitter (10 μM) in the presence or absence (control) of a range of inhibitor concentrations (10 nM–0.5 mM) and the rate of uptake was determined as described in Methods. Data are presented as the mean±s.e.mean of at least four independent determinations performed in quadruplicate.
Hydroxylation of the primary amine significantly reduces analogue affinity at both NET and SERT
3,4-Methylenedioxy-N-hydroxyamphetamine (MDOH), which differs from MDMA in that the methyl group of the primary amine has been replaced by a hydroxyl group (Figure 1), was an extremely weak inhibitor of specific monoamine transport and reduced the inhibitory potency of MDMA by almost 13- and 6-fold at NET and SERT, respectively (Table 1).
Discussion
The structural analogues of MDMA tested in this study, many of which have been identified in so-called ‘ecstasy' tablets, are all inhibitors of either noradrenaline and/or 5-HT transport. All of the analogues tested, with the exception of 2CB at SERT, displayed Hill slopes that were not significantly different from –1 and indicates that they are all competitive inhibitors of each transporter. It is probable, therefore, that the inhibitory potency of these compounds for either transporter would be greater at a physiological concentration of the substrate (that is, in the nanomolar range) than was observed under the saturating substrate conditions selected for these experiments. This was noted to be the case for MDMA, as its IC50 was approximately 15-fold lower at 100 nM [3H]5-HT than at 10 μM.
One of the main findings of this study is that both SERT and NET possess very different requirements for inhibition of uptake and that certain common structural motifs confer greater inhibitory potency at one, but not the other transporter. For instance, modifications to the amphetamine side chain α-methyl group significantly reduce inhibitory potency at NET, but not at SERT. MBDB and BDB were notably less potent than MDMA at inhibiting [3H]NA uptake via NET, yet both are very effective inhibitors of 5-HT uptake at SERT, where they share a very similar inhibitory profile to that seen with MDMA. These results support the findings of Pifl et al. (2005), which showed that replacement of the α-methyl group with a 3,4-methylenedioxybenzyl group greatly inhibited inhibitory potency at NET, but not SERT. MBDB has recently been listed as a controlled substance in the United States and France due to its highly addictive and unique empathogenic properties and its abuse has been noted throughout Europe and the United States (Nagai et al., 2002). In addition, it is often sold in place of MDMA and is a common contaminant of illegal ‘ecstasy' tablets (Furnari et al., 1998; Van Aerts et al., 2000; Freudenmann and Spitzer, 2004). Like the ‘entactogen' MDMA, MBDB can be clearly distinguished from psychostimulants, such as amphetamine, and hallucinogens (for example, mescaline, 2,5-dimethoxy-4-methylamphetamine) in rat discrimination studies. However, it is also known to cause less euphoria and to lack the stimulant properties that are commonly associated with MDMA (Oberlender and Nichols, 1988, 1990; Van Aerts et al., 2000). Although MBDB is known to stimulate little or no DA release and is a weak inhibitor of DAT (Steele et al., 1987; Van Aerts et al., 2000), this is unlikely to be the reason behind its apparent reduction in MDMA-like effects, because dopamine release is more commonly associated with the reinforcing qualities of amphetamines and not the stimulatory properties (Van Aerts et al., 2000). Therefore, since increases in extracellular NA are thought to contribute significantly to amphetamine-induced stimulatory action (Pifl et al., 1999; Rothman et al., 2001), it is more likely to be the weak inhibitory potency of MBDB at NET that is responsible for its modest stimulatory and euphoric effects.
3,4-Methylenedioxyphenyl-2-butanamine (BDB) is the N-demethylated derivative of MBDB and has been found in the urine of MBDB abusers (Kronstrand, 1996; Kintz, 1997). In 1978, Shulgin (1978) demonstrated that N-methylation of hallucinogenic phenylethylamine derivatives resulted in a decrease in hallucinogenic activity. This finding was later confirmed by Bronson et al. (1995) when examining the increased hallucinogenic properties demonstrated by BDB in comparison to MBDB. In this study, BDB was also shown to be significantly more potent than MBDB via the demonstration of a greater inhibitory potency of [3H]5-HT uptake at SERT. Given the association of hallucinogenic manifestation with the activation of 5-HT2 receptors (Titeler et al., 1988), our data support a role of SERT inhibition and subsequent increases in extracellular 5-HT in the manifestation of hallucinogenic episodes.
During the preparation of this manuscript, Nagai et al. (2007) published comparative data on the inhibition of monoamine transport in crude synaptosomes by a range of amphetamine derivatives, including BDB and MBDB. Direct comparison of their results and ours is difficult, since the experimental systems are not the same. The much lower IC50 values quoted by Nagai et al. (2007) for these compounds in crude synaptosomes reflects the fact that these are competitive inhibitors and both [3H]NA and [3H]5-HT were employed at a considerably lower concentration than the saturating dose of 10 μM selected for our experiments. Nagai et al. (2007) show that BDB and MBDB were equipotent in their inhibition of synaptosomal [3H]NA and [3H]5-HT uptake, which is the same result as we obtained in our experiments (although we have deliberately not compared potencies of the drugs to each transporter, because different cell lines were used for each). We also agree that both compounds are less potent than MDMA as inhibitors of [3H]NA transport. However, although Nagai et al. concluded that BDB and MBDB were both less potent than MDMA as inhibitors of [3H]5-HT uptake, we show that MDMA and BDB are equipotent.
2,5-Dimethoxy-4-bromophenylethylamine (2CB) is similar to MBDB and BDB in that it too carries a modification of the α-methyl group on the amphetamine side chain. In this case, the α-methyl group has been removed resulting in a two-carbon, as opposed to a three-carbon, amphetamine side chain. In addition, the substitution pattern on the phenyl ring of 2CB is very different to that of the other analogues tested, that is, a methoxy group at positions 2 and 5 and a bromo substituent at position 4. Interestingly, these modifications result in a greatly reduced inhibitory potency at NET, but not at SERT and are in sharp contrast to DMMA and HMMA (which also carry modifications to the phenyl ring, although at positions 3 and 4), which were extremely weak inhibitors of both NET and SERT. It should be noted that 2CB was the only analogue analysed that did not demonstrate competitive binding at SERT. This suggests that 2CB binds to the transporter independently of the substrate site. Structural motifs that are relevant for competitive binding at SERT may be less important if the inhibitor binds at a different site on the transporter.
2,5-Dimethoxy-4-bromophenylethylamine is an illicit, synthetic schedule 1 hallucinogen and is thought to be active in humans at levels as low as 0.1–0.2 mg kg−1, thus rendering this compound approximately 10 times more potent than its three-carbon amphetamine analogues (Shulgin and Carter, 1975). Severe hallucinogenic properties of this compound at higher doses have been recorded (Giroud et al., 1998; Munehiro and Hitoshi, 2002). 2CB is typically regarded as a 5-hydroxytryptaminergic agent as it is a known agonist of 5-HT2 receptors (Glennon et al., 1988). Our results support the view that 2CB targets the 5-HT system, as 2CB was a potent inhibitor of 5-hydroxytryptaminergic, but not noradrenergic, neurotransmission when compared at the level of the two transporters.
A second observation drawn from this study, in regard to analogue recognition at the NA and 5-HT transporters, is that while the positioning of the methylenedioxy group may not be a strict requirement for substrate affinity at NET, alternative positioning of the 3,4-methylenedioxy bridge on the MDMA phenyl ring significantly reduces analogue or drug-binding affinity at SERT. For instance, 2,3-MDMA and MDMA are equipotent at NET, whereas MDMA is clearly twice as potent as 2,3-MDMA in inhibiting [3H]5-HT uptake at SERT. However, removal or replacement of the 3,4-methylenedioxy bridge with alternative substituents (HMMA, HMA and DMMA) greatly diminishes MDMA inhibitory potency at both NET and SERT. This suggests that while the methylenedioxy functionality is a requirement for inhibitory potency at both NET and SERT, NET permits greater flexibility with regard to the actual positioning of the methylenedioxy bridge on the phenyl ring.
Other findings indicate that the hydroxylation of the primary amine in the MDMA analogue MDOH (Figure 1) severely decreases its inhibitory potency at both NET and SERT (Table 1). In fact, the simple substitution of a hydroxyl group for a methyl group at the primary amine reduced the inhibitory potency of MDMA by a factor of 13 and 6 at NET and SERT, respectively (Table 1). Previous studies involving amphetamine discriminative analysis in rats have shown that MDOH possesses even less of an amphetamine-like component of action than MDMA itself (Glennon and Misenheimer, 1989). Taken together, these findings suggest that MDOH is a surprisingly weak mediator of MDMA-like effects.
In summary, this study demonstrates the following in vitro structure–activity relationships (SAR) at rat NET and SERT using, among others, the previously unknown MDMA analogue, 2,3-MDMA: (1) MDMA methylenedioxy functionality is a requirement for effective MDMA inhibitory action at both SERT and NET; (2) the actual positioning of the methylenedioxy bridge on the phenyl ring is a more significant requirement at SERT than NET; (3) alterations to the amphetamine side chain significantly reduce inhibitory potency at NET, but have little effect on transporter recognition of MDMA at SERT; and (4) MDMA metabolites (HMA and HMMA) are less potent than the parent compound MDMA at inhibiting NA and 5-HT transport. In addition, we provide new information regarding the pharmacological activities of the common MDMA contaminants 2CB, MBDB and BDB (Giroud et al., 1998; Kintz and Samyn, 1999; Vaiva et al., 2001b) at NET and SERT. The data presented in this report provide crucial information with regard to the mode of action of previously unknown MDMA analogues, all of which have a high-risk potential for abuse among the global youth of today. Further studies will determine the carrier-mediated releasing properties and the toxicity of these compounds in vitro.
Acknowledgments
The support of the Health Research Board of Ireland is gratefully acknowledged. The Conway Institute is funded by the Programme for Research in Third Level Institutions in Ireland (PRTLI), administered by the Higher Education Authority (HEA).
Abbreviations
- BDB
3,4-methylenedioxyphenyl-2-butanamine
- 2CB
2,5-dimethoxy-4-bromophenylethylamine
- DMMA
3,4-dimethoxymethamphetamine
- HMA
4-hydroxy-3-methoxyamphetamine
- HMMA
4-hydroxy-3-methoxymethamphetamine
- MBDB
3,4-methylenedioxyphenyl-N-methyl-2-butanamine
- MDMA
3,4-methylenedioxymethamphetamine
- 2,3-MDMA
2,3-methylenedioxymethamphetamine
- MDOH
3,4-methylenedioxy-N-hydroxyamphetamine
- NA
noradrenaline
- NET
noradrenaline transporter
- SERT
5-hydroxytryptamine transporter
Conflict of interest
The authors state no conflict of interest.
References
- Anderson WK, Dabrah TT, Houston MD. Synthesis of erbstatin, a naturally occurring inhibitor of tyrosine-specific protein kinase. J Org Chem. 1987;52:1945–1947. [Google Scholar]
- Becker J, Neis P, Rohrich J, Zorntlein S. A fatal paramethoxymethamphetamine intoxication. Leg Med (Tokyo) 2003;5 Suppl 1:S138–S141. doi: 10.1016/s1344-6223(02)00096-2. [DOI] [PubMed] [Google Scholar]
- Braun U, Shulgin AT, Braun G. Centrally active N-substituted analogs of 3,4-methylenedioxyphenylisopropylamine (3,4-methylenedioxyamphetamine) J Pharm Sci. 1980;69:192–195. doi: 10.1002/jps.2600690220. [DOI] [PubMed] [Google Scholar]
- Breier JM, Bankson MG, Yamamoto BK. L-tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. J Neurosci. 2006;26:290–299. doi: 10.1523/JNEUROSCI.3353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson ME, Jiang W, DeRuiter J, Clark CR. Structure–activity relationships of BDB and its monomethyl and dimethyl derivatives. Pharmacol Biochem Behav. 1995;51:477–479. doi: 10.1016/0091-3057(95)00012-l. [DOI] [PubMed] [Google Scholar]
- Cheng JY, Chan MF, Chan TW, Hung MY. Impurity profiling of ecstasy tablets seized in Hong Kong by gas chromatography–mass spectrometry. Forensic Sci Int. 2006;162:87–94. doi: 10.1016/j.forsciint.2006.02.055. [DOI] [PubMed] [Google Scholar]
- Colado MI, O'Shea E, Green AR. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology (Berl) 2004;173:249–263. doi: 10.1007/s00213-004-1788-8. [DOI] [PubMed] [Google Scholar]
- de Boer D, Gijzels MJ, Bosman IJ, Maes RAA. More data about the new psychoactive drug 2C-B. J Anal Toxicol. 1999;23:227–228. doi: 10.1093/jat/23.3.227. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Roset PN, Pizarro N, Abanades S, Segura M, et al. Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit. 2004;26:137–144. doi: 10.1097/00007691-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Escobedo I, O'Shea E, Orio L, Sanchez V, Segura M, de la Torre R, et al. A comparative study on the acute and long-term effects of MDMA and 3,4-dihydroxymethamphetamine (HHMA) on brain monoamine levels after i.p. or striatal administration in mice. Br J Pharmacol. 2005;144:231–241. doi: 10.1038/sj.bjp.0706071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsling ML, Fallon JK, Shah D, Tilbrook GS, Cowan DA, Kicman AT, et al. The effect of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy') and its metabolites on neurohypophysial hormone release from the isolated rat hypothalamus. Br J Pharmacol. 2002;135:649–656. doi: 10.1038/sj.bjp.0704502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenmann RW, Spitzer M. The neuropsychopharmacology and toxicology of 3,4-methylenedioxy-N-ethyl-amphetamine (MDEA) CNS Drug Rev. 2004;10:89–116. doi: 10.1111/j.1527-3458.2004.tb00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Snoddy HD, Molloy BB. Blockade of amine depletion by nisoxetine in comparison to other uptake inhibitors. Psychopharmacol Commun. 1975;1:455–464. [PubMed] [Google Scholar]
- Furnari C, Ottaviano V, Rosati F, Tondi V. Identification of 3,4-methylenedioxyamphetamine analogs encountered in clandestine tablets. Forensic Sci Int. 1998;92:49–58. doi: 10.1016/s0379-0738(98)00005-x. [DOI] [PubMed] [Google Scholar]
- Giroud C, Augsburger M, Rivier L, Mangin P, Sadeghipour F, Varesio E, et al. 2C-B: a new psychoactive phenylethylamine recently discovered in Ecstasy tablets sold on the Swiss black market. J Anal Toxicol. 1998;22:345–354. doi: 10.1093/jat/22.5.345. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Misenheimer BR. Stimulus effects of N-monoethyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane (MDE) and N-hydroxy-1-(3,4-methylenedioxyphenyl)-2-aminopropane (N-OH MDA) in rats trained to discriminate MDMA from saline. Pharmacol Biochem Behav. 1989;33:909–912. doi: 10.1016/0091-3057(89)90491-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Lyon RA. A preliminary investigation of the psychoactive agent 4-bromo-2,5-dimethoxyphenethylamine: a potential drug of abuse. Pharmacol Biochem Behav. 1988;30:597–601. doi: 10.1016/0091-3057(88)90071-8. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy') Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Duvauchelle C, Ikegami A, Olsen CM, Lau SS, de la Torre R, et al. Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J Pharmacol Exp Ther. 2005;313:422–431. doi: 10.1124/jpet.104.077628. [DOI] [PubMed] [Google Scholar]
- Kintz P. Excretion of MBDB and BDB in urine, saliva, and sweat following single oral administration. J Anal Toxicol. 1997;21:570–575. doi: 10.1093/jat/21.7.570. [DOI] [PubMed] [Google Scholar]
- Kintz P, Samyn N. Determination of ‘Ecstasy' components in alternative biological specimens. J Chromatogr B Biomed Sci Appl. 1999;733:137–143. doi: 10.1016/s0378-4347(98)00521-0. [DOI] [PubMed] [Google Scholar]
- Kronstrand R. Identification of N-methyl-1-(3,4-methylenedioxyphenyl)-2-butanamine (MBDB) in urine from drug users. J Anal Toxicol. 1996;20:512–516. doi: 10.1093/jat/20.6.512. [DOI] [PubMed] [Google Scholar]
- Lemberger L, Terman S, Rowe H, Billings R. The effect of nisoxetine (Lilly compound 94939), a potential antidepressant on biogenic amine uptake in man. Br J Clin Pharmacol. 1976;3:215–220. doi: 10.1111/j.1365-2125.1976.tb00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. Recreational ecstasy use and the neurotoxic potential of MDMA: current status of the controversy and methodological issues. Drug Alcohol Rev. 2006;25:269–276. doi: 10.1080/09595230600657758. [DOI] [PubMed] [Google Scholar]
- Milhazes N, Cunha-Oliveira T, Martins P, Garrido J, Oliveira C, Cristina Rego A, et al. Synthesis and cytotoxic profile of 3,4-methylenedioxymethamphetamine (‘ecstasy') and its metabolites on undifferentiated PC12 Cells: a putative structure–toxicity relationship. Chem Res Toxicol. 2006;19:1294–1304. doi: 10.1021/tx060123i. [DOI] [PubMed] [Google Scholar]
- Monks TJ, Jones DC, Bai F, Lau SS. The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit. 2004;26:132–136. doi: 10.1097/00007691-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Morton J. Ecstasy: pharmacology and neurotoxicity. Curr Opin Pharmacol. 2005;5:79–86. doi: 10.1016/j.coph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Munehiro K, Hitoshi T. Update on clandestine amphetamines and their analogues recently seen in Japan. J Health Sci. 2002;48:14–21. [Google Scholar]
- Nagai F, Nonaka R, Satoh K, Kamimura H. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Nagai T, Matsushima K, Suzuki A, Saotome A, Kurosu A, Nihei H, et al. Enantiomer analysis of a new street drug, 3,4-methylenedioxy-N-methyl-butanamine, in rat urine. J Anal Toxicol. 2002;26:104–109. doi: 10.1093/jat/26.2.104. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Hoffman AJ, Oberlender RA, Jacob P, III, Shulgin AT. Derivatives of 1-(1,3-benzodioxol-5-yl)-2-butanamine: representatives of a novel therapeutic class. J Med Chem. 1986;29:2009–2015. doi: 10.1021/jm00160a035. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE. Drug discrimination studies with MDMA and amphetamine. Psychopharmacology (Berl) 1988;95:71–76. doi: 10.1007/BF00212770. [DOI] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE. N-methyl-1-(1,3-benzodioxol-5-yl)-2-butanamine as a discriminative stimulus in studies of 3,4-methylenedioxy-methamphetamine-like behavioral activity. J Pharmacol Exp Ther. 1990;255:1098–1106. [PubMed] [Google Scholar]
- Pifl C, Agneter E, Drobny H, Sitte HH, Singer EA. Amphetamine reverses or blocks the operation of the human noradrenaline transporter depending on its concentration: superfusion studies on transfected cells. Neuropharmacology. 1999;38:157–165. doi: 10.1016/s0028-3908(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Pifl C, Nagy G, Berenyi S, Kattinger A, Reither H, Antus S. Pharmacological characterization of ecstasy synthesis byproducts with recombinant human monoamine transporters. J Pharmacol Exp Ther. 2005;314:346–354. doi: 10.1124/jpet.105.084426. [DOI] [PubMed] [Google Scholar]
- Refstad S. Paramethoxyamphetamine (PMA) poisoning; a ‘party drug' with lethal effects. Acta Anaesthesiol Scand. 2003;47:1298–1299. doi: 10.1046/j.1399-6576.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioural effects of amphetamine-type drugs. Ann NY Acad Sci. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schifano F, Corkery J, Deluca P, Oyefeso A, Ghodse AH. Ecstasy (MDMA, MDA, MDEA, MBDB) consumption, seizures, related offences, prices, dosage levels and deaths in the UK (1994–2003) J Psychopharmacol. 2006;20:456–463. doi: 10.1177/0269881106060147. [DOI] [PubMed] [Google Scholar]
- Shulgin AT.Psychotomimetic drugs: structure–activity relationships Handbook of Psychopharmacology 1978Plenum: New York; 243–333.In: Iversen LL, Iversen SD, Snyder SH (eds) [Google Scholar]
- Shulgin AT, Carter MF. Centrally active phenethylamines. Psychopharmacol Commun. 1975;1:93–98. [PubMed] [Google Scholar]
- Siedel S, Singer EA, Just H, Farhan H, Scholze P, Kudlacek O, et al. Amphetamines take two to tango: an oligomer-based counter-transport model of neurotransmitter transport explores amphetamine action. Mol Pharmacol. 2005;67:140–151. doi: 10.1124/mol.67.1.. [DOI] [PubMed] [Google Scholar]
- Simonsen KW, Kaa E. Designer drugs in Jutland. Ugeskr Laeger. 2001;163:2248–2252. [PubMed] [Google Scholar]
- Steele TD, Nichols DE, Yim GK. Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharmacol. 1987;36:2297–2303. doi: 10.1016/0006-2952(87)90594-6. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Tanner-Smith EE. Pharmacological content of tablets sold as ‘ecstasy': results from an online testing service. Drug Alcohol Depend. 2006;83:247–254. doi: 10.1016/j.drugalcdep.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Tate CG, Haase J, Baker C, Boorsma M, Magnani F, Vallis Y, et al. Comparison of seven different heterologous protein expression systems for the production of the serotonin transporter. Biochim Biophys Acta. 2003;1610:141–153. doi: 10.1016/s0005-2736(02)00719-8. [DOI] [PubMed] [Google Scholar]
- Teng SF, Wu SC, Liu C, Li JH, Chien CS. Characteristics and trends of 3,4-methylenedioxymethamphetamine (MDMA) tablets found in Taiwan from 2002 to February 2005. Forensic Sci Int. 2006;161:202–208. doi: 10.1016/j.forsciint.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Nelson DR, Johnson AM. Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology. 1987;93:193–200. doi: 10.1007/BF00179933. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology (Berl) 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- UNODC World drug report 2006United Nations publications: New York; Vol. 1, [Google Scholar]
- Vaiva G, Bailly D, Boss V, Thomas P, Lestavel P, Goudemand M. A case of acute psychotic episode after a single dose of ecstasy. Encephale. 2001a;27:198–202. [PubMed] [Google Scholar]
- Vaiva G, Boss V, Bailly D, Thomas P, Lestavel P, Goudemand M. An ‘accidental' acute psychosis with ecstasy use. J Psychoactive Drugs. 2001b;33:95–98. doi: 10.1080/02791072.2001.10400473. [DOI] [PubMed] [Google Scholar]
- Van Aerts LA, Mallaret M, Rigter H. N-methyl-1- (1,3-benzodioxol-5-yl)-2-butanamine (MBDB): its properties and possible risks. Addict Biol. 2000;5:269–282. doi: 10.1111/j.1369-1600.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Varma RS, Kabalka GW. Synthesis of alkylamines via reduction of nitroalkenes using in situ prepared BH3·THF. Synthetic Commun. 1985;15:843–847. [Google Scholar]
- Verrico CD, Miller GM, Madras BK. MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology (Berl) 2005;189:489–503. doi: 10.1007/s00213-005-0174-5. [DOI] [PubMed] [Google Scholar]